You are here: Home > Medical Countermeasures Database > N-Acetylcysteine
N-Acetylcysteine - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
N- Acetylcysteine
2. Chemical Defense therapeutic area(s)
— including key possible usesTreatment of chemical induced lung injury and respiratory toxicity including exposure to nerve agents, sulfur mustard gas, chlorine, phosgene.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
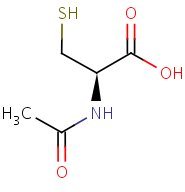
HSDB. N-acetylcysteine
Mechanism of action
-
N-acetylcysteine (NAC) is an amino acid with a MW of 163.2. It acts as an antioxidant, both directly as a glutathione substitute and indirectly as a precursor for glutathione. It also causes vasodilation by increasing cyclic guanosine monophosphate levels, inhibits platelet aggregation, acts as a sulphydryl donor to regenerate endothelial-derived relaxing factor and reduces IL-8 and TNF-alpha production. While there is evidence for its effectiveness as an antidote to paracetamol poisoning, its use in other disorders has only experimental or anecdotal support. For example, in hepatic failure, there are few studies in man showing improved outcome following NAC therapy. There is also conflicting evidence for the use of NAC in sepsis or ARDS and while there is some evidence to suggest that NAC may be of benefit in acute myocardial infarction, the patient numbers are small. It may also be of use in ameliorating nitrate tolerance. It is also possible that NAC may confer benefit in reducing the risks of radiographic contrast nephropathy, although the study suggesting this was probably insufficiently powered to review all patient subsets (e.g. diabetics). N-acetylcysteine would also appear to enhance T cell function in HIV infected patients. However, the use of NAC for immunomodulation in HIV patients has not yet undergone prospective randomised controlled trials and therefore cannot be recommended as routine therapy in HIV infected, or other immune deficient, patients. There is currently insufficient evidence to propose NAC for the treatment of carbon monoxide poisoning. Whilst there is experimental evidence for a variety of novel roles for NAC, further clinical studies are required before it can be recommended for the routine management of any disorders other than that of paracetamol poisoning.
Atkinson MC. The use of N-acetylcysteine in intensive care. Crit Care Resusc. 2002 Mar;4(1):21-7. [PubMed Citation]
-
Chlorine (Cl(2)) is a reactive oxidant gas used extensively in industrial processes. Exposure of both humans and animals to high concentrations of Cl(2) results in acute lung injury, which may resolve spontaneously or progress to acute respiratory failure. Injury to airway and alveolar epithelium may result from chemical reactions of Cl(2), from HOCl (the hydrolysis product of Cl(2)), and/or from the various reaction products, such as chloramines, that are formed from the reactions of these chlorinating species with biological molecules. Subsequent reactions may initiate self-propagating reactions and induce the production of inflammatory mediators compounding injury to pulmonary surfactant, ion channels, and components of lung epithelial and airway cells. Low-molecular-weight antioxidants, such as ascorbate, glutathione, and urate, present in the lung epithelial lining fluid and tissue, remove Cl(2) and HOCl and thus decrease injury to critical target biological targets. However, levels of lung antioxidants of animals exposed to Cl(2) in concentrations likely to be encountered in the vicinity of industrial accidents decrease rapidly and irreversibly. Our measurements show that prophylactic administration of a mixture containing ascorbate and desferal N-acetyl-cysteine, a precursor of reduced glutathione, prevents Cl(2)-induced injury to the alveolar epithelium of rats exposed to Cl(2). The clinical challenge is to deliver sufficient quantities of antioxidants noninvasively, after Cl(2) exposure, to decrease morbidity and mortality.
Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc. 2010;7:278-83. [PubMed Citation]
-
The chemical warfare agent analog, 2-chloroethyl ethyl sulfide, known as 'half-mustard gas' (HMG), is less toxic and less of an environmental hazard than the full molecule and has been shown to produce an acute lung injury in rats when instilled via intrapulmonary injection. This injury is characterized by massive, localized hemorrhage and edema into the alveolar compartment and can be quantitated by measuring extravasation of (125)I-bovine serum albumin into the extravascular compartment. Employing this rat model of HMG-induced lung injury, we observed significant attenuation of the pulmonary injury when experimental animals were complement or neutrophil depleted prior to HMG challenge. Significant protection also was provided by the use of antioxidants such as catalase, dimethyl sulfoxide, dimethyl thiourea, resveratrol and N-acetyl-L-cysteine (NAC). The last compound showed protection from lung injury as high as 70% and was still effective even when given up to 90 min after exposure of the lungs to HMG. These data suggest that acute lung injury caused by exposure to HMG may be related partially to complement mediated pathways and the generation by neutrophils of toxic oxygen species The data indicate that NAC is an effective antidote against HMG-induced acute lung injury in the rat.
McClintock SD, Till GO, Smith MG, Ward PA. Protection from half-mustard-gas-induced acute lung injury in the rat. J Appl Toxicol. 2002 Jul-Aug;22(4):257-62. [PubMed Citation]
-
NAC can stimulate GSH synthesis, enhance glutathione-S-transferase (GST) activity, promote detoxification, and act directly on reactive oxidant radicals. Moreover, NAC reduces the formation of proinflammatory cytokines, such as interleukin-8 and tumor necrosis factor-[alpha].
Moradi M, Mojtahedzadeh M, Mandegari A, Soltan-Sharifi MS, Najafi A, Khajavi MR, Hajibabayee M, Ghahremani MH. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine. Respir Med. 2009 Mar;103(3):434-441. [PubMed Citation]
-
N-acetylcysteine (NAC), as a thiol compound, often acts as an antioxidant or reactive oxygen species (ROS) scavenger to prevent lung injury in animal models. In addition, NAC has the potential to interact directly with oxidants or regulate the activation of nuclear factor (NF)-κB and hypoxia-inducible factor-1 (HIF-1) to attenuate acute lung injury.
Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593-597. [PubMed Citation]
-
1. Acute lung injury (ALI) or acute respiratory distress syndrome is a serious clinical problem with high mortality. N-Acetylcysteine (NAC) is an anti-oxidant and a free radical scavenger. It has been reported recently that NAC ameliorates organ damage induced by endotoxin (lipopolysaccharide; LPS) in conscious rats. The present study was designed to evaluate the effects of NAC on LPS-induced ALI and other changes in anaesthetized rats. 2. Sprague-Dawley rats were anaesthetized with pentobarbital (40 mg/kg, i.p.). Endotracheal intubation was performed to provide artificial ventilation. Arterial pressure and heart rate were monitored. The extent of ALI was evaluated with the lung weight (LW)/bodyweight ratio, LW gain, exhaled nitric oxide (NO) and protein concentration in bronchoalveolar lavage (PCBAL). Haematocrit, white blood cells, plasma nitrate/nitrite, methyl guanidine (MG), tumour necrosis factor (TNF)-alpha and interleukin (IL)-1b were measured. Pathological changes in the lung were examined and evaluated. 3. Endotoxaemia was produced by injection of 10 mg/kg, i.v., LPS (Escherichia coli). Animals were randomly divided into three groups. In the vehicle group, rats received an i.v. drip of physiological saline solution (PSS) at a rate of 0.3 mL/h. The LPS group received an i.v. drip of PSS for 1 h, followed by LPS (10 mg/kg by slow blous injection, i.v., over 1-2 min). Rats in the LPS + NAC group received NAC by i.v. drip at a rate of 150 mg/kg per h (0.3 mL/h) for 60 min starting 10 min before LPS administration (10 mg/kg by slow blous injection, i.v., over 1-2 min). Each group was observed for a period of 6 h. 4. N-Acetylcysteine treatment improved the LPS-induced hypotension and leukocytopenia. It also reduced the extent of ALI, as evidenced by reductions in LW changes, exhaled NO, PCBAL and lung pathology. In addition, NAC diminished the LPS-induced increases in nitrate/nitrite, MG, TNF-a and IL-1b. 5. In another series of experiments, LPS increased the mortality rate compared with the vehicle group (i.v. drip of PSS at a rate of 0.3 mL/h) during a 6 h observation period. N-Acetylcysteine, given 10 min prior to LPS, significantly increased the survival rate. 6. The results of the present study suggest that NAC exerts a protective effect on the LPS-induced ALI. The mechanisms of action may be mediated through the reduction of the production of NO, free radicals and pro-inflammatory cytokines.
Kao SJ, Wang D, Lin HI, Chen HI. (2006). N-acetylcysteine abrogates acute lung injury induced by endotoxin. Clin Exp Pharmacol Physiol. 2006;33:33-40. [PubMed]
-
Reactive oxygen species (ROS) play an important role in the pathogenesis of airway inflammation and hyperresponsiveness. Recent studies have demonstrated that antioxidants are able to reduce airway inflammation and hyperreactivity in animal models of allergic airway disease. A newly developed antioxidant, small molecular weight thiol compound, N-acetylcysteine amide (AD4) has been shown to increase cellular levels of glutathione and to attenuate oxidative stress related disorders such as Alzheimer's disease, Parkinson's disease, and multiple sclerosis. However, the effects of AD4 on allergic airway disease such as asthma are unknown. We used ovalbumin (OVA)-inhaled mice to evaluate the role of AD4 in allergic airway disease. In this study with OVA-inhaled mice, the increased ROS generation, the increased levels of Th2 cytokines and VEGF, the increased vascular permeability, the increased mucus production, and the increased airway resistance in the lungs were significantly reduced by the administration of AD4. We also found that the administration of AD4 decreased the increases of the NF-kappaB and hypoxia-inducible factor-1alpha (HIF-1alpha) levels in nuclear protein extracts of lung tissues after OVA inhalation. These results suggest that AD4 attenuates airway inflammation and hyperresponsiveness by regulating activation of NF-kappaB and HIF-1alpha as well as reducing ROS generation in allergic airway disease.
Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, Choe YH, Hong SH, Han HJ, Lee YR, Kim JS, Atlas D, Lee YC. (2007). A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp Mol Med. 2007 Dec;39(6):756-768. [PubMed Citation]
-
Previous studies indicated that oxidative stress was involved in phosgene-induced acute lung injury (ALI) and many antioxidants had been used to prevent ALI. N-acetylcysteine (NAC) had been used to protect ALI induced by various types of oxidative stress. Considering the limited information of NAC on phosgene-induced ALI, the purpose of this study was to elucidate the molecular mechanisms of phosgene-induced ALI and the protective effects of NAC. This study discovered that intraperitoneal administration of NAC significantly alleviated phosgene-induced pulmonary edema, as confirmed by decreased lung wet to dry weight ratio and oxidative stress markers. The content of l-gamma-glutamyl-l-cysteinyl-glycine (glutathione; GSH) and the ratio of the reduced and disulfide forms (GSH/GSSG), significant indicators of the antioxidative ability, were apparently inhibited by phosgene exposure. However, both indicators could be reversed by NAC administration, indicating that dysregulation of redox status of glutathione might be the cause of phosgene-induced ALI. The nuclear factor (NF)-E2-related factor 2 (Nrf2), which has been proven to up-regulate the expression of glutathione reductase (GR), was obviously decreased by phosgene exposure. However, NAC administration elevated Nrf2 expression significantly. In conclusion, these data provided the first evidences showing that it was the transcriptional factor Nrf2 that connected phosgene-induced ALI with GSH metabolism. NAC protected against oxidative stress through acting on this newly disclosed Nrf2/GR/GSH pathway, by which NAC elevated the biosynthesis of protective GSH to repair and reconstitute the defense system destroyed by phosgene.
Ji L, Liu R, Zhang XD, Chen HL, Bai H, Wang X, Zhao HL, Liang X, Hai CX. N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal Toxicol. 2010 Jun;22(7):535-42. [PubMed Citation]
Summary of clinical and non-clinical studies
Exposure to chemical warfare agents (CWAs) may result in oxidative stress and subsequent acute lung injury and respiratory toxicity. Evidence from several animal studies demonstrates the safe and effective use of antioxidants such as N-acetylcysteine (NAC), a thiol compound and precursor of glutathione (GSH), to overcome CWA-induced oxidative stress and associated acute lung injuries. In the isolated perfused rabbit lung model (IPRLM), intratracheal NAC (40 mg/kg for 10 minutes) was administered 45 minutes to 60 minutes after phosgene exposure (650 mg/m3) (Sciuto and Hurt, 2004). Pulmonary artery pressure, lung weight gain, pulmonary edema formation, leukotriene (C4, D4, and E4) concentration, lipid peroxidation, and oxidized GSH, which were increased following phosgene exposure, were decreased in the NAC-treated group compared with the untreated group (Sciuto et al., 1995; Sciuto and Hurt, 2004). It appears that the antioxidant properties of NAC maintained GSH levels and reduced both peroxidation of lipids and synthesis of arachidonic acid metabolites. However, NAC administration was not effective in blocking the phosgene-induced increase in tracheal pressure. In another study, phosgene-exposed animals (500 ppm for 1 minute) received saline (positive control), a low (50 mg/kg), moderate (100 mg/kg), or high (200 mg/kg) dose of NAC, or were exposed to phosgene and untreated (control) (Ji et al., 2010). Intraperitoneal (IP) administration of NAC caused a significant dose-dependent decrease in lung wet to dry weight ratio, which increased 3 hours after phosgene exposure. A significant decrease in oxidative stress markers was also observed after NAC administration at moderate and high doses. NAC was also able to significantly restore lung superoxide dismutase (SOD) and catalase (CAT) levels, which decreased 3 hours after phosgene exposure. The protective efficacy of NAC and other potential pulmonary therapeutics (surfactant, liquivent, dexamethasone, and anti-sense syk oligonucleotides) was evaluated in a nerve agent-exposed animal model (Nambiar et al., 2007). Guinea pigs exposed to VX (27.03 mg/m3) for 4 minutes had a 24-hour survival rate of 52% (10 of 19 guinea pigs) and a significant increase in bronchoalveolar lavage (BAL) protein, total cell number, and cell death. Treatment with NAC (75 mg/kg) 2 minutes after exposure to VX yielded the highest survival rate (75%, 3 of 4 guinea pigs) of all pulmonary therapeutics tested, but the increase in survival rate was only moderate. The protective efficacy of NAC (and the other pulmonary therapeutics) was significantly enhanced by the addition of atropine, likely due to inhibition of VX-induced mucous secretion; there was 100% survival of VX-exposed animals treated with all pulmonary agents tested plus atropine, except in the case of anti-sense syk (83% survival rate). The combination regimen also decreased BAL protein levels, total cell number, and cell death. Prophylactic NAC administration (5-40 mg/kg) demonstrated a protective efficacy as high as 70% (20 mg/kg dose) in rats with half mustard gas (HMG)-induced lung injuries (McClintock et al., 2002). Even when NAC treatment was delayed for as much as 90 minutes in HMG-exposed animals, a protective efficacy of 54% was observed. Protection after delayed treatment was not observed with any of the other tested antioxidants (catalase, dimethyl sulfoxide, dimethyl thiourea, and resveratrol). Prophylactic treatment with a mixture of NAC, ascorbate, and deferoxamine (150 mg/kg, 200 mg/kg, and 15 mg/kg, respectively; IP) in animals with chlorine-induced lung injury stabilized both ascorbate levels and arterial blood gas values, as well as decreased BAL fluid albumin levels (Leustik et al., 2008). A 30-minute chlorine gas challenge (184 ppm or 400 ppm) significantly induced hypoxemia and hypercapnia, increased BAL fluid protein concentration, and significantly decreased BAL fluid ascorbate levels in a rat model (Yadav et al., 2010). The aforementioned antioxidant mixture, but with a lower dose of ascorbate (80 mg/kg), was administered 18 hours intramuscularly and 1 hour intravenously before chlorine exposure to chlorine-challenged animals. The partial pressure of oxygen in arterial blood (Pao2) and ascorbate were restored to normal levels, and BAL fluid protein concentration decreased by about 35%. Another chlorine-gas exposed rat model revealed higher GSH levels and less lung tissue damage in animals treated with NAC 6 hours and 24 hours post-exposure compared to untreated animals (Akdur et al., 2008).
B. Link to clinical studies
Pediatric studies
-
Smoke-inhalation injury causes a destruction of the ciliated epithelium that lines the tracheobronchial tree. Casts produced from these cells, polymorphonuclear leukocytes and mucus, can cause upper-airway obstruction, contributing to pulmonary failure. We have reported that a combination of aerosolized heparin and a mucolytic agent, N-acetylcystine [corrected], can ameliorate cast formation and reduce pulmonary failure secondary to smoke inhalation. In this study, 90 consecutive pediatric patients between 1985 and 1995 who had bronchoscopically diagnosed inhalation injury requiring ventilatory support were studied. Forty-three children admitted between 1985 and 1989 acted as controls. Forty-seven children admitted between 1990 and 1994 received 5000 units of heparin and 3 ml of a 20% solution of N-acetylcystine [corrected] aerosolized every 4 hours the first 7 days after the injury. All patients were extubated when they were able to maintain spontaneously a PaO2/FIO2 ratio of more than 400. The number of patients requiring reintubation for successive pulmonary failure was recorded, as was mortality. The results indicate a significant decrease in reintubation rates, in incidence of atelectasis, and in mortality for patients treated with the regimen of heparin and N-acetylcystine [corrected] when compared with controls. Heparin/N-acetylcystine [corrected] nebulization in children with massive burn injury and smoke-inhalation injury results in a significant decrease in incidence of reintubation for progressive pulmonary failure and a reduction in mortality (Class III).
Desai MH, Mlcak R, Richardson J, Nichols R, Herndon DN. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine [correction of acetylcystine] therapy. J Burn Care Rehabil. 1998 May-Jun;19(3):210-2. [PubMed Citation]
Pregnancy, breastfeeding studies
-
There are no adequate and well-controlled studies of Acetadote in pregnant women. However, limited case reports of pregnant women exposed to acetylcysteine during various trimesters did not report any adverse maternal, fetal or neonatal outcomes (Class IV).
-
There are published reports on four pregnant women with acetaminophen toxicity, who were treated with oral or intravenous acetylcysteine at the time of delivery. Acetylcysteine crossed the placenta and was measurable following delivery in serum and cord blood of three viable infants and in cardiac blood of a fourth infant at autopsy (22 weeks gestational age who died 3 hours after birth). No adverse sequelae developed in the three viable infants. All mothers recovered and none of the infants had evidence of acetaminophen poisoning (Class IV).
-
It is not known whether Acetadote is present in human milk Class IV).
Product label: ACETADOTE (acetylcysteine) injection, solution [Cumberland Pharmaceuticals Inc.] Last revised: January 2011 [DailyMed]
Clinical reviews
-
Oxidative stress has been implicated in the pathogenesis and progression of chronic obstructive pulmonary disease. Both reactive oxidant species from inhaled cigarette smoke and those endogenously formed by inflammatory cells constitute an increased intrapulmonary oxidant burden. Structural changes to essential components of the lung are caused by oxidative stress, contributing to irreversible damage of both parenchyma and airway walls. In addition, oxidative stress results in alterations in the local immune response, increasing the risk of infections and exacerbations, which, in turn, may accelerate lung function decline. The antioxidant N-acetylcysteine, a glutathione precursor, has been applied in these patients in order to reduce symptoms, exacerbations and the accelerated lung function decline. This article reviews the presently available experimental and clinical data on the antioxidative effects of N-acetylcysteine in chronic obstructive pulmonary disease (Class IV).
Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004 Apr;23(4):629-36. [PubMed Citation]
-
The efficacy, safety, and cost issues that should be considered when deciding on the appropriate route of acetylcysteine for the treatment of patients with acetaminophen poisoning are reviewed. Oral and i.v. acetylcysteine appear to be equally effective when given within 8-10 hours of acetaminophen overdose. Anaphylactoid reactions to i.v. acetylcysteine have generally been reported in 3-6% of acetaminophen-poisoned patients. Dosing errors and hyponatremia have occurred in pediatric patients receiving i.v. acetylcysteine. Several investigators found an increased rate of anaphylactoid reactions in patients treated with i.v. acetylcysteine whose pretreatment serum acetaminophen levels were nontoxic. Compounding i.v. acetylcysteine from the oral preparation is less expensive than using premade i.v. solution. State pharmacy laws dictate whether extemporaneous compounding of acetylcysteine from the oral formulation is allowed. Oral acetylcysteine administration has resulted in minimal anaphylactoid reactions and is safer than i.v. acetylcysteine. Oral therapy should preferentially be considered in patients with asthma or atopic histories. The most important factors to consider when selecting the route of acetylcysteine administration include individual susceptibility, the severity of acetaminophen toxicity, and the time interval between acetaminophen ingestion and initiation of acetylcysteine therapy. Oral acetylcysteine administered within 8-10 hours of acetaminophen overdose prevents liver toxicity in the majority of patients who tolerate it and have no contraindications to therapy. I.V. acetylcysteine should be administered when patients are treated more than 10 hours postingestion of acetaminophen overdose or have underlying conditions preventing oral treatment. Anaphylactoid reactions are rare and occur more frequently in patients treated with the i.v. preparation (Class IV).
Kanter MZ. Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am J Health Syst Pharm. 2006 Oct 1;63(19):1821-7. [PubMed Citation]
C. Link to non-clinical (e.g. animal) studies
Adult animal studies
-
The two most common gas inhalation injuries encountered in emergency departments are carbon monoxide and chlorine inhalations. In this study, chlorine was produced through a method different to the previous experimental models. Rats were subjected to inhale chlorine, after which the effects of N-acetylcysteine on pulmonary damage were evaluated. A total of 50 rats were equally divided into five groups. Group 1 received nothing. Groups 2 and 3 were taken as 6 h, groups 4 and 5 as 24 h control and N-acetylcysteine groups, respectively. Firstly, 200 ppm chlorine gas was given for 20 min. Then, 40 mg/kg N-acetylcysteine was given intraperitoneally. The same procedure with the same dose was repeated 3 h later. The same procedures were applied to the control group but this time saline was used. Tissue samples of lungs were taken. Glutathione levels of the rats in the N-acetylcysteine group sacrificed at 24 h were significantly higher than those of the control group. Histopathological evaluation of the pulmonary tissues of the rats sacrificed at 6 and 24 h revealed mild-to-moderate degrees of tissue damage. The degree of tissue damage at 6 h and 24 h N-acetylcysteine group rats was lower than that in the control group. As a result, tissue damage resulting from experimental chlorine inhalation can be alleviated by N-acetylcysteine. This is mainly the result of the antioxidant effects of the N-acetylcysteine.
Akdur O, Sozuer EM, Ikizceli I, Avsarogullari L, Ozturk F, Muhtaroglu S, Ozkan S, Durukan P. Experimental inhalation of chlorine gas produced with a different method; effects of N-acetyl cysteine on acute pulmonary damage. Toxicol Mech Methods. 2008 Jan;18(9):739-43. [PubMed Citation]
-
Previous studies indicated that oxidative stress was involved in phosgene-induced acute lung injury (ALI) and many antioxidants had been used to prevent ALI. N-acetylcysteine (NAC) had been used to protect ALI induced by various types of oxidative stress. Considering the limited information of NAC on phosgene-induced ALI, the purpose of this study was to elucidate the molecular mechanisms of phosgene-induced ALI and the protective effects of NAC. This study discovered that intraperitoneal administration of NAC significantly alleviated phosgene-induced pulmonary edema, as confirmed by decreased lung wet to dry weight ratio and oxidative stress markers. The content of l-gamma-glutamyl-l-cysteinyl-glycine (glutathione; GSH) and the ratio of the reduced and disulfide forms (GSH/GSSG), significant indicators of the antioxidative ability, were apparently inhibited by phosgene exposure. However, both indicators could be reversed by NAC administration, indicating that dysregulation of redox status of glutathione might be the cause of phosgene-induced ALI. The nuclear factor (NF)-E2-related factor 2 (Nrf2), which has been proven to up-regulate the expression of glutathione reductase (GR), was obviously decreased by phosgene exposure. However, NAC administration elevated Nrf2 expression significantly. In conclusion, these data provided the first evidences showing that it was the transcriptional factor Nrf2 that connected phosgene-induced ALI with GSH metabolism. NAC protected against oxidative stress through acting on this newly disclosed Nrf2/GR/GSH pathway, by which NAC elevated the biosynthesis of protective GSH to repair and reconstitute the defense system destroyed by phosgene.
Ji L, Liu R, Zhang XD, Chen HL, Bai H, Wang X, Zhao HL, Liang X, Hai CX. N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal Toxicol. 2010 Jun;22(7):535-42. [PubMed Citation]
-
Chlorine (Cl(2)) is a highly reactive oxidant gas used extensively in a number of industrial processes. Exposure to high concentrations of Cl(2) results in acute lung injury that may either resolve spontaneously or progress to acute respiratory failure. Presently, the pathophysiological sequelae associated with Cl(2)-induced acute lung injury in conscious animals, as well as the cellular and biochemical mechanisms involved, have not been elucidated. We exposed conscious Sprague-Dawley rats to Cl(2) gas (184 or 400 ppm) for 30 min in environmental chambers and then returned them to room air. At 1 h after exposure, rats showed evidence of arterial hypoxemia, respiratory acidosis, increased levels of albumin, IgG, and IgM in bronchoalveolar lavage fluid (BALF), increased BALF surfactant surface tension, and significant histological injury to airway and alveolar epithelia. These changes were more pronounced in the 400-ppm-exposed rats. Concomitant decreases of ascorbate (AA) and reduced glutathione (GSH) were also detected in both BALF and lung tissues. In contrast, heart tissue AA and GSH content remained unchanged. These abnormalities persisted 24 h after exposure in rats exposed to 400 ppm Cl(2). Rats injected systemically with a mixture of AA, deferoxamine, and N-acetyl-L-cysteine before exposure to 184 ppm Cl(2) had normal levels of AA, lower levels of BALF albumin and normal arterial Po(2) and Pco(2) values. These findings suggest that Cl(2) inhalation damages both airway and alveolar epithelial tissues and that resulting effects were ameliorated by prophylactic administration of low-molecular-weight antioxidants.
Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol. 2008 Nov;295(5):L733-43. [PubMed Citation]
-
The chemical warfare agent analog, 2-chloroethyl ethyl sulfide, known as 'half-mustard gas' (HMG), is less toxic and less of an environmental hazard than the full molecule and has been shown to produce an acute lung injury in rats when instilled via intrapulmonary injection. This injury is characterized by massive, localized hemorrhage and edema into the alveolar compartment and can be quantitated by measuring extravasation of (125)I-bovine serum albumin into the extravascular compartment. Employing this rat model of HMG-induced lung injury, we observed significant attenuation of the pulmonary injury when experimental animals were complement or neutrophil depleted prior to HMG challenge. Significant protection also was provided by the use of antioxidants such as catalase, dimethyl sulfoxide, dimethyl thiourea, resveratrol and N-acetyl-L-cysteine (NAC). The last compound showed protection from lung injury as high as 70% and was still effective even when given up to 90 min after exposure of the lungs to HMG. These data suggest that acute lung injury caused by exposure to HMG may be related partially to complement mediated pathways and the generation by neutrophils of toxic oxygen species The data indicate that NAC is an effective antidote against HMG-induced acute lung injury in the rat.
McClintock SD, Till GO, Smith MG, Ward PA. Protection from half-mustard-gas-induced acute lung injury in the rat. J Appl Toxicol. 2002 Jul-Aug;22(4):257-62. [PubMed Citation]
-
To develop therapeutics against lung injury and respiratory toxicity following nerve agent VX exposure, we evaluated the protective efficacy of a number of potential pulmonary therapeutics. Guinea pigs were exposed to 27.03 mg/m(3) of VX or saline using a microinstillation inhalation exposure technique for 4 min and then the toxicity was assessed. Exposure to this dose of VX resulted in a 24-h survival rate of 52%. There was a significant increase in bronchoalveolar lavage (BAL) protein, total cell number, and cell death. Surprisingly, direct pulmonary treatment with surfactant, liquivent, N-acetylcysteine, dexamethasone, or anti-sense syk oligonucleotides 2 min post-exposure did not significantly increase the survival rate of VX-exposed guinea pigs. Further blocking the nostrils, airway, and bronchioles, VX-induced viscous mucous secretions were exacerbated by these aerosolized treatments. To overcome these events, we developed a strategy to protect the animals by treatment with atropine. Atropine inhibits muscarinic stimulation and markedly reduces the copious airway secretion following nerve agent exposure. Indeed, post-exposure treatment with atropine methyl bromide, which does not cross the blood-brain barrier, resulted in 100% survival of VX-exposed animals. Bronchoalveolar lavage from VX-exposed and atropine-treated animals exhibited lower protein levels, cell number, and cell death compared to VX-exposed controls, indicating less lung injury. When pulmonary therapeutics were combined with atropine, significant protection to VX-exposure was observed. These results indicate that combinations of pulmonary therapeutics with atropine or drugs that inhibit mucous secretion are important for the treatment of respiratory toxicity and lung injury following VX exposure.
Nambiar MP, Gordon RK, Rezk PE, Katos AM, Wajda NA, Moran TS, Steele KE, Doctor BP, Sciuto AM. Medical countermeasure against respiratory toxicity and acute lung injury following inhalation exposure to chemical warfare nerve agent VX. Toxicol Appl Pharmacol. 2007 Mar;219(2-3):142-50. [PubMed Citation]
-
We examined the effects of treatment with N-acetylcysteine (NAC) on pulmonary edema formation in isolated perfused rabbit lungs following in vivo phosgene exposure. This study focused on posttreatment intratracheal administration of NAC after exposure. Rabbits, 2 to 3 kg, were exposed to a cumulative dose of phosgene to attain a concentration x time exposure effect of 1,500 ppm/min. Lungs were perfused with Krebs-Henseleit buffer at 40 ml/min from 70 to 150 min after exposure. Pulmonary artery pressure (Ppa), tracheal pressure (Pt), and the rate of lung weight gain (LWG) were measured continuously. Perfusate concentration of peptide leukotrienes LTC4, D4, and E4 were measured every 20 min during perfusion. At the conclusion of the experiment, lung tissue was analyzed for reduced and oxidized glutathione (GSH and GSSG) and lipid peroxidation (thiobarbituric acid-reactive substances, TBARS). Exposure to phosgene significantly increased Pt, LWG, LTC4, D4, and E4, TBARS, and GSSG over time compared with controls. Compared with phosgene, intratracheal NAC lowered Ppa, LWG, LTC4, D4, and E4, TBARS, and GSSG. We conclude that NAC protected against phosgene-induced lung injury by acting as an antioxidant by maintaining protective levels of glutathione, reducing both lipid peroxidation and production of arachidonic acid metabolites.
Sciuto AM, Strickland PT, Kennedy TP, Gurtner GH. Protective effects of N-acetylcysteine treatment after phosgene exposure in rabbits. Am J Respir Crit Care Med. 1995 Mar;151(3 Pt 1):768-72. [PubMed Citation]
-
A series of studies was performed to address treatment against the former chemical warfare edemagenic gas phosgene. Both in situ and in vivo models were used to assess the efficacy of postexposure treatment of phosgene-induced lung injury using clinically existing drugs. The degree of efficacy was judged by examining treatment effects on pulmonary edema formation (PEF) as measured by wet/dry weight (WW/DW) ratios, real-time (in situ) lung weight gain (LWG), survival rates (SR), odds ratios, and glutathione (GSH) redox states. Drugs included N-acetylcysteine (NAC), ibuprofen (IBU), aminophylline (AMIN), and isoproterenol (ISO). Using the in situ isolated perfused rabbit lung model (IPRLM), intratracheal (IT) NAC (40 mg/kg bolus) delivered 45-60 min after phosgene exposure (650 mg/m(3)) for10 min lowered pulmonary artery pressure, LWG, leukotrienes (LT) C(4)/D(4)/E(4), lipid peroxidation, and oxidized GSH. We concluded that NAC protected against phosgene-induced lung injury by acting as an antioxidant by maintaining protective levels of GSH, reducing both lipid peroxidation and production of arachidonic acid metabolites. Also in IPRLM, administration of AMIN (30 mg/kg) 80-90 min after phosgene exposure significantly reduced lipid peroxidation and perfusate LTC(4)/D(4)/E(4), reduced LWG, and prevented phosgene-induced decreases in lung tissue cAMP. These data suggest that protective mechanisms observed with AMIN involve decreased LTC(4)/D(4)/E(4) mediated pulmonary capillary permeability and attenuated lipid peroxidation. Direct antipermeability effects of AMIN-induced upregulation of cAMP on cellular contraction may also be important in protection against phosgene-induced lung injury. Posttreatment with ISO in the IPRLM by either combined intravascular (iv; infused into pulmonary artery at 24 microg/min infused) + IT (24 microg bolus) or IT route alone 50-60 min after phosgene exposure significantly lowered pulmonary artery pressure, tracheal pressure, and LWG. ISO treatment significantly enhanced GSH products or maintained protective levels when compared with results from phosgene-exposed only rabbits. These data suggest that protective mechanisms for ISO involve reduction in vascular pressure, decreased LTC(4)/D(4)/E(4)-mediated pulmonary capillary permeability, and favorably maintained lung tissue GSH redox states. For in vivo male mouse (CD-1, 25-30 g) studies IBU was administered ip within 20 min after a lethal dose of phosgene (32 mg/m(3) for 20 min) at 0 (saline), 3, 9, or 15 mg/mouse. Five hours later, a second IBU injection was given but at half the original doses (0, 1.5, 4.5, and 7.5 mg/mouse); therefore, these treatment groups are now referred to as the 0/0, 3/1.5, 9/4.5, and 15/7.5 mg IBU/mouse groups. SRs and odds ratios were calculated for each dose at 12 and 24 h. The 12-h survival was 63% for 9/4.5 mg IBU and 82% for the 15/7.5 mg IBU groups, compared with 25% for saline-treated phosgene-exposed mice. At 24 h, those survival rates were reduced to 19%, 19%, and 6%, respectively. In the 15/7.5 mg IBU group, lung WW/DW ratios were significantly lower than in saline-treated mice at 12 h. Lipid peroxidation was lower only for the 9/4.5 mg IBU dose; however, nonprotein sulfhydryls (a measure of GSH) were greater across all IBU doses. The odds ratio was 5 for the 9/4.5 IBU group at 12 h and 13 for the 15/7.5 mg IBU group, compared with 3.5 for both groups at 24 h. IBU posttreatment increased the survival of mice at 12 h by reducing PEF, lipid peroxidation, and GSH depletion. In conclusion, effective treatment of phosgene-induced lung injury involves early postexposure intervention that could reduce free radical species responsible for lipid peroxidation, correct the imbalance in the GSH redox state, and prevent the release of biological mediators such as leukotrienes, which are accountable for increased permeability.
Sciuto AM, Hurt HH. Therapeutic treatments of phosgene-induced lung injury. Inhal Toxicol. 2004 Jul;16(8):565-80. [PubMed Citation]
Pregnant animal studies
-
Reproductive and developmental toxicity studies performed in rats at oral doses up to 6.7 times the recommended human intravenous dose and in rabbits at doses up to 3.3 times the recommended human intravenous dose revealed no evidence of impaired fertility or embryofetal toxicity.
Product label: ACETADOTE (acetylcysteine) injection, solution [Cumberland Pharmaceuticals Inc.] Last revised: January 2011 [DailyMed]
Non-clinical reviews
-
Chlorine (Cl(2)) is a reactive oxidant gas used extensively in industrial processes. Exposure of both humans and animals to high concentrations of Cl(2) results in acute lung injury, which may resolve spontaneously or progress to acute respiratory failure. Injury to airway and alveolar epithelium may result from chemical reactions of Cl(2), from HOCl (the hydrolysis product of Cl(2)), and/or from the various reaction products, such as chloramines, that are formed from the reactions of these chlorinating species with biological molecules. Subsequent reactions may initiate self-propagating reactions and induce the production of inflammatory mediators compounding injury to pulmonary surfactant, ion channels, and components of lung epithelial and airway cells. Low-molecular-weight antioxidants, such as ascorbate, glutathione, and urate, present in the lung epithelial lining fluid and tissue, remove Cl(2) and HOCl and thus decrease injury to critical target biological targets. However, levels of lung antioxidants of animals exposed to Cl(2) in concentrations likely to be encountered in the vicinity of industrial accidents decrease rapidly and irreversibly. Our measurements show that prophylactic administration of a mixture containing ascorbate and desferal N-acetyl-cysteine, a precursor of reduced glutathione, prevents Cl(2)-induced injury to the alveolar epithelium of rats exposed to Cl(2). The clinical challenge is to deliver sufficient quantities of antioxidants noninvasively, after Cl(2) exposure, to decrease morbidity and mortality.
Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc. 2010 Jul;7(4):278-83. [PubMed Citation]
-
Sulfur mustard (SM) is highly toxic to the lung inducing both acute and chronic effects including upper and lower obstructive disease, airway inflammation, and acute respiratory distress syndrome, and with time, tracheobronchial stenosis, bronchitis, and bronchiolitis obliterans. Thus it is essential to identify effective strategies to mitigate the toxicity of SM and related vesicants. Studies in animals and in cell culture models have identified key mechanistic pathways mediating their toxicity, which may be relevant targets for the development of countermeasures. For example, following SM poisoning, DNA damage, apoptosis, and autophagy are observed in the lung, along with increased expression of activated caspases and DNA repair enzymes, biochemical markers of these activities. This is associated with inflammatory cell accumulation in the respiratory tract and increased expression of tumor necrosis factor-a and other proinflammatory cytokines, as well as reactive oxygen and nitrogen species. Matrix metalloproteinases are also upregulated in the lung after SM exposure, which are thought to contribute to the detachment of epithelial cells from basement membranes and disruption of the pulmonary epithelial barrier. Findings that production of inflammatory mediators correlates directly with altered lung function suggests that they play a key role in toxicity. In this regard, specific therapeutic interventions currently under investigation include anti-inflammatory agents (e.g., steroids), antioxidants (e.g., tocopherols, melatonin, N-acetylcysteine, nitric oxide synthase inhibitors), protease inhibitors (e.g., doxycycline, aprotinin, ilomastat), surfactant replacement, and bronchodilators. Effective treatments may depend on the extent of lung injury and require a multi-faceted pharmacological approach.
Weinberger B, Laskin JD, Sunil VR, Patrick J. Sinko PJ, Heck DE, Debra L. Laskin DL. Sulfur mustard-induced pulmonary injury: Therapeutic approaches to mitigating Toxicity. Pulm Pharmacol Ther. 2011 Feb; 24(1):92-9 [PubMed Citation].
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
N-Acetylcysteine was given intravenously and as three fast dissolving and one slow-release formulation, on separate occasions, as a single dose of 600 mg to 10 fasting (5 men and 5 women) healthy volunteers. Blood and urine were sampled for the following 12 h. Renal clearance constituted around 30% of total body clearance, which was 0.21 l/h/kg. Volume of distribution was 0.33 l/kg, consistent with distribution mainly to extracellular water. The late elimination half-life was 2.27 h and the mean residence time 1.62 h. The slow-release tablet resulted in a flattened plasma concentration-time curve typical of slow release formulations, while the other three oral formulations were rapidly absorbed. The oral availability of N-acetylcysteine varied between 6 and 10%, with the slow-release tablet having the lowest and the fast dissolving tablet the highest availability.
Borgström L, Kågedal B, Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31(2):217-22. [PubMed Citation]
The pharmacokinetics and bioavailability of N-acetylcysteine (NAC) have been determined after its intravenous and oral administration to 6 healthy volunteers. According to a randomized cross-over design each subject received NAC 200 mg i.v. and 400 mg p.o., and blood samples were collected for 30 h. Reduced NAC had a volume of distribution (VSS) of 0.59 l.kg-1 and a plasma clearance of 0.84 l.h-1.kg-1. The terminal half-life after intravenous administration was 1.95 h. The oral bioavailability was 4.0%. Based on total NAC concentration, its volume of distribution (VSS) was 0.47 l.kg-1 and its plasma clearance was 0.11 l.h-1.kg-1. The terminal half-life was 5.58 h after intravenous administration and 6.25 h after oral administration. Oral bioavailability of total NAC was 9.1%.
Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77-82. [PubMed Citation]
The plasma pharmacokinetics of oral acetylcysteine(N-acetylcysteine, NAC) after the administration of single 600 mg and repeated 200 mg doses and the relative bioavailability of the two regimens were studied in 12 adult subjects. On two different occasions in a cross-over, balanced fashion the subjects were administered orally either a single dose of NAC 600 mg as effervescent tablets or 4 repeated doses of NAC as granules in sachets at the regimen of 200 mg t.i.d. Venous blood samples were obtained just before dosing and 20, 40 min, 1, 1.5, 2, 3, 4, 6, 8, 10 and 12 h after the administration of NAC 600 mg; with the 1st, the 2nd and the 4th doses of NAC 200 mg samples were taken just before dosing and after 20, 40 min, 1, 1.5, 2, 3, 4, 6 and 8 h, the last sampling after the 1st dose being the one before the 2nd dose. A detailed description of the assaying methods of NAC is given in the text. As indexes of bioavailability Cmax' tmax and AUC of NAC plasma concentrations were considered and MRT was taken as an estimate of its persistence in plasma. NAC was quickly absorbed without any significant difference in tmax among the doses. With the 600 mg dose Cmax' AUC and MRT were greater than with a single 200 mg dose; after summing up the values of these parameters for the 200 mg doses no significant differences were observed in comparison to the single 600 mg dose in Cmax and AUC, while MRT resulted significantly higher.(ABSTRACT TRUNCATED AT 250 WORDS)
De Caro L, Ghizzi A, Costa R, Longo A, Ventresca GP, Lodola E. Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteers. Arzneimittelforschung. 1989 Mar;39(3):382-6. [PubMed Citation]
The pharmacokinetics after oral administration of 200, 600 or 1200 mg of N-acetylcysteine (NAC) were studied in 10 healthy subjects. Normalized maximal plasma concentration was significantly higher after a 600 mg dose than after a 200 mg dose. Bioavailability of NAC significantly increased with increasing dose. Time for maximal plasma concentration also increased with increasing dose. The observations can be explained by a capacity-limited presystemic elimination of NAC. In an extension of the study, 600 mg of NAC was given twice a day for 5 days and the plasma concentrations were followed after the morning dose on day 6. No differences in the pharmacokinetic parameters were observed in comparison with the single 600 mg dose. This indicates that the beneficial clinical effects observed after repeated dosing can not be ascribed to an accumulation of NAC in plasma.
Borgström L, Kågedal B. Dose dependent pharmacokinetics of N-acetylcysteine after oral dosing to man. Biopharm Drug Dispos. 1990 Mar;11(2):131-6. [PubMed Citation]
N-Acetylcysteine is useful as a mucolytic agent for treatment of chronic bronchitis and other pulmonary diseases complicated by the production of viscous mucus. It is also used as an antidote to paracetamol (acetaminophen) poisoning and found to be effective for the prevention of cardiotoxicity by doxorubicin and haemorrhagic cystitis from oxazaphosphorines. After an oral dose of N-acetylcysteine 200 to 400 mg the peak plasma concentration of 0.35 to 4 mg/L is achieved within 1 to 2 hours. Although the data are conflicting, it appears that the administration of charcoal may interfere with drug absorption, with up to 96% of the drug adsorbed on to the charcoal. Information on absorption in the presence of food or other drugs is not available. The volume of distribution ranges from 0.33 to 0.47 L/kg and protein binding is significant, reaching approximately 50% 4 hours after the dose. Pharmacokinetic information is not available as to whether or not N-acetylcysteine crosses the blood-brain barrier or placenta, or into breast milk. Renal clearance has been reported as 0.190 to 0.211 L/h/kg and approximately 70% of the total body clearance is nonrenal. Following oral administration, reduced N-acetylcysteine has a terminal half-life of 6.25h. Little is known of the metabolism of this agent, although it is believed to be rapidly metabolised and incorporated on to proteins. The major excretory product is inorganic sulphate. Frequently reported side effects are nausea, vomiting and diarrhoea. Biochemical and haematological adverse effects are observed but are not clinically relevant. Drug interactions of clinical significance have been observed with paracetamol, glutathione and anticancer agents.
Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991 Feb;20(2):123-34. [PubMed Citation]
Hepatic Impairment
The threshold plasma paracetamol concentration at which N-acetylcysteine (NAC) treatment is recommended to treat paracetamol poisoning in a patient with induced liver enzymes (for example, with chronic liver disease or taking anticonvulsant drugs) is 50% lower than in a patient without induced liver enzymes. More patients with chronic liver disease might therefore be expected to be exposed to NAC treatment than previously. In addition, there is increasing use of NAC in patients with chronic liver disease for multiorgan failure or hepatorenal syndrome. Little is known of NAC's pharmacokinetic properties in patients with cirrhosis. The aim was to determine if the pharmacokinetics of NAC are altered by chronic liver disease. NAC was given intravenously in a dose of 600 mg over 3 min to nine patients with biopsy-proven cirrhosis (Child's grade; 1 A, 4 B, 4 C: aetiology: 7 alcohol-related, 1 primary biliary cirrhosis, 1 secondary biliary stenosis) and six healthy matched controls. Venous blood was taken at 20, 40, 60 and 90 min then at 2, 3, 4, 6, 8 and 10 h after NAC administration. Serum NAC was estimated by HPLC. The data were normalized to a standard body weight of 70 kg. The area under the serum concentration-time curve was increased (152.34 mg/L.h +/- 50.38 s.d.) in cirrhotics compared with normal controls (93.86 mg/L.h +/- 9.60 s.d.) (P < 0.05). The clearance of NAC was reduced in patients with chronic liver disease (4.52 L/h +/- 1.87 s.d.) compared with controls (6.47 L/h +/- 0.78: P < 0.01). Increased vigilance for untoward anaphylactoid reactions is necessary in cirrhotics as they may have higher plasma NAC concentrations. Further studies to determine the optimum dosage regimen in such patients are required.
Jones AL, Jarvie DR, Simpson D, Hayes PC, Prescott LF. Pharmacokinetics of N-acetylcysteine are altered in patients with chronic liver disease. Aliment Pharmacol Ther. 1997 Aug;11(4):787-91. [PubMed Citation]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
Mucolytic: Patients ≥40 kg
Loading Dose: 150 mg/kg in 200 mL of diluent administered over 60 min
Dose 2: 50 mg/kg in 500 mL of diluent administered over 4 hr
Dose 3: 100 mg/kg in 1000 mL of diluent administered over 16 hr
Patients >20- <40 kg
Loading Dose: 150 mg/kg in 100 mL of diluent administered over 60 min
Dose 2: 50 mg/kg in 250 mL of diluent administered over 4 hr
Dose 3: 100 mg/kg in 500 mL of diluent administered over 16 hr
Product label: ACETADOTE (acetylcysteine) injection, solution [Cumberland Pharmaceuticals Inc.] Last revised: January 2011 [DailyMed]
Nebulized: When nebulized into a face mask, mouth piece or tracheostomy, 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution may be given every 2 to 6 hours; the recommended dose for most patients is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times a day.
Direct instillation: When used by direct instillation, 1 to 2 mL of a 10% to 20% solution may be given as often as every hour. When used for the routine nursing care of patients with tracheostomy, 1 to 2 mL of a 10% to 20% solution may be given every 1 to 4 hours by instillation into the tracheostomy.
Diagnostic bronchial studies: two to three administrations of 1 to 2 mL of the 20% solution or 2 to 4 mL of the 10% solution should be given by nebulization or by instillation intratracheally, prior to the procedure.
Product label: acetylcysteine (ACETYLCYSTEINE) solution [Fresenius Kabi USA, LLC] Last revised: July 2006 [DailyMed]
Children (FDA)
Mucolytic: Patients ≤20 kg
Loading Dose: 150 mg/kg in 3 mL/kg of body weight of diluent administered over 60 min
Dose 2: 50 mg/kg in 7 mL/kg of body weight of diluent administered over 4 hr
Dose 3: 100 mg/kg in 14 mL/kg of body weight of diluent administered over 16 hr
Product label: ACETADOTE (acetylcysteine) injection, solution [Cumberland Pharmaceuticals Inc.] Last revised: January 2011 [DailyMed]
Pregnancy (FDA)
Pregnancy category B
Product label: ACETADOTE (acetylcysteine) injection, solution [Cumberland Pharmaceuticals Inc.] Last revised: January 2011 [DailyMed]
Nursing Mothers (FDA)
It is not known whether Acetadote is present in human milk. Because many drugs are excreted in human milk, caution should be exercised when acetylcysteine is administered to a nursing woman. Based on the pharmacokinetics of acetylcysteine, it should be nearly completely cleared 30 hours after administration. Nursing women may consider resuming nursing 30 hours after administration.
Product label: ACETADOTE (acetylcysteine) injection, solution [Cumberland Pharmaceuticals Inc.] Last revised: January 2011 [DailyMed]
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for N-acetylcysteine has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Formulation
Solution; inhalation: 10%. May contain disodium edetate. In 4, 10 and 30 mL vials.
Solution, concentrate; inhalation: 20%. May contain disodium edetate. In 4, 10 and 30 mL vials.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1147-9
Parenteral For injection concentrate, for IV infusion 200 mg/mL
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.3619-3622
Shelf life
Stability
-
Acetylcysteine is a reducing agent and is incompatible with oxidizing agents. Solutions of acetylcysteine become discolored and liberate hydrogen sulfide upon contact with rubber, some metals, particularly iron and copper, and/or when subjected to autoclaving. Acetylcysteine does not react with glass, plastic, aluminum, anodized aluminum, chromed metal, tantalum, sterling silver, or stainless steel. Although silver may become tarnished after exposure to acetylcysteine, this does not affect potency of the drug. The presence of a light purple color in acetylcysteine sodium oral and oral inhalation solutions does not appreciably affect potency of the drug; however, it is best to utilize equipment constructed with plastic or glass and with stainless steel or another nonreactive metal when administering acetylcysteine by nebulization. The presence of a light pink to light purple color in acetylcysteine injection does not affect the quality of the product.
-
The manufacturers state that solutions of acetylcysteine sodium are physically and/or chemically incompatible with solutions containing amphotericin B, tetracyclines, erythromycin lactobionate, or ampicillin sodium. When one of these anti-infectives is to be administered by aerosol inhalation, it should be nebulized separately. Acetylcysteine solutions are also physically incompatible with iodized oil, trypsin, chymotrypsin, and hydrogen peroxide.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.3619-3622
Storage
Unopened vials of acetylcysteine sodium solution should be stored at 15-30°C. Following exposure to air, oral and oral inhalation solutions should be stored at 2-8°C to retard oxidation and should be used within 96 hours.
Commercially available acetylcysteine concentrate for injection should be stored at 20-25°C. When diluted with 5% dextrose, resultant solutions are stable for 24 hours at controlled room temperature.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.3619-3622
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
PURPOSE: To investigate the effect of N-acetylcysteine on preventing pump-induced oxidoinflammatory response during cardiopulmonary bypass (CPB). METHODS: Forty patients undergoing coronary artery bypass grafting (CABG) were randomly divided into a study group (n = 20), given 50 mg kg (-1) N-acetylcysteine intravenously for 3 days, and a control group (n = 20) given saline. Serum samples were collected for measurement of myeloperoxidase (MPO), malondialdehyde (MDA), interleukin-6, Alpha1-acid glycoprotein (AAGP), and C-reactive protein (CRP) during surgery and postoperatively. RESULTS: The MPO and MDA values showed a similar pattern during and after CPB in the study group, with significantly less variance than in the control group. Interleukin-6 showed similar patterns in the two groups, but the data from 30 min after the start of CPB and from 6 h post-CPB were significantly different. The AAGP and CRP values were both elevated during CPB in the two groups without a significant difference, but 6 and 24 h post-CPB, the values were significantly higher in the control group than in the study group. CONCLUSIONS: N-Acetylcysteine decreased pump-induced oxidoinflammatory response during CPB, suggesting that it could be a novel therapy for assisting in the prevention of CBP-induced oxidoinflammatory damage.
Sucu N, Cinel I, Unlu A, Aytacoglu B, Tamer L, Kocak Z, Karaca K, Gul A, Dikmengil M, Atik U, Oral U. N-acetylcysteine for preventing pump-induced oxidoinflammatory response during cardiopulmonary bypass. Surg Today. 2004;34(3):237-42. [PubMed Citation]
-
Cardiopulmonary bypass (CPB) has been implicated in causing poor pulmonary gas exchange postoperatively in patients undergoing coronary artery bypass grafting (CABG) procedures. In this prospective, randomized, double-blind, placebo-controlled study, we examined the pulmonary effects of N-acetylcysteine (NAC) in patients undergoing CABG. Twenty patients undergoing elective CABG and early tracheal extubation were randomized into two groups. Group I (ten patients) received a physiologic salt solution as a placebo in a continuous intravenous infusion for one hour before CPB and 24 hours after CPB; Group II (ten patients) received 100 mg/ kg NAC intravenously for one hour before CPB and 40 mg/kg/day at 24 hours after CPB. Perioperative hemodynamic and pulmonary data were recorded. Postoperative tracheal extubation was accomplished at the earliest appropriate time. The postoperative clinical course was similar in the two groups. Both groups exhibited significant postoperative increases in A-a oxygen gradient (p < 0.01), but patients in Group II exhibited significantly lower increases in postoperative A-a oxygen gradient (p < 0.006). Other hemodynamic and pulmonary data (pulmonary capillary wedge pressure, pulmonary vascular resistance (PVR), cardiac index (CI), shunt flow, dynamic lung compliance and static lung compliance) exhibited no differences between the groups. There was no significant difference in terms of intubation time. The malondialdehyde (MDA) increase in Group II following CPB was found to be significantly lower than in Group I (p = 0.043). This clinical study reveals that administration of NAC to patients undergoing elective CABG with CPB improves systemic oxygenation. There was no effect in other pulmonary parameters and in terms of intubation time.
Eren N, Cakir O, Oruc A, Kaya Z, Erdinc L. Effects of N-acetylcysteine on pulmonary function in patients undergoing coronary artery bypass surgery with cardiopulmonary bypass. Perfusion. 2003 Nov;18(6):345-50. [PubMed Citation]
-
BACKGROUND: Volume supplementation by saline infusion combined with N-acetylcysteine (NAC) represents an effective strategy to prevent contrast agent-induced nephrotoxicity (CIN). Preliminary data support the concept that sodium bicarbonate and ascorbic acid also may be effective in preventing CIN. METHODS AND RESULTS: Three hundred twenty-six consecutive patients with chronic kidney disease, referred to our institutions for coronary and/or peripheral procedures, were randomly assigned to prophylactic administration of 0.9% saline infusion plus NAC (n=111), sodium bicarbonate infusion plus NAC (n=108), and 0.9% saline plus ascorbic acid plus NAC (n=107). All enrolled patients had serum creatinine > or = 2.0 mg/dL and/or estimated glomerular filtration rate < 40 mL x min(-1) x 1.73 m(-2). Contrast nephropathy risk score was calculated in each patient. In all cases, iodixanol (an iso-osmolar, nonionic contrast agent) was administered. The primary end point was an increase of > or = 25% in the creatinine concentration 48 hours after the procedure (CIN). The amount of contrast media administered (179+/-102, 169+/-92, and 169+/-94 mL, respectively; P=0.69) and risk scores (9.1+/-3.4, 9.5+/-3.6, and 9.3+/-3.6; P=0.21) were similar in the 3 groups. CIN occurred in 11 of 111 patients (9.9%) in the saline plus NAC group, in 2 of 108 (1.9%) in the bicarbonate plus NAC group (P=0.019 by Fisher exact test versus saline plus NAC group), and in 11 of 107 (10.3%) in the saline plus ascorbic acid plus NAC group (P=1.00 versus saline plus NAC group). CONCLUSIONS: The strategy of volume supplementation by sodium bicarbonate plus NAC seems to be superior to the combination of normal saline with NAC alone or with the addition of ascorbic acid in preventing CIN in patients at medium to high risk.
Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, Ricciardelli B, Colombo A; Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007 Mar 13; 115 (10): 1211-7. [PubMed Citation]
Children
-
Mucolytic (inhalation): children 13 to 17 years of age - 5 to 10 mL of 10 % or 20% acetylcysteine 3 or 4 times per day.
-
Mucolytic (inhalation): children 1 to 12 years of age- 6 to 10 mL of 10% acetylcysteine or 3 to 5 mL of 20% acetylcysteine 3 or 4 times per day.
-
Mucolytic (inhalation): 30 days to 1 year of age - 2 to 4 mL of 10% acetylcysteine or 1 to 2 mL of 20% acetylcysteine 3 or 4 times per day.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1147-9
8. Route of Administration/Monitoring
As an antidote for acetaminophen overdosage, acetylcysteine is administered orally as a 5% solution or by IV infusion. As a mucolytic agent, acetylcysteine, usually in a 10-20% solution, may be administered by nebulization, direct application, or intratracheal instillation.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.3619-3622
9. Adverse effects
-
To identify risk factors in the development of side-effects to N-acetylcysteine (NAC) in patients with paracetamol poisoning. A retrospective study was carried out based upon the hospital charts of 529 consecutive patients admitted with paracetamol poisoning, all treated with NAC, at the Department of Hepatology, Copenhagen University Hospital (the tertiary care centre of liver disease in Denmark). Forty-five patients (8.5%; 95% confidence intervals (CI) 6.4, 11%) developed side-effects to NAC and 18 patients (3.4%; 95% CI 2.1, 5.4%) developed systemic side-effects. Asthmatics were 2.9 times (95% CI 2.1, 4.7) more likely to develop side-effects (Chi-square: P = 0.004). Side-effects were of similar severity in asthmatics and nonasthmatics. A history of medical allergy was not a risk factor. Serum paracetamol was lower in patients with side-effects than in those without (Mann-Whitney: P = 0.00006). Asthma must be considered a risk factor in the development of side-effects to NAC. However, the side-effects are easily managed and there is no reason to withhold NAC from any patient with paracetamol poisoning. Paracetamol itself seems to offer some protection against the development of side-effects to NAC.
Schmidt LE, Dalhoff K. Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning. Br J Clin Pharmacol. 2001 Jan;51(1):87-91. [PubMed Citation]
-
Paracetamol overdose is a common reason for presentation to the emergency department and N-acetylcysteine is frequently used in the treatment of toxic paracetamol ingestions. Adverse reactions to N-acetylcysteine are common though usually mild and easily treated. Serious reactions to N-acetylcysteine however, are rare and there have been no previous reported fatalities with its therapeutic use. This report describes the case of a 40 year old brittle asthmatic patient who died after treatment with intravenous N-acetylcysteine. Asthma is a risk factor for adverse reactions to N-acetylcysteine and special caution should be exercised in its use in brittle asthmatic patients.
Appelboam AV, Dargan PI, Knighton J. Fatal anaphylactoid reaction to N-acetylcysteine: caution in patients with asthma. Emerg Med J. 2002 Nov;19(6):594-5. [PubMed Citation]
-
Clinical details of seven patients who suffered adverse reactions to N-acetylcysteine as Parvolex are documented. Skin testing was carried out to diluted Parvolex, and its individual components N-acetylcysteine and ethylenediaminetetra-acetate, in five reacting patients and five patients who had received Parvolex with no ill-effects. Weal responses to high concentrations (20 mg/ml) of acetylcysteine as Parvolex were significantly greater (p less than 0.02) in reactors. There were no other significant differences between the groups. In two patients who reacted, the effects of intradermal Parvolex could be inhibited by prior therapy with the antihistamine terfenadine. These results suggest a 'pseudo-allergic' rather than an immunological aetiology for adverse reactions to Parvolex.
Bateman DN, Woodhouse KW, Rawlins MD. Adverse reactions to N-acetylcysteine. Hum Toxicol. 1984 Oct;3(5):393-8. [PubMed Citation]
-
To review the potential for anaphylactoid reactions to intravenously administered acetylcysteine when used in the treatment of acetaminophen overdose. This case is unique in that electrocardiographic changes, including ST segment depression and T-wave inversion were associated with the episode and complicated the diagnosis. Intravenous administration of acetylcysteine has been used in the treatment of acetaminophen overdose. This route may be considered in some clinical situations where oral therapy is complicated. Anaphylactoid reactions, including cutaneous eruptions, flushing, chest pain, tachycardia, and fever have been reported in up to three percent of patients receiving intravenous acetylcysteine. The nature of these reactions and evidence concerning their etiology suggest a histamine-release phenomenon. Response to intervention with antihistamines and the safety of further acetylcysteine administration are discussed. This case illustrates a variant anaphylactoid reaction to intravenously administered acetylcysteine and emphasizes the need for practitioners to consider the potential for these reactions prior to initiation of therapy and indicates appropriate treatment of these reactions.
Bonfiglio MF, Traeger SM, Hulisz DT, Martin BR. Anaphylactoid reaction to intravenous acetylcysteine associated with electrocardiographic abnormalities. Ann Pharmacother. 1992 Jan;26(1):22-5. [PubMed Citation]
-
Nausea, vomiting, and other GI symptoms may occur following oral administration of acetylcysteine in the treatment of acetaminophen overdosage. The drug may also aggravate vomiting associated with acetaminophen overdosage. Administration of dilute acetylcysteine solutions may minimize the tendency of the drug to aggravate vomiting.
-
Pediatrics: Generalized urticaria, sometimes accompanied by mild fever, has occurred rarely following oral administration of acetylcysteine. Marked elevations in liver function test results (e.g., AST [SGOT] and ALT [SGPT]), occurred on 2 occasions following administration of high doses (total doses: 106 and 250 g over 3-4 days) of acetylcysteine rectally and via nasogastric tube in a 3-year-old boy with cystic fibrosis; these abnormalities were noted within a few days of initiation of acetylcysteine therapy and resolved gradually following discontinuance of the drug.
-
Anaphylactoid reactions (i.e., acute hypersensitivity reactions such as rash, hypotension, wheezing, and/or dyspnea) have been reported in patients receiving IV acetylcysteine for the treatment of acetaminophen overdosage; in some cases, the anaphylactoid reactions were serious, including death in a patient with asthma. Rash, urticaria, and pruritus are the most frequently reported adverse reactions in patients receiving IV acetylcysteine. Acute flushing and erythema also have occurred; these reactions generally occur 30-60 minutes after initiating the infusion and resolve despite continued infusion of the drug. Reactions to acetylcysteine that involve manifestations other than flushing and erythema should be considered anaphylactoid reactions and treated as such.
-
Pediatrics: No adverse effects were noted following IV infusion of acetylcysteine at a mean rate of 4.2 mg/kg per hour for 24 hours in 10 preterm infants (gestational age of 25-31 weeks and weight of 500-1380 g) or following infusion of acetylcysteine 0.1-1.3 mg/kg per hour for 6 days in 6 neonates (gestational age of 26-30 weeks and weight of 520-1335 g).
-
Acetylcysteine appears to have a wide margin of safety. Adverse effects of acetylcysteine following oral inhalation or intratracheal instillation may include stomatitis, nausea, vomiting, drowsiness, clamminess, severe rhinorrhea, and fever. Acetylcysteine solutions have a slight, disagreeable odor which may contribute to the incidence of nausea. When a face mask is used for nebulization of acetylcysteine, there may be stickiness on the face afterwards which can be easily removed by washing with water.
-
Sensitization to acetylcysteine has been reported rarely, but has not been confirmed by patch testing. Sensitization to acetylcysteine and dermal eruptions have been reported in several inhalation therapists after frequent and extended exposure to the drug.
-
Irritation of the tracheal and bronchial tracts and hemoptysis have occurred following administration of acetylcysteine; however, such findings are not uncommon in patients with bronchopulmonary disease and a causal relationship has not been established.
-
Chest tightness and bronchoconstriction have been reported with acetylcysteine. Clinically overt acetylcysteine-induced bronchospasm occurs rarely and unpredictably, even in patients with asthmatic bronchitis or bronchitis complicating bronchial asthma. Occasionally, patients receiving oral inhalation of acetylcysteine develop increased airway obstruction of varying and unpredictable severity. Patients who have had such reactions to previous therapy with acetylcysteine may not react during subsequent therapy with the drug, and patients who have had inhalation treatments with acetylcysteine without incident may react to subsequent therapy.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.3619-3622
10. Contraindication(s)
-
Since oral administration of acetylcysteine may result in vomiting or aggravate vomiting associated with acetaminophen overdosage, patients at risk of gastric hemorrhage (e.g., those with esophageal varices or peptic ulcers) should be evaluated with regard to the relative risks of upper GI hemorrhage and acetaminophen-induced hepatotoxicity and treatment with acetylcysteine given accordingly. If generalized urticaria or other allergic symptoms occur during oral acetylcysteine therapy, the drug should be discontinued unless it is considered essential and the allergic symptoms can be otherwise controlled. The manufacturers state that if encephalopathy resulting from hepatic failure occurs during oral acetylcysteine therapy, the drug should be discontinued to avoid further administration of nitrogenous substances; there are no data indicating that acetylcysteine adversely affects hepatic failure, but it is a theoretical possibility.
-
If an anaphylactoid reaction (rash, hypotension, wheezing, dyspnea, or any dermatologic manifestations other than flushing and erythema) occurs during IV acetylcysteine therapy, the drug should be temporarily interrupted in order to administer antihistamines and, in severe reactions, epinephrine. Once treatment of the anaphylactoid reaction has been initiated, IV acetylcysteine can be reinstituted cautiously. If the anaphylactoid reaction recurs or increases in severity, IV acetylcysteine should be discontinued and alternative management considered.
-
Acetylcysteine should be used with caution in patients with asthma or a history of bronchospasm.
-
To avoid fluid overload (which can result in hyponatremia, seizure, and death), the volume of 5% dextrose used to dilute acetylcysteine injection should be adjusted as needed. The total volume of 5% dextrose should be reduced in patients weighing less than 40 kg and those requiring fluid restriction. IV acetylcysteine is contraindicated in patients with known hypersensitivity or previous anaphylactoid reaction to acetylcysteine or any ingredient in the formulation.
-
Asthmatic patients receiving acetylcysteine by oral inhalation or intratracheal instillation should be observed closely during such therapy; if bronchospasm occurs, a bronchodilator should be given by nebulization. If bronchospasm progresses, acetylcysteine should be discontinued immediately.
-
An increased volume of liquefied bronchial secretions may develop after administration of acetylcysteine and the airway may become occluded. If cough is inadequate to maintain an open airway during acetylcysteine therapy, mechanical suction or endotracheal aspiration should be instituted.
-
Acetylcysteine solutions are contraindicated in patients hypersensitive to the drug.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.3619-3622
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations- No data available at this time.
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations- No data available at this time.
15. Study-related ethical concerns
— including review panel recommendations- No data available at this time.
16. Global regulatory status
U.S.
Acetylcysteine is used orally or IV as an antidote for the treatment of acetaminophen overdosage.
Acetylcysteine is used as a mucolytic agent in the adjunctive treatment of patients with abnormal, viscid, or inspissated mucous secretions in such conditions as acute and chronic bronchopulmonary disorders (e.g., pneumonia, bronchitis, emphysema, tracheobronchitis, chronic asthmatic bronchitis, tuberculosis, bronchiectasis, primary amyloidosis of the lung); atelectasis caused by mucus obstruction; pulmonary complications of cystic fibrosis; pulmonary complications of thoracic and cardiovascular surgery; and post-traumatic chest conditions.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
U.K.
Tear deficiency, impaired or abnormal mucus production.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009
Paracetamol overdosage
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009
17. Other potentially useful information
-
In experimental studies, the old mucolytic agent N-acetylcysteine (NAC) has had beneficial effects in disorders supposedly linked to oxidative stress. Numerous, mainly small clinical trials with variable doses have yielded inconsistent results in a wide variety of diseases. NAC added to the conventional therapy of human immunodeficiency virus infection might be of benefit; in respect of chronic obstructive pulmonary disease, systematic reviews and meta-analyses suggested that prolonged treatment with NAC is efficacious, but a recent multicentre study has questioned this. In a large intervention trial on cancer recurrence, NAC was ineffective. NAC infusions have been widely used in acute hepatic failure but convincing evidence of its benefits is lacking. A preliminary study reported that NAC is effective in preventing radiocontrast-induced nephropathy but thereafter highly mixed results have been published, and even meta-analyses disagree on its efficacy. In intensive care NAC has mostly been a disappointment but recently it has 'given promises' in surgery with cardiopulmonary bypass. NAC therapy is routine only in paracetamol intoxication.
Aitio ML. N-acetylcysteine -- passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006 Jan;61(1):5-15. [PubMed Citation]
-
To develop management guidelines for the treatment of anaphylactoid reactions to intravenous N-acetylcysteine (NAC) and to assess the safety of restarting the infusion after a reaction. In phased 1, we used a 6-year retrospective case series of hospitalized patients and a review of the literature to develop the management guidelines for anaphylactoid reactions to intravenous NAC. In phase 2, these guidelines were evaluated prospectively in our poison-control center. In phase 1, the charts of 11 patients with anaphylactoid reactions (9 cutaneous and 2 systemic) were reviewed. In most cases, no treatment or treatment with diphenhydramine alone or with salbutamol was sufficient to continue or restart NAC infusion safely. On the basis of our findings in those patients and on published experience, we concluded that anaphylactoid reactions to intravenous NAC are dose-related and the antihistamines are useful in controlling and in preventing recurrence of anaphylactoid symptoms. We developed the following guidelines: flushing requires no treatment, urticaria should be treated with diphenhydramine, and NAC infusion should be continued in both cases. Angioedema and respiratory symptoms each require the administration of diphenhydramine and symptomatic therapy. In these cases, NAC infusion should be stopped but, when necessary, can be started 1 hour after the administration of diphenhydramine in the absence of symptoms. In phase 2, 50 patients (31 cutaneous and 19 systemic reactions) were treated prospectively with the use of these guidelines. Recurrence of symptoms occurred in only one case involving a deviation from the guidelines. The NAC infusion was restarted immediately after the administration of diphenhydramine in a patient who sustained a systemic reaction. Non-life-threatening anaphylactoid reactions to intravenous NAC are treated easily and the infusion may be continued or restarted safely after the administration of diphenhydramine.
Bailey B, McGuigan MA. Management of anaphylactoid reactions to intravenous N-acetylcysteine. Ann Emerg Med. 1998 Jun;31(6):710-5. [PubMed Citation]
-
Octanol/Water Partition Coefficient: log Kow = -0.66 /Estimated/
HSDB. N-acetylcysteine
18. Publications
Aitio ML. N-acetylcysteine -- passe-partout or much ado about nothing? Br J Clin Pharmacol. 2006 Jan;61(1):5-15. [PubMed Citation]
Akdur O, Sozuer EM, Ikizceli I, Avsarogullari L, Ozturk F, Muhtaroglu S, Ozkan S, Durukan P. Experimental inhalation of chlorine gas produced with a different method; effects of N-acetyl cysteine on acute pulmonary damage. Toxicol Mech Methods. 2008 Jan;18(9):739-43. [PubMed Citation]
Appelboam AV, Dargan PI, Knighton J. Fatal anaphylactoid reaction to N-acetylcysteine: caution in patients with asthma. Emerg Med J. 2002 Nov;19(6):594-5. [PubMed Citation]
Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593-597. [PubMed Citation]
Atkinson MC. The use of N-acetylcysteine in intensive care. Crit Care Resusc. 2002 Mar;4(1):21-7. [PubMed Citation]
Bailey B, McGuigan MA. Management of anaphylactoid reactions to intravenous N-acetylcysteine. Ann Emerg Med. 1998 Jun;31(6):710-5. [PubMed Citation]
Bateman DN, Woodhouse KW, Rawlins MD. Adverse reactions to N-acetylcysteine. Hum Toxicol. 1984 Oct;3(5):393-8. [PubMed Citation]
Bonfiglio MF, Traeger SM, Hulisz DT, Martin BR. Anaphylactoid reaction to intravenous acetylcysteine associated with electrocardiographic abnormalities. Ann Pharmacother. 1992 Jan;26(1):22-5. [PubMed Citation]
Borgström L, Kågedal B. Dose dependent pharmacokinetics of N-acetylcysteine after oral dosing to man. Biopharm Drug Dispos. 1990 Mar;11(2):131-6. [PubMed Citation]
Borgström L, Kågedal B, Paulsen O. Pharmacokinetics of N-acetylcysteine in man. Eur J Clin Pharmacol. 1986;31(2):217-22. [PubMed Citation]
Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, Ricciardelli B, Colombo A; Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007 Mar 13; 115 (10): 1211-7. [PubMed Citation]
Carbonell N, Blasco M, SanjuáR, Péz-Sancho E, Sanchis J, Insa L, Bodí, NúJ, GarcíRamó, Miguel A. Intravenous N-acetylcysteine for preventing contrast-induced nephropathy: a randomised trial. Int J Cardiol. 2007 Jan 31;115(1):57-62. [PubMed Citation]
De Caro L, Ghizzi A, Costa R, Longo A, Ventresca GP, Lodola E. Pharmacokinetics and bioavailability of oral acetylcysteine in healthy volunteers. Arzneimittelforschung. 1989 Mar;39(3):382-6. [PubMed Citation]
Dekhuijzen PN. Antioxidant properties of N-acetylcysteine: their relevance in relation to chronic obstructive pulmonary disease. Eur Respir J. 2004 Apr;23(4):629-36. [PubMed Citation]
Desai MH, Mlcak R, Richardson J, Nichols R, Herndon DN. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine [correction of acetylcystine] therapy. J Burn Care Rehabil. 1998 May-Jun;19(3):210-2. [PubMed Citation]
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
Eren N, Cakir O, Oruc A, Kaya Z, Erdinc L. Effects of N-acetylcysteine on pulmonary function in patients undergoing coronary artery bypass surgery with cardiopulmonary bypass. Perfusion. 2003 Nov;18(6):345-50. [PubMed Citation]
Holdiness MR. Clinical pharmacokinetics of N-acetylcysteine. Clin Pharmacokinet. 1991 Feb;20(2):123-34. [PubMed Citation]
HSDB. N-acetylcysteine
Hynninen MS, Niemi TT, Pöä, Raininko EI, SalmenperäT, Lepäalo MJ, Railo MJ, Tallgren MK. N-acetylcysteine for the prevention of kidney injury in abdominal aortic surgery: a randomized, double-blind, placebo-controlled trial. Anesth Analg. 2006 Jun;102(6):1638-45. [PubMed Citation]
Ji L, Liu R, Zhang XD, Chen HL, Bai H, Wang X, Zhao HL, Liang X, Hai CX. N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhal Toxicol. 2010 Jun;22(7):535-42. [PubMed Citation]
Jones AL, Jarvie DR, Simpson D, Hayes PC, Prescott LF. Pharmacokinetics of N-acetylcysteine are altered in patients with chronic liver disease. Aliment Pharmacol Ther. 1997 Aug;11(4):787-91. [PubMed Citation]
Kanter MZ. Comparison of oral and i.v. acetylcysteine in the treatment of acetaminophen poisoning. Am J Health Syst Pharm. 2006 Oct 1;63(19):1821-7. [PubMed Citation]
Kao SJ, Wang D, Lin HI, Chen HI. (2006). N-acetylcysteine abrogates acute lung injury induced by endotoxin
Clin Exp Pharmacol Physiol. 2006;33:33-40. [PubMed]
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1147-9
Lee KS, Kim SR, Park HS, Park SJ, Min KH, Lee KY, Choe YH, Hong SH, Han HJ, Lee YR, Kim JS, Atlas D, Lee YC. (2007). A novel thiol compound, N-acetylcysteine amide, attenuates allergic airway disease by regulating activation of NF-kappaB and hypoxia-inducible factor-1alpha. Exp Mol Med. 2007 Dec;39(6):756-768. [PubMed Citation]
Leustik M, Doran S, Bracher A, Williams S, Squadrito GL, Schoeb TR, Postlethwait E, Matalon S. Mitigation of chlorine-induced lung injury by low-molecular-weight antioxidants. Am J Physiol Lung Cell Mol Physiol. 2008 Nov;295(5):L733-43. [PubMed Citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.3619-3622
McClintock SD, Till GO, Smith MG, Ward PA. Protection from half-mustard-gas-induced acute lung injury in the rat. J Appl Toxicol. 2002 Jul-Aug;22(4):257-62. [PubMed Citation]
Moradi M, Mojtahedzadeh M, Mandegari A, Soltan-Sharifi MS, Najafi A, Khajavi MR, Hajibabayee M, Ghahremani MH. The role of glutathione-S-transferase polymorphisms on clinical outcome of ALI/ARDS patient treated with N-acetylcysteine. Respir Med. 2009 Mar;103(3):434-441. [PubMed Citation]
Nambiar MP, Gordon RK, Rezk PE, Katos AM, Wajda NA, Moran TS, Steele KE, Doctor BP, Sciuto AM. Medical countermeasure against respiratory toxicity and acute lung injury following inhalation exposure to chemical warfare nerve agent VX. Toxicol Appl Pharmacol. 2007 Mar;219(2-3):142-50. [PubMed Citation]
Olsson B, Johansson M, Gabrielsson J, Bolme P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur J Clin Pharmacol. 1988;34(1):77-82. [PubMed Citation]
Ristikankare A, Kuitunen T, Kuitunen A, Uotila L, Vento A, Suojaranta-Ylinen R, Salmenpera M, Poyhia R. Lack of renoprotective effect of i.v. N-acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. Br J Anaesth. 2006 Nov;97(5):611-6. [PubMed Citation]
Schmidt LE, Dalhoff K. Risk factors in the development of adverse reactions to N-acetylcysteine in patients with paracetamol poisoning. Br J Clin Pharmacol. 2001 Jan;51(1):87-91. [PubMed Citation]
Sciuto AM, Hurt HH. Therapeutic treatments of phosgene-induced lung injury. Inhal Toxicol. 2004 Jul;16(8):565-80. [PubMed Citation]
Sciuto AM, Strickland PT, Kennedy TP, Gurtner GH. Protective effects of N-acetylcysteine treatment after phosgene exposure in rabbits. Am J Respir Crit Care Med. 1995 Mar;151(3 Pt 1):768-72. [PubMed Citation]
Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, Hardman JG, Jamdar S. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut. 2007 Oct;56(10):1439-44. [PubMed Citation]
Sklar GE, Subramaniam M. Acetylcysteine treatment for non-acetaminophen-induced acute liver failure. Ann Pharmacother. 2004 Mar;38(3):498-500. [PubMed Citation]
Sucu N, Cinel I, Unlu A, Aytacoglu B, Tamer L, Kocak Z, Karaca K, Gul A, Dikmengil M, Atik U, Oral U. N-acetylcysteine for preventing pump-induced oxidoinflammatory response during cardiopulmonary bypass. Surg Today. 2004;34(3):237-42. [PubMed Citation]
Weinberger B, Laskin JD, Sunil VR, Patrick J. Sinko PJ, Heck DE, Debra L. Laskin DL. Sulfur mustard-induced pulmonary injury: Therapeutic approaches to mitigating Toxicity. Pulm Pharmacol Ther. 2011 Feb; 24(1):92-9 [PubMed Citation].
Yadav AK, Bracher A, Doran SF, Leustik M, Squadrito GL, Postlethwait EM, Matalon S. Mechanisms and modification of chlorine-induced lung injury in animals. Proc Am Thorac Soc. 2010 Jul;7(4):278-83. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
