You are here: Home > Medical Countermeasures Database > Albuterol
Albuterol -
Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Albuterol
2. Chemical Defense therapeutic area(s)
— including key possible usesAlbuterol has been investigated as a potential medical mitigation agent against both upper and lower chemical-induced pulmonary injury by the agents such as chlorine gas, mustard gas, phosgene and nerve agents sarin and soman.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
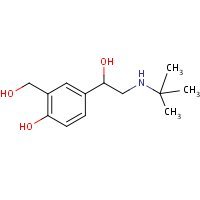
HSDB. Albuterol
Mechanism of action
-
Albuterol is a selective beta2-adrenergic receptor agonist with pharmacological properties and therapeutic indications similar to terbutaline. The mechanism of the antiasthmatic action of beta-adrenergic receptor agonists is undoubtedly linked to the direct relaxation of airway smooth muscle and consequent bronchodilation. Although human bronchial smooth muscle receives little or no sympathetic innervations, it nevertheless contains large numbers of beta2-adrenergic receptors. Stimulation of these receptors activates the Gs adenylyl cyclase cyclic AMP pathway with consequent reduction in smooth muscle tone. Beta2-adrenergic receptor agonist (e.g. albuterol, isoproterenol) also increase the conductance of large Ca2+-sensitive K+ channels in airway smooth muscle, leading to membrane hyperpolerization and relaxation. This occurs at least partly by mechanisms independent of adenylyl cyclase activity and cyclic AMP production and may involve the regulation of capacitative Ca2+ entry by small G proteins.
-
There are beta2-adrenergic receptors on cell types in the airways other than bronchial smooth muscle. Of particular interest, stimulation of beta2-adrenergic receptors inhibits the function of numerous inflammatory cells, including mast cells, basophiles, eosinophils, neutrophils, and lymphocytes. In general, stimulating beta2-adrenergic receptors in these cell types increases intracellular cyclic AMP, activating a signaling cascade that inhibits the release of inflammatory mediators and cytokines.
Brunton, LL, et al. (eds.). (2006). (Goodman & Gilman's) The Pharmacological Basis of Therapeutics (11th Ed.). McGraw-Hill-Medical Publishing Division, New York, NY. 720.
-
In vitro studies and in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on beta2-adrenergic receptors compared with isoproterenol. While it is recognized that beta2-adrenergic receptors are the predominant receptors in bronchial smooth muscle, data indicate that there is a population of beta2-receptors in the human heart existing in a concentration between 10% and 50%. The precise function of these receptors has not been established.
Product label Albuterol aerosol, metered [Rebel Distributors Corp] Last revised: July 2011 [DailyMed]
Summary of clinical and non-clinical studies
Albuterol (Salbutamol) is a β-adrenergic agonist used in the management of bronchoconstriction in acute respiratory distress syndrome (ARDS) (Perkins et al., 2007; Perkins et al., 2011) and asthma (Harvey and Tattersfield, 1982) with possible implications as a chemical defense therapeutic agent. Twenty-five soldiers admitted to the emergency department for accidental exposure to chlorine gas were treated with warmed humidified oxygen and nebulized albuterol (Cevik et al., 2009). Twelve patients were hospitalized and received additional treatment with ampicillin-sulbactam, methylprednisolone and nebulized albuterol and were discharged within 4 days in good health with no mortality. Reports of managing mustard gas casualties during the Iran-Iraq conflict in the 1980s reveal that casualties were treated with salbutamol orally (2 mg three times daily), high dosage prednisone (40-60 mg per day), and broad spectrum antibiotics. It was reported that nebulized bronchodilators, such as albuterol, were ineffectual in five such casualties, presenting with cough productive of sputum, inspiratory crackles, air flow limitation, and hypoxaemia; yet all five casualities had remarkable, if not complete, recovery of lung function according to Newman-Taylor. Albuterol was given in tablet form over 6 weeks. (Russell, et al., 2006; Newman-Taylor, 1991). Airway responsiveness to albuterol was also measured in 22 war victims exposed to chemical warfare agents (60% sulfur mustard and 40% nerve agents such as sarin) 17 to 19 years after exposure. They all had confirmed gas exposure and suffered respiratory symptoms including chest tightness, breathlessness, cough and wheezing. When presented with an albuterol challenge, there was an increased airway responsiveness to albuterol in subjects that had been exposed to chemical warfare agents compared to 15 normal controls. In vitro, in canine smooth muscle tissue, albuterol produced a partial relaxation (20±4.6% at 1μM and 24±8% at 10 μM) of tension induced by the nerve agent soman but relaxation was not sustained (Filbert, Moore and Adler, 1992). Albuterol was not effective in the treatment of phosgene induced acute lung injury and did not improve survival when tested in pigs, although it was noted clinically that 5 mg albuterol by nebulizer every 4 hours can reduce lung inflammation if administered within one hour of phosgene-exposure in patients that require oxygen (Grainge et al., 2009). The lung is one of the organs most damaged during chemical warfare agents and these articles suggest the possible feasibility and limits that exist in the use of albuterol as a treatment option following exposure. (Albuterol solution and tablets have been noted to have limited therapeutic effect when compared to inhalation based formulations in children. In addition systemic side effects such as tachycardia and jitteriness are much more commonplace with solution and tablets. - CHEMM Editor)
B. Link to clinical studies
Studies involving multiple populations
-
RATIONALE: β2-Adrenergic receptor agonists accelerate resolution of pulmonary edema in experimental and clinical studies. OBJECTIVES: This clinical trial was designed to test the hypothesis that an aerosolized β2-agonist, albuterol, would improve clinical outcomes in patients with acute lung injury (ALI). METHODS: We conducted a multicenter, randomized, placebo-controlled clinical trial in which 282 patients with ALI receiving mechanical ventilation were randomized to receive aerosolized albuterol (5 mg) or saline placebo every 4 hours for up to 10 days. The primary outcome variable for the trial was ventilator-free days. MEASUREMENTS AND MAIN RESULTS: Ventilator-free days were not significantly different between the albuterol and placebo groups (means of 14.4 and 16.6 d, respectively; 95% confidence interval for the difference, -4.7 to 0.3 d; P = 0.087). Rates of death before hospital discharge were not significantly different between the albuterol and placebo groups (23.0 and 17.7%, respectively; 95%confidence interval for the difference,-4.0 to 14.7%; P = 0.30). In the subset of patients with shock before randomization, the number of ventilator-free days was lower with albuterol, although mortality was not different. Overall, heart rates were significantly higher in the albuterol group by approximately 4 beats/minute in the first 2 days after randomization, but rates of new atrial fibrillation (10% in both groups) and other cardiac dysrhythmias were not significantly different. CONCLUSIONS: These results suggest that aerosolized albuterol does not improve clinical outcomes in patients with ALI. Routine use of β2-agonist therapy in mechanically ventilated patients with ALI cannot be recommended (Class II).
Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT. Randomized, placebo-controlled clinical trial of an aerosolized beta2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011 Sep 1; 184 (5): 561-8. [PubMed Citation]
-
Background: The Acute Respiratory Distress Syndrome (ARDS) is a common cause of respiratory failure in critically ill patients. Experimental studies suggest that treatment with beta agonists may be helpful in ARDS. The Beta Agonist Lung Injury Trial (BALTI-2) is a multicenter, pragmatic, randomized, double-blind, placebo-controlled clinical trial which aims to determine if sustained treatment with intravenous (IV) salbutamol will improve survival in ARDS. Methods/Design: Patients fulfilling the American-European Consensus Conference Definition of ARDS will be randomized in a 1:1 ratio to receive an IV infusion either of salbutamol (15 μg kg ideal body weight-1 hr-1) or placebo (0.9% sodium chloride solution), for a maximum of seven days. Allocation to randomized groups will use minimization to ensure balance with respect to hospital of recruitment, age group (<64, 65-84, >85 years) and PaO2/FiO2 ratio (≤6.7, 6.8- 13.2, ≥13.3 kPa). Data will be recorded by participating ICUs until hospital discharge, and all surviving patients will be followed up by post at six and twelve months post randomization. The primary outcome is mortality at 28 days after randomization; secondary outcomes are mortality in ICU, mortality in hospital, number of ventilator-free days, number of organ failure-free days, mortality at twelve months post-randomization, quality of life at six and twelve months, length of stay in ICU, length of stay in hospital, adverse effects (tachycardia, arrhythmia or other side effects sufficient to stop treatment drug). 1,334 patients will be recruited from about fifty ICUs in the UK. An economic evaluation will be conducted alongside the trial (Class II).
Perkins GD, Gates S, Lamb SE, McCabe C, Young D, Gao F. Beta Agonist Lung Injury Trial (BALT-2) trial protocol: a randomized, double-blind, placebo controlled of intravenous infusion of albutamol in the acute respiratory distress syndrome. Trials. 2011 May 9; 12: 113. [PubMed Citation]
Adult
-
Objectives: Chlorine gas is a potent pulmonary irritant that affects the mucous membranes and induces severe disturbances of pulmonary gas exchange within minutes of inhalation. The present study evaluated an extraordinary type of mass inhalational exposure. Material and Methods: Clinical reports of 25 soldiers who were admitted to the emergency department of Maresal Cakmak Military Hospital, Erzurum were retrospectively evaluated. All patients were exposed to chlorine gas as a result of mixing sodium hypochlorite with hydrochloric acid during cleaning activities. Results: All patients were male and the mean age of patients was 22.04±2.98 years. The main symptoms were coughing and dyspnea in 18 patients (72%). Forced expiratory volume in 1 second (FEV1) and FEV1/forced volume capacity (FVC) ratio were found to be normal in all patients but FVC and peak expiratory flow (PEF) were below the normal range (80%) in 9 patients (36%). All patients received warmed humidified oxygen combined with nebulized salbutamol. Inhaled budesonide and nebulized sodium bicarbonate were ordered additionally for 19 patients (76%). Thirteen patients (52%) were discharged from the emergency department and 12 patients (48%) were hospitalized. No mortality was observed. Conclusion: Chlorine gas is a potent pulmonary irritant that causes acute damage in both the upper and lower respiratory tract. We suggest that inhaled steroids combined with nebulized sodium bicarbonate could be a safe and effective alternative for the treatment of symptomatic patients. Education of the public about the dangers of mixing of hypochlorite bleach with acidic cleaning agents is also very important (Class IV).
Cevik Y, Onay M, Akmaz I, Sezigen S. Mass casualties from acute inhalation of chlorine gas. South Med J. 2009 Dec; 102 (12): 1209-13. [PubMed Citation]
-
Background and objectives: Increased airway responsiveness to b-agonists is noted in asthmatics and smokers. The lung may be exposed to chemical warfare agents such as mustard gas and pulmonary complications of exposure range from no effect to severe bronchial stenosis. There is little understanding of airway hyperresponsiveness to b-agonist drugs in chemical war victims and this study examined airway responsiveness to salbutamol in victims of chemical warfare. Methods: The threshold concentrations of inhaled salbutamol required for a 20% change in FEV1 as PC20, or a 35% change in specific airway conductance (sGaw) as PC35 were measured in 22 persons exposed to chemical warfare and 15 normal control subjects. Results: In 11 of the 22 subjects PC20 salbutamol could be measured and in 15 of the 22 subjects PC35 salbutamol could be measured. This group of patients was the responder group (PC20 = 10.79 and PC35 = 8.55 mg/L) and in them the concentration of salbutamol needed for a response was significantly lower than that required in normal controls (PC20 = 237.68 and PC35 = 88.72 mg/L, P < 0.001). There was a significant correlation between FEV1 and PC20 salbutamol (r = 0.815, P < 0.001). Conclusions: These results showed increased airway responsiveness to salbutamol in most subjects exposed to chemical warfare; this was correlated with airway caliber (Class III).
Boskabady MH, Attaran D, Shaffei MN. Airway responses to salbutamol after exposure to chemical warfare. Respirology. 2008 Mar; 13 (2): 288-93. [PubMed citation]
-
Background: Intravenous salbutamol (albuterol) reduces lung water in patients with the acute respiratory distress syndrome (ARDS). Experimental data show that it also reduces pulmonary neutrophil accumulation or activation and inflammation in ARDS. Aim: To investigate the effects of salbutamol on neutrophil function. Methods: The in vitro effects of salbutamol on neutrophil function were determined. Blood and bronchoalveolar lavage (BAL) fluid were collected from 35 patients with acute lung injury (ALI)/ARDS, 14 patients at risk from ARDS and 7 ventilated controls at baseline and after 4 days' treatment with placebo or salbutamol (ALI/ARDS group). Alveolar-capillary permeability was measured in vivo by thermodilution (PiCCO). Neutrophil activation, adhesion molecule expression and inflammatory cytokines were measured. Results: In vitro, physiological concentrations of salbutamol had no effect on neutrophil chemotaxis, viability or apoptosis. Patients with ALI/ARDS showed increased neutrophil activation and adhesion molecule expression compared with at risk-patients and ventilated controls. There were associations between alveolar-capillary permeability and BAL myeloperoxidase (r = 0.4, p = 0.038) and BAL interleukin 8 (r = 0.38, p = 0.033). In patients with ALI/ARDS, salbutamol increased numbers of circulating neutrophils but had no effect on alveolar neutrophils. Conclusion: At the onset of ALI/ARDS, there is increased neutrophil recruitment and activation. Physiological concentrations of salbutamol did not alter neutrophil chemotaxis, viability or apoptosis in vitro. In vivo, salbutamol increased circulating neutrophils, but had no effect on alveolar neutrophils or on neutrophil activation. These data suggest that the beneficial effects of salbutamol in reducing lung water are unrelated to modulation of neutrophil-dependent inflammatory pathways (Class III)
Perkins GD, Nathani N, McAuley DF, Gao F, Thickett DR. In vitro and in vivo effects of salbutamol on neutrophil function in acute lung injury. Thorax. 2007 Jan; 62 (1): 36-42. [PubMed Citation]
-
This study was designed to determine whether resistance to the airway effects of the beta-agonist, salbutamol, would develop in three groups of subjects while taking large doses of inhaled salbutamol. Six normal non-atopic, six atopic non-asthmatic, and eight atopic asthmatic subjects were studied by an identical technique. The development of resistance was assessed from salbutamol dose-response studies in which the airway response was measured as specific airway conductance (sGaw). Further evidence was sought in the atopic and asthmatic subjects by measuring the airway response to a standard histamine inhalation challenge and the protective effect of 100 micrograms salbutamol on this challenge, and by six-hourly peak flow recordings. Subjects were assessed before and during four weeks in which they took inhaled salbutamol regularly in doses increasing to 500 microgram qid in week 4. Normal subjects showed a progressive reduction in the bronchodilator (sGaw) response to salbutamol during the four weeks, indicating the progressive development of resistance. The atopic subjects, both asthmatic and non-asthmatic, showed no reduction in the response to salbutamol during the four weeks, nor any change in the response to histamine challenge or in regular peak flow readings. These results demonstrate that asthmatic patients do not develop bronchial beta-adrenoceptor resistance easily and suggests that they and atopic non-asthmatic subjects are less susceptible to its development than normal subjects (Class III).
Harvey JE, Tattersfield AE. Airway response to salbutamol: effect of regular salbutamol inhalations in normal, atopic, and asthmatic subjects. Thorax. 1982 Apr; 37 (4): 280-7. [PubMed Citation]
Pregnancy, breastfeeding studies
-
OBJECTIVES: Approximately 4% to 12% of pregnant women have asthma; few studies have examined the effects of maternal asthma medication use on birth defects. We examined whether maternal asthma medication use during early pregnancy increased the risk of selected birth defects.
METHODS:National Birth Defects Prevention Study data for 2853 infants with 1 or more selected birth defects (diaphragmatic hernia, esophageal atresia, small intestinal atresia, anorectal atresia, neural tube defects, omphalocele, or limb deficiencies) and 6726 unaffected control infants delivered from October 1997 through December 2005 were analyzed. Mothers of cases and controls provided telephone interviews of medication use and additional potential risk factors. Exposure was defined as maternal periconceptional (1 month prior through the third month of pregnancy) asthma medication use (bronchodilator or anti-inflammatory). Associations between maternal periconceptional asthma medication use and individual major birth defects were estimated by using adjusted odds ratios (aOR) and 95% confidence intervals (95%CI).
RESULTS:No statistically significant associations were observed for maternal periconceptional asthma medication use and most defects studied; however, positive associations were observed between maternal asthma medication use and isolated esophageal atresia (bronchodilator use: aOR = 2.39, 95%CI = 1.23, 4.66), isolated anorectal atresia (anti-inflammatory use: aOR = 2.12, 95%CI = 1.09, 4.12), and omphalocele (bronchodilator and anti-inflammatory use: aOR = 4.13, 95%CI = 1.43, 11.95).
CONCLUSIONS: Positive associations were observed for anorectal atresia, esophageal atresia, and omphalocele and maternal periconceptional asthma medication use, but not for other defects studied. It is possible that observed associations may be chance findings or may be a result of maternal asthma severity and related hypoxia rather than medication use.
Lin S, Munsie JP, Herdt-Losavio ML, Druschel CM, Campbell K, Browne ML, Romitti PA, Olney RS, Bell EM; Maternal asthma medication use and the risk of selected birth defects. National Birth Defects Prevention Study. Pediatrics. 2012 Feb;129(2):e317-24. [PubMed Citation]
-
BACKGROUND: Few epidemiological studies have explored the relationship between orofacial clefts and bronchodilators. We assessed whether mothers who used bronchodilators during early pregnancy were at an increased risk of delivering infants with orofacial clefts.
METHODS:We used National Birth Defects Prevention Study case-control data from mothers of 2711 infants with orofacial clefts and 6482 mothers of live born infants without birth defects, delivered during 1997 through 2005. Information on medication use from 3 months before pregnancy through delivery was collected using a standardized interview. Logistic regression was used to estimate adjusted odds ratios (aOR) and 95% confidence intervals (CIs) for maternal bronchodilator use during the periconceptional period (1 month before pregnancy through the third month of pregnancy) while controlling for other covariates.
RESULTS:We observed an association between maternal bronchodilator use during the periconceptional period and cleft lip only (CLO) (aOR = 1.77, 95% CI: 1.08-2.88). The risk of cleft palate only (CPO) (aOR = 1.53, 95% CI: 0.99-2.37) was elevated but was not statistically significant. No association was observed for maternal bronchodilator use and the risk of cleft lip with cleft palate (aOR = 0.78, 95% CI: 0.46-1.31). The most commonly used bronchodilator was albuterol (88.7%). Maternal albuterol use was associated with CLO (aOR = 1.79, 95% CI: 1.07-2.99) and CPO (aOR = 1.65, 95% CI: 1.06-2.58).
CONCLUSIONS:We observed a statistically significant association between maternal bronchodilator use during the periconceptional period and the risk of CLO after controlling for other risk factors. It is unclear whether the increased odds ratios observed in this study are due to the bronchodilators, the severity of asthma, or both, or to chance alone. Further studies to disentangle the role of asthma or asthma medications would help clarify these findings.
Munsie JW, Lin S, Browne ML, Campbell KA, Caton AR, Bell EM, Rasmussen SA, Romitti PA, Druschel CM; Maternal bronchodilator use and the risk of orofacial clefts. National Birth Defects Prevention Study. Hum Reprod. 2011 Nov;26(11):3147-54. [PubMed Citation]
-
A 2009 report examined the evidence for the use and toxicity of beta2-agonists in pregnancy, particularly for terbutaline and ritodrine as tocolytic agents and albuterol for asthma. Based on animal and human reports, the authors concluded that there was a biologic plausible basis for associating prolonged in utero exposure to beta2-agonists with functional and behavioral teratogenesis, specifically increased risks for autism spectrum disorders, psychiatric disorders, poor cognitive and motor function, and poor school performance. There also were increased risks in offspring for elevated heart rate and hypertension. The mechanisms for these effects appeared to be a decrease in parasympathetic activity resulting in an increase in sympathetic activity. A genetic predisposition for the toxicity was supported by data. The available data suggested that albuterol use in the 1st and 2nd trimesters was associated with a modest increase in the risk of autism spectrum disorders. The authors concluded that excessive treatment with albuterol should be avoided (Class IV).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.32-34
-
In a surveillance study of Michigan Medicaid recipients conducted between 1985 and 1992 involving 229,101 completed pregnancies, 1090 newborns had been exposed to albuterol during the 1st trimester (F. Rosa, personal communication, FDA, 1993). A total of 48 (4.4%) major birth defects were observed (43 expected). Specific data were available for six defect categories, including (observed/expected) 9/11 cardiovascular defects, 2/2 oral clefts, 2/0.6 spina bifida, 1/2 limb reduction defects, 0/3 hypospadias, and 6/3 polydactyly. Only with the latter defect is there a suggestion of a possible association, but other factors, including the mother's disease, concurrent drug use, and chance, may be involved (Class IV).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.32-34
-
A double-blind, controlled study involving 144 women (74 treated with albuterol and 70 treated with placebo) observed no difference between the groups in the length of gestation, birth weight, or fetal outcome, except fewer infants in the albuterol group had respiratory distress syndrome (14) (Class II).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.32-34
-
No reports describing the use of albuterol during human lactation have been located. Other drugs in the class (see Terbutaline) are considered compatible with breastfeeding, and albuterol most likely is compatible (Class IV).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.32-34
Clinical reviews
-
There is no specific antidote for the treatment of casualties exposed to chlorine, phosgene, or mustards; therefore, management is largely supportive. Corticosteroid treatment has been given to casualties accidentally exposed to chlorine. Clinical data on efficacy are inconclusive as the numbers given steroids have been small and the indications for administration unclear. There have been no clinical controlled studies. There is a stronger evidence base from animal studies, particularly from porcine and rodent models. Lung injury induced by phosgene and mustard appears to be mediated by glutathione depletion, lipid peroxidation, free radical generation, and subsequent cellular toxicity. There is limited evidence to suggest that repletion of glutathione reduces and/or prevents lung damage by these agents. This may provide an opportunity for therapeutic intervention (Class IV).
Russell D, Blain PG, Rice P. Clinical management of casualties exposed to lung damaging agents: a critical review. Emerg Med J. 2006 Jun; 23 (6): 421-4. [PubMed Citation]
-
Context. Phosgene is a substance of immense importance in the chemical industry. Because of its widespread industrial use, there is potential for small-scale exposures within the workplace, large-scale accidental release, or even deliberate release into a built-up area. Objective. This review aims to examine all published studies concerning potential treatments for phosgene-induced acute lung injury and incorporate them into up-to date clinical guidance. In addition, it aims to contrast the approaches when dealing with small numbers of patients known to be exposed (possibly with dose information) with the presentation of a large and heterogeneous population of casualties following a significant industrial accident or deliberate release; no published guidelines have specifically addressed this second problem. Methods. PubMed and Embase were searched for all available years till April 2010 and 584 papers were identified and considered. Experimental studies. Because of the nature of the injury, there have been no human trials of patients exposed to phosgene. Multiple small and large animal studies have been performed to examine potential treatments of phosgene-induced acute lung injury, but many of these used isolated organ models, pretreatment regimens, or clinically improbable doses. Recent studies in large animals using both realistic time frames and dosing regimens have improved our knowledge, but clinical guidance remains based on incomplete data. Management of a small-scale, confirmed exposure. In the circumstance of a small-scale, confirmed industrial release where a few individuals are exposed and present rapidly, an intravenous bolus of high-dose corticosteroid (e.g., methylprednisolone 1 g) should be considered, although there are no experimental data to support this recommendation. The evidence is that there is no benefit from nebulized steroid even when administered 1 h after exposure, or methylprednisolone if administered intravenously ≥6 h after exposure. Consideration should also be given to administration of nebulized acetylcysteine 1-2 g, though there is no substantive evidence of benefit outside a small animal, isolated lung model and there is a possibility of adverse effects. If the oxygen saturation falls below 94%, patients should receive the lowest concentration of supplemental oxygen to maintain their SaO2 in the normal range. Once patients require oxygen, nebulized β-agonists [e.g., salbutamol (albuterol) 5 mg by nebulizer every 4 h] may reduce lung inflammation if administered within 1 h of exposure. Elective intubation should be considered early using an ARDSnet protective ventilation strategy. Management of a large-scale, non-confirmed exposure. In the circumstances of a large-scale industrial or urban release, not all patients presenting will have been exposed and health services are likely to be highly stretched. In this situation, patients should not be treated immediately as there is no evidence that delaying therapy causes harm, rather they should be rested and observed with regular physical examination and measurement of peripheral oxygen saturations. Once a patient's oxygen saturation falls below 94%, treatment with the lowest concentration of oxygen required to maintain their oxygen saturations in the normal range should be started. Once oxygen has been started, nebulized β-agonists [e.g., salbutamol (albuterol) 5 mg by nebulizer every 4 h] may reduce lung inflammation if administered within 1 h of exposure, though delayed administration which is likely following a large-scale release has not been tested formally. There is no benefit from nebulized steroid even when administered 1 h after exposure, or high-dose corticosteroid if administered intravenously ≥6 h after exposure. Although there are no experimental data to support this recommendation, an intravenous bolus of high-dose corticosteroid (e.g., methylprednisolone 1 g) may be considered if presentation is <6 h and resources allow. Depending on the numbers of casualties presenting, invasive ventilation should be initiated either electively once symptoms present (especially where there is a short latent period, indicating likelihood of more significant injury), or delayed until required. Ventilation should be with high positive end expiratory pressure, ARDSnet recommended ventilation. Conclusions. The mechanisms underlying the phosgene-induced acute lung injury are not well understood. Future experimental work should ensure that potential treatments are tested in a large animal model using realistic dosing regimens and clinically relevant timings, such as those that might be found in a mass casualty situation (Class IV).
Grainge C, Rice P. Management of phosgene-induced acute lung injury. Clin Toxicol (Phila). 2010 Jul; 48 (6): 497-508. [PubMed Citation]
-
The acute respiratory distress syndrome (ARDS) is a devastating constellation of clinical, radiological and pathological signs characterized by failure of gas exchange and refractory hypoxia. Despite nearly 30 years of research, no specific pharmacological therapy has yet proven to be efficacious in manipulating the pathophysiological processes that underlie this condition. Several in vitro and in vivo animal or human studies suggest a potential role for beta2-agonists in the treatment of ARDS. These agents have been shown to reduce pulmonary neutrophil sequestration and activation, accelerate alveolar fluid clearance, enhance surfactant secretion, and modulate the inflammatory and coagulation cascades. They are also used widely in clinical practice and are well tolerated in critically ill patients. The present review examines the evidence supporting a role for beta2-agonists as a specific pharmacological intervention in patients with ARDS (Class IV).
Perkins GD, McAuley DF, Richter A, Thickett DR, Gao F. Bench-to-bedside review: beta2-Agonists and the acute respiratory distress syndrome. Crit Care. 2004 Feb; 8 (1): 25-32. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
Objectives: To examine the effectiveness of nebulized salbutamol in the treatment of phosgene induced acute lung injury. Method: Using previously validated methods, 12 anaesthetized large white pigs were exposed to phosgene (Ct 1978 ± 8 mg min m(-3)), established on mechanical ventilation and randomized to treatment with either nebulized salbutamol (2.5 mg per dose) or saline control. Treatments were given 1, 5, 9, 13, 17 and 21 hours following phosgene exposure. The animals were followed to 24 hours following phosgene exposure. Results: Salbutamol treatment had no effect on mortality and had a deleterious effect on arterial oxygenation, shunt fraction and heart rate. There was a reduction in the number of neutrophils from 24.0% ± 4.4 to 12.17% ± 2.1 (p<0.05) in bronchoalveolar lavage, with some small decreases in inflammatory mediators in bronchoalveolar lavage but not in plasma. Conclusion: Nebulized salbutamol treatment following phosgene induced acute lung injury does not improve survival, and worsens various physiological parameters including arterial oxygen partial pressure and shunt fraction. Salbutamol treatment reduces neutrophil influx into the lung. Its sole use following phosgene exposure is not recommended.
Grainge C, Brown R, Jugg BJ, Smith AJ, Mann TM, Jenner J, Rice P, Parkhouse DA. Early Treatment with nebulized salbutamol worsens physiological measures and does not improve survival following phosgene induced acute lung injury. J R Army Med Corps. 2009 Jun; 155 (2): 105-9. [PubMed Citation]
-
The purpose of the present study was to investigate: (1) the acute effects of sulfur mustard on airway, lung, and surface tension of bronchoalveolar lavage fluid (BALfluid) in guinea pigs following intratracheal (i.t.) exposure to 1LD50 of an aerosolized solution of sulfur mustard in saline, and (2) the therapeutic efficacy of i.t. administration of the natural surfactant Curosurf and the broncholytic Salbutamol. Intratracheally aerosolized sulfur mustard solution induced two clinically relevant symptoms, that is, asthma-like symptoms reflected by an early bronchoconstriction and "late asthmatic responses" (LAR), and ARDS-like symptoms, that is, pulmonary edema and damage to the lung surfactant. The respiratory minute volume (RMV) was enhanced. Histologically, inflammation and severe epithelial injury in the upper airways were observed, whereas the lungs were homogeneously affected. The surface tension of BAL fluid derived at 24 h after sulfur mustard exposure was much higher (20 ± 1 mN/m) than that of unexposed control animals (about 1.0 ± 0.5 mN/m), indicating that the lung surfactant had been altered, and justifying treatment with exogenous surfactant. Intratracheal nebulization of a Salbutamol solution (10 μg/kg), or i.t. bolus administration of Curosurf (62.5 or 125 mg/kg), tended to reduce mortality, although Salbutamol appeared to be more effective than Curosurfin this respect. Although the present study does not give a definite answer to the question of whether the animal model used would be the most relevant for humans, a number of considerations in favor of i.t. aerosolization of sulfur mustard are discussed. Since it was noticed that sulfur mustard exposure induced damage to the lung surfactant, severe bronchoconstriction, and inflammation of the respiratory tract, the effectiveness of a combined treatment consisting of exogenous surfactant, anti-inflammatory drugs, and broncholytics is recommended to be further investigated.
van Helden HP, Kuijpers WC, Diemel RV. Asthma-like symptoms following intratracheal exposure of Guinea pigs to sulfur mustard aerosol: therapeutic efficacy of exogenous lung surfactant curosurf and salbutamol. Inhal Toxicol. 2004 Jul; 16 (8): 537-48. [PubMed Citation]
-
OBJECTIVE: To determine whether clinically relevant airspace concentrations of beta2-adrenergic agonists stimulated maximal alveolar fluid clearance rates and to determine whether beta2 agonist therapy decreased pulmonary edema in experimental acute lung injury. DESIGN: Prospective randomized laboratory investigation. SETTING: University-affiliated laboratory. SUBJECTS: Sprague Dawley rats. INTERVENTIONS: Dibutyryl cyclic adenosine monophosphate (cAMP), salmeterol, albuterol, and isoproterenol in normal rat lung. Salmeterol in a rat model of acid-induced lung injury. MEASUREMENTS AND MAIN RESULTS: Basal alveolar fluid clearance was 7.6 +/- 2.2 %/hr. Maximal cAMP-dependent alveolar fluid clearance rate was 32.9 +/- 10.9 %/hr (p <.05). Racemic albuterol 10(-5) M, salmeterol 10(-6) M, and isoproterenol 10(-6) M each stimulated alveolar fluid clearance to a level comparable to maximal cAMP-dependent alveolar fluid clearance. Compared with basal rates, alveolar fluid clearance was increased by both racemic albuterol 10(-6) M (14.5 +/- 3.0%, p <.05) and R-enantiomer 10(-6) M (15.0 +/- 4.6%, p <.05), but there was no difference between the two groups. Intra-alveolar salmeterol 10 (-6) M attenuated the degree of pulmonary edema following acid-induced lung injury. Extravascular lung water increased to only 180 +/- 30 microL with salmeterol treatment, compared with 296 +/- 65 microL in saline-treated rats 4 hrs after acid injury (p <.05). This decrease in lung water was accompanied by a 2.4-fold increase in the rate of alveolar fluid clearance at 4 hrs in the salmeterol-treated group. Lung endothelial permeability, expressed as extravascular plasma equivalents, was reduced to 64 +/- 9 microL with salmeterol compared with 119 +/- 51 microL in saline-treated rats 4 hrs after acid injury (p <.05). CONCLUSIONS: Clinically relevant airspace concentrations of beta2-adrenergic agonists a) stimulate maximal cAMP-dependent airspace fluid clearance in normal lungs and b) reduce pulmonary edema in acid aspiration-induced lung injury by increasing alveolar fluid clearance and decreasing endothelial permeability. Clinical studies are required to determine whether beta2-adrenergic agonists improve outcome in patients with acute lung injury.
McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004 Jul; 32 (7): 1470-6. [PubMed Citation]
-
The effects of the beta 2-adrenoceptor agonists, salbutamol and formoterol, on the increase of microvascular permeability induced by histamine or bradykinin in guinea-pig airways have been studied in vivo. Extravasation of intravenously injected Evans blue dye was used as an index of permeability. The effects of salbutamol and formoterol on the increase in pulmonary airway resistance induced by histamine or bradykinin have also been studied. The increase in pulmonary airway resistance induced by histamine or bradykinin was totally inhibited by salbutamol and formoterol. The ED50 of the two mediators were 0.59 +/- 0.21 (n = 5) and 0.20 +/- 0.14 (n = 5) micrograms kg-1 respectively for salbutamol, and 0.13 +/- 0.12 (n = 6) and 0.02 +/- 0.01 (n = 6) micrograms kg-1 respectively for formoterol. Salbutamol (10 and 30 micrograms kg-1) and formoterol (1 and 10 micrograms kg-1) inhibited the increase of microvascular permeability induced by histamine (30 micrograms kg-1) in the guinea-pig airways. The inhibitory effect was predominant in the trachea and the main bronchi, with a maximum inhibition of 20 to 50%. The two drugs had little or no inhibitory effect on the other structures studied, viz. nasal mucosa, larynx, proximal and distal intrapulmonary airways. Salbutamol and formoterol (1 and 10 micrograms kg-1) abolished the increase in microvascular permeability induced by bradykinin (0.3 micrograms kg-1). This inhibitory effect of two beta-adrenoceptor stimulants was predominant in the trachea and the nasal mucosa where it was observed with 1 microgram kg-1 of the beta-adrenoceptor agonists. In the main bronchi, and in the proximal and distal intrapulmonary airways, the effects of bradykinin were abolished by 10 pg kg-1 of formoterol and salbutamol. The effects of bradykinin, but not those of histamine, were significantly reduced (nasal mucosa, main bronchi and distal intrapulmonary airways) or abolished (trachea, proximal intrapulmonary airways) by morphine 10mgkg-1, i.v. These results suggest that an indirect effect, through non-adrenergic noncholinergic (NANC) nerves is involved in the action of bradykinin on the microvascular permeability. In conclusion, intravenously injected beta-adrenoceptor stimulants can inhibit, partially or totally, the increase of airways microvascular permeability induced by intravenous histamine or bradykinin. However, these effects require doses that are higher than those that inhibit the increase in pulmonary airway resistance induced by these mediators. As suggested by the results obtained with morphine, the higher efficacy of beta2-adrenoceptor agonists versus bradykinin may occur through activation of presynaptic receptors of the non-adrenergic non-cholinergic (NANC) nerves preventing release of inflammatory neuropeptides such as substance P and neurokinin A.
Advenier C, Qian Y, Koune JD, Molimard M, Candenas ML, Naline E. Formoterol and salbutamol inhibit bradykinin- and histamine-induced airway microvascular leakage in guinea-pig. Br J Pharmacol. 1992 Apr; 105 (4): 792-798. [PubMed Citation]
-
SUMMARY. The potential of the potassium channel activator cromakalim and its active enantiomer BRL 38227 as inhaled bronchodilators has been evaluated in the guinea-pig, in comparison with nifedipine, salbutamol and aminophylline. Inhaled cromakalim and BRL 38227 prolonged the time before histamine-induced collapse in conscious guinea-pigs, BRL 38227 (ED 50 250 to 500 pg/mL, roughly 10 to 20 μg per animal) being twice as potent as cromakalim. In anaesthetized guinea-pigs, BRL 38227 (inhaled and i.v.) and aminophylline (i.v.) caused similar percentage inhibitions of the increase in airways resistance and decrease in dynamic lung compliance elicited by histamine, whereas salbutamol (inhaled and i.v.) was more effective against resistance. Inhaled BRL 38227 and salbutamol were more potent against inhaled than against i.v. histamine. BRL 38227 inhibited the effects of i.v. and inhaled histamine by 67-78% when nebulized from solutions of 250 and 31 pg/mL respectively, but the lowest concentration that lowered blood pressure significantly was 500 pg/mL. In contrast, nifedipine had no effect on compliance and caused only a marginal (21%) inhibition of resistance at a dose (200 μg/kg i.v.) which lowered blood pressure by 44%. These results show that BRL 38227 is an effective bronchodilator when given by inhalation. It differs from salbutamol in its effects on airways dynamics, and its effect on lung compliance cannot be attributed to a pulmonary vasodilator effect. Furthermore, L-type calcium channels are not significantly involved in histamineinduced bronchoconstriction or therefore in the bronchodilator effect of BRL 38227.
Bowring NE, Buckle DR, Clarke GD, Taylor JF, Arch JR. Evaluation of the Potassium Channel Activator BRL 38227 as an Inhaled Bronchodilator in the Guinea-pig: Contrast with Nifedipine and Salbutamol. Pulm Pharmacol. 1991; 4 (2): 99-105. [PubMed Citation]
-
The rate of aqueous humor formation was determined in the cynomolgus monkey eyes by a tracer dilution technique. One 25-gauge needle was inserted into the posterior chamber and a solution of fluorescein labeled dextran with a molecular weight of 40,000 was infused at a constant rate. The aqueous humor was collected through a needle inserted into the anterior chamber, while the intraocular pressure (IOP) was maintained at a constant level. The aqueous humor formation rate and the dye distribution volume were calculated from the time profile of the dye concentration in the effluent aqueous humor. By means of this technique, the effects of pilocarpine, salbutamol, and timolol on the aqueous humor formation rate were studied. The test drug solution was administered into the conjunctival reservoir during a 90-min period before measurements and also during the measurement so that a steady-state drug concentration was maintained in the anterior chamber during the measurement. Pilocarpine 0.1% reduced the aqueous humor formation rate to approximately 50% of the control without significantly changing the IOP or the distribution volume. Salbutamol 0.5%, a beta-adrenergic agonist, increased the rate by about 38%, but timolol 0.1%, a beta-adrenergic antagonist, reduced the rate by an average of 36%. Timolol caused a statistically significant lowering of the IOP by about 2 mmHg. Simultaneous administration of salbutamol 0.5% and timolol 0.2% caused no change in the aqueous humor formation rate or the IOP. The barrier function of the blood aqueous barrier was not altered by these drugs as revealed by aqueous protein determinations.
Miichi H, Nagataki S. Effects of pilocarpine, salbutamol, and timolol on aqueous humor formation in cynomolgus monkeys. Investigative Ophthalmolofy & Visual Science September 1983, Vol. 24 1269-75. [PubMed Citation]
Pregnant animal studies
-
Albuterol has been shown to be teratogenic in mice. A study in CD-1 mice at subcutaneous (sc) doses of 0.025, 0.25, and 2.5 mg/kg (approximately 3/1000, 3/100, and 3/10 times, respectively, the maximum recommended daily oral dose for adults on a mg/m2 basis), showed cleft palate formation in 5 of 111 (4.5%) fetuses at 0.25 mg/kg and in 10 of 108 (9.3%) fetuses at 2.5 mg/kg. The drug did not induce cleft palate formation at the lowest dose, 0.025 mg/kg. Cleft palate also occurred in 22 of 72 (30.5%) fetuses from females treated with 2.5 mg/kg of isoproterenol (positive control) subcutaneously (approximately 3/10 times the maximum recommended daily oral dose for adults on a mg/m2 basis).
Product label: Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
-
A reproduction study in Stride Dutch rabbits revealed cranioschisis in 7 of 19 (37%) fetuses when albuterol was administered orally at a 50 mg/kg dose (approximately 25 times the maximum recommended daily oral dose for adults on a mg/m2 basis).
Product label: Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
Other non-clinical studies
Human non-clinical studies-
A phosgene exposure that resulted in the patient's death. A 58 year-old man arrived to the emergency department 1 hour after exposure to phosgene with complaints of a sore throat. Initial vital signs were blood pressure 175/118 mmHg, heart rate 98/min, respirations 12/min, and oxygen saturation of 93% on room air. Physical exam revealed few scattered rhonchi, without signs of distress. Initial arterial blood gases (ABG's) revealed pH 7.42, pCO2 43 mmHg, pO2 68 mmHg, HCO3 27 meq/L, and oxygen saturation of 93% on room air. Initial chest x-ray 2 hours after the exposure demonstrated clear lung fields. Approximately 2.5 hours after the exposure, he began complaining of dyspnea, restlessness and his oxygen saturation dropped below 90%. He received nebulized albuterol, 1 gram intravenous methylprednisolone, and 100 % oxygen via face mask. Minimal improvement was noted and he was intubated. The post intubation chest x-ray, 3.5 hours after the exposure, revealed diffuse alveolar infiltrates. Acetylcysteine, terbutaline, and IV steroids were administered without improvement. The patient died 30 hours after exposure. DISCUSSION: There are many misunderstandings concerning phosgene due to its rare presentation. Traditional treatment modalities are often unproven in human trials and were unsuccessful in this case. CONCLUSION: This case highlights the significant toxicity that results from phosgene exposure and the challenges of the limited treatment modalities. There is concern for the use of this agent in chemical terrorism.
Hardison LS Jr, Wright E, Pizon AF. Phosgene exposure: a case of accidental industrial exposure. J Med Toxicol. 2014; 10(1): 51-6. [PubMed Citation]
Animal in vitro studies-
A possible role for beta-adrenergic agonists in the management of bronchoconstriction resulting from exposure to anticholinesterase compounds was investigated in vitro in canine tracheal smooth muscle. Norepinephrine, salbutamol and isoproterenol produced partial relaxation of soman-induced contractures. However, the relaxation induced was not sustained; muscle tensions returned to pretreatment levels within minutes despite the continued presence of beta-agonists. Increasing cAMP levels with the non beta-agonist bronchodilators such as theophylline, a phosphodiesterase inhibitor, or forskolin, a specific stimulnator of adenylate cyclase, resulted in more complete and longer lasting relaxation, suggesting that beta-adrenoceptor desensitization may contribute to the failure by beta-agonists to produce sustained relaxation.
Filbert MG, Moore DH, Adler M. Relaxation of soman-induced contracture of airway smooth muscle in vitro. Drug Chem Toxicol. 1992; 15 (3): 203-15. [PubMed Citation]
Non-clinical reviews
-
Despite extensive research into its pathophysiology, acute lung injury/acute respiratory distress syndrome (ALI/ARDS) remains a devastating syndrome with mortality approaching 40%. Pharmacologic therapies that reduce the severity of lung injury in vivo and in vitro have not yet been translated to effective clinical treatment options, and innovative therapies are needed. Recently, the use of beta2 adrenergic agonists as potential therapy has gained considerable interest due to their ability to increase the resolution of pulmonary edema. However, the results of clinical trials of beta agonist therapy for ALI/ARDS have been conflicting in terms of benefit. In the previous issue of Critical Care, Briot and colleagues present evidence that may help clarify the inconsistent results. The authors demonstrate that, in oleic acid lung injury in dogs, the inotropic effect of beta agonists may recruit damaged pulmonary capillaries, leading to increased lung endothelial permeability.
Lee JW. Beta2 adrenergic agonists in acute lung injury? The heart of the matter. Crit Care. 2009; 13 (6): 1011. [PubMed Citation]
-
This paper reviews the published toxicology of salbutamol. Salbutamol is a relatively selective beta 2-adrenoreceptor stimulant with rapid, potent bronchodilator activity and only minor inotropic or chronotropic effects. It was not found to be mutagenic. LD50 values and other acute studies indicated low toxicity. Findings published for repeat dose studies were mainly uneventful. Tachycardia and flushing of the skin were observed in dogs. There were several findings peculiar to the rat--growth of the salivary gland, enlargement of the Harderian gland, an increase in colloid in the pituitary, and mesovarian leiomyomas. Increases in heart weights associated with inflammation, hypertrophy of muscle fibres, focal myocardial necrosis and fibrosis were seen in rats. Malformation, in the form of cleft palate, was reported in mice but not in rats or rabbits. These treatment related effects reported for salbutamol are not compound-related but rather are class-related. They are an expression of pharmacological activity brought about by the excessive beta stimulant action of high dosage with the drug.
Libretto SE. A review of the toxicology of salbutamol (albuterol). Arch Toxicol. 1994; 68 (4): 213-6. [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
AIM: To study the pharmacokinetics and relative bioavailability of salbutamol metered-dose inhaler (MDI) in healthy volunteers. METHODS: An HPLC method for the determination of salbutarnol in human plasma was improved. Ten healthy male Chinese volunteers were enrolled in a randomized crossover study. After the subjects inhaled or orally administered 1.2 mg salbutamol, fourteen blood samples were collected at predetermined time points. The concentrations of salbutamol in plasma were assessed with non-compartment model to obtain the pharmacokinetic parameters. The relative bioavailability of MDI versus water solution was calculated. RESULTS: The HPLC assay was sensitive, specific, accurate, and precise. The pharmacokinetics of salbutamol MDI was described well with two-compartment model. The parameters for salbutamol inhaled and orally administered were as following: T (max) (0.22±0.07) and (1.8±0.6) h, C(max)(3.4± 1.1) and (3.9± 1.4) microg/L. T(1/2) (4.5± 1.5) and (4.6± 1.1) h. AC 0-20min(0.9±0.3) and (0.16±0.10) microg/LxhxL (-1), respectively. There were significant differences in T(max) and AUC 0-20 min between the two dosage forms. The AUC 0-20 min (inhal) was 8 times as high as the AUC 0-20 min (po). The relative bioavailability of salbutamol MDI was 57 % ± 24 % compared with oral solution. CONCLUSION: The absorption process of salbutamol MDI in human was significantly different from that of oral solution.
Du XL, Zhu Z, Fu Q, Li DK, Xu WB. Pharmacokinetics and relative bioavailability of salbutamol metered-dose inhaler in healthy volunteers. Acta Pharmacol Sin. 2002 Jul; 23 (7): 663-6. [PubMed Citation]
-
We evaluated the effects of race and gender on albuterol pharmacokinetics in 30 patients with moderate asthma (15 blacks, 15 whites, 16 men, 14 women). Subjects received a single dose of albuterol 8 mg oral solution and had blood samples collected at various times for 12 hours after the dose. Albuterol plasma concentrations were determined by HPLC with fluorescence detection, and pharmacokinetics were determined by compartmental analysis. The apparent volume of distribution of albuterol was significantly higher in men than in women (631+/-171 and 510+/-109 L, respectively, p<0.05). Consequently, the maximum concentration was lower in men than women (10.3+/-2.1 and 12.0+/-1.9 ng/ml, respectively, p<0.05). Elimination rates were 0.136+/-0.008 and 0.160+0.012 hour(-1), respectively (p<0.10). When corrected for ideal body weight, apparent volume of distribution was not different by gender. No differences between blacks and whites other than lag time were noted in albuterol kinetics. The greater apparent volume of distribution in men is likely explained by differences in ideal body weight or lean body mass.
Mohamed MH, Lima JJ, Eberle LV, Self TH, Johnson JA. Effects of gender and race on albuterol pharmacokinetics. Pharmacotherapy. 1999 Feb; 19 (2): 157-61. [PubMed Citation]
-
The pharmacokinetics of inhaled (R,S)-albuterol following pulmonary absorption were studied in healthy human subjects. Ten subjects (5 females and 5 males) inhaled two puffs (180 microg) of albuterol via a metered-dose inhaler and spacer device. All subjects were nonsmoking and had normal pulmonary function. Charcoal slurries were ingested to block gastrointestinal absorption of drug. Venous samples were obtained from each subject at thirteen time points from 0 through 12 h post dose. (R,S)-Albuterol concentration in plasma was measured using a gas chromatography-mass spectrometry (GC-MS) assay. The plasma concentration-time profiles conformed to a two-compartment extravascular model with first-order absorption kinetics. The drug levels reached maximum in 12.6 +/- 2.2 (SD) minutes, which is in contrast with previous reports that maximum plasma concentrations occur within 2 to 4 h. The mean peak plasma level was 1469 +/- 410 pg/mL. The mean half-life of distribution was 17.9 +/- 8.2 min. The mean half-life of elimination was 4.4 +/- 1.5 h. Female subjects achieved peak concentration more rapidly than male subjects (10.4 vs 14.8 min, p = 0.01) and had a higher mean peak concentration (1778 vs 1159 pg/mL, p = 0.04).
Anderson PJ, Zhou X, Breen P, Gann L, Logsdon TW, Compadre CM, Hiller FC. Pharmacokinetics of (R,S)-Albuterol after aerosol inhalation in healthy adult volunteers. J Pharm Sci. 1998 Jul; 87 (7): 841-4. [PubMed Citation]
-
Salbutamol is a beta 2-adrenoceptor stimulant used clinically as a racemate where the activity resides predominantly in the (-)R enantiomer with little or no activity attributed to the (+)S enantiomer. Salbutamol undergoes extensive pre-systemic metabolism and active renal excretion. The pharmacokinetics of the enantiomers of salbutamol have been investigated after intravenous (1.6 mg) and oral (4 mg) dosing with racemic drug to seven normal male volunteers. Plasma and urine samples were analyzed by chiral h.p.l.c. after solid phase extraction. The ratio of (-)R/(+)S salbutamol in plasma and urine following intravenous administration ranged from near unity soon after dosing to about 0.66 after 8 h. The ratio remained at about 0.3 in both plasma and urine over the 8 h following an oral dose. The following pharmacokinetic parameters for (+)S and (-)R salbutamol were found to be significantly different (P < 0.05) after intravenous administration (clearance 0.39 +/- 0.12 vs 0.62 +/- 0.18 1 h-1 kg-1, terminal phase half-life 2.85 +/- 0.83 vs 2.00 +/- 0.49 h, amount excreted unchanged in urine 55 +/- 11 vs 46 +/- 8%) and following oral administration (amount excreted unchanged in urine 32 +/- 11 vs 8 +/- 4% and bioavailability 0.71 +/- 0.09 vs 0.30 +/- 0.07). 5 The active (-)R enantiomer of salbutamol undergoes significantly faster metabolism in man than the inactive (+)S enantiomer resulting in considerably lower bioavailability of the active enantiomer following oral administration.
Boulton DW, Fawcett JP. Enantioselective disposition of salbutamol in man following oral and intravenous administration. Br J Clin Pharmacol. 1996 Jan; 41 (1): 35-40. [PubMed Citation]
-
A new extended-release formulation of albuterol, Volmax, was developed based on the oral osmotic drug delivery system. Proventil Repetabs, another extended-release formulation of albuterol, is a repeat-action tablet. The steady-state pharmacokinetic profile of Volmax 8 mg orally every 12 hours for 3 days was compared with that of Proventil Repetabs 8 mg orally every 12 hours for 3 days in a randomized, two-way, crossover study in 23 healthy men. Results were determined for both daytime and nighttime dosing intervals. Volmax demonstrated significantly less peak-trough fluctuation during both dosing intervals than Proventil Repetabs (p less than 0.05). Peak plasma concentrations were higher and occurred earlier with Proventil Repetabs. There were no significant differences between the two formulations with respect to reported adverse experiences. Volmax exhibited a more controlled release of albuterol during both daytime and nighttime dosing intervals than Proventil Repetabs.
Hussey EK, Donn KH, Powell JR. Albuterol extended-release products: a comparison of steady-state pharmacokinetics. Pharmacotherapy. 1991; 11 (2): 131-5. [PubMed Citation]
-
The pharmacokinetics of salbutamol and its sulphate conjugate metabolite were investigated after intravenous and steady-state oral administration of salbutamol to 10 healthy volunteers. With intravenous administration, total plasma clearance was 480 ± 123 ml min-1, elimination half-life was 3.86 ± 0.83 h and apparent volume of distribution was 156 ± 381. Urinary excretion of unchanged drug and sulphate conjugate were 64.2 ± 7.1% and 12.0 ± 3.1% of the dose, respectively. 3 With oral administration, systemic availability was 0.50 ± 0.04, and urinary excretion of unchanged drug and sulphate conjugate were 31.8 ± 1.9% and 48.2 ± 7.3% of the dose, respectively. The drug eliminated on the first-pass could be accounted for entirely as sulphate conjugate formed, presumably, in the intestinal wall. 4 Renal clearance of salbutamol was 291 ± 70 ml min-1 after intravenous and 272 ± 38 ml min-1 after oral administration, while the renal clearance of the sulphate conjugate was 98.5 ± 23.5 ml min-1 after oral administration. Heart rate increased with increasing plasma salbutamol concentration, although a lag was evident. The effect on heart rate was lower after 24 h continuous oral salbutamol administration.
Morgan DJ, Paull JD, Richmond BH, Wilson-Evered E, Ziccone SP. Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol. 1986 Nov; 22 (5): 587-593. [PubMed Citation]
-
Albuterol sulfate, alpha'[[1,1-dimethyl)amino]methyl]-4-hydroxy-1,3-benzenedimethanol sulfate, is a relatively selective beta-2-adrenergic bronchodilator used for the relief of bronchospasm. The bioavailability of two 4-mg tablet formulations, differing in their inactive excipients, and a syrup formulation, was evaluated. The three dosage forms were orally administered to 12 normal male volunteers in a randomized three-way crossover study. Plasma samples were collected at frequent time points through 12 h and analyzed for albuterol content by a specific GC-MS method. The drug was rapidly absorbed from all three formulations. Maximum drug concentrations were comparable for the three formulations and were obtained between 1.8-2.0 h. The areas under the plasma concentration-time curves were 68-78 h X ng/mL. The drug elimination phase half-live (t1/2 beta) ranged from 4.8 to 5.5 h. Analysis of the data showed that the bioavailability of albuterol from a tablet formulation is equivalent to that from a solution.
Powell ML, Weisberger M, Gural R, Chung M, Patrick JE, Radwanski E, Symchowicz SS. Comparative bioavailability and pharmacokinetics of three formulations of albuterol. J Pharm Sci. 1985 Feb; 74 (2): 217-9. [PubMed Citation]
Children
-
OBJECTIVE: To characterize the population pharmacokinetics (PK) of (R)- and (S)-albuterol in pediatric asthmatics using a model that supports a sparse blood sampling strategy. METHODS: The data for this analysis were collected from patients enrolled in a randomized, double-blind, multicenter, placebo- and active-controlled study evaluating the safety and efficacy of levalbuterol in asthmatic children aged 4-11 years. Patients received either levalbuterol 0.31 mg, levalbuterol 0.63 mg, racemic albuterol 1.25 mg, or racemic albuterol 2.5 mg via nebulizer. Separate population pharmacokinetic models were developed for (R)- and (S)-albuterol using the NOMNEM((R)) computer program. Covariate models were developed to identify significant predictors of inter-patient variability. RESULTS: A total of 995 samples and 262 patients were used for the (R)-albuterol population PK model while a total of 496 samples and 128 patients were used for the (S)-albuterol population PK model. The apparent clearance of (R)-albuterol was much more rapid than that of (S)-albuterol (approximately four-fold higher), and the apparent volume of distribution was much larger for (R)-albuterol (in part due to pre-systemic metabolism) than for (S)-albuterol (approximately four-fold higher). CONCLUSIONS: In this study of pediatric patients, the models were able to demonstrate using two to four samples per patient that the apparent clearance and volume of distribution of (R)-albuterol were several fold higher than that of (S)-albuterol. The pharmacokinetics of (R)-albuterol were similar after administration of levalbuterol or racemic albuterol and were linear over the examined dose range (0.31-0.63 mg nebulized dose). The presence of (S)-albuterol did not significantly alter the pharmacokinetics of (R)-albuterol, suggesting that effects of (S)-albuterol may be due to the intrinsic pharmacology of this isomer.
Maier G, Rubino C, Hsu R, Grasela T, Baumgartner RA. Population pharmacokinetics of (R)-albuterol and (S)-albuterol in pediatric patients aged 4-11 years with asthma. Pulm Pharmacol Ther. 2007; 20 (5): 534-42. [PubMed Citation]
Pregnancy
-
Objective: We sought to determine whether subcutaneous administration of salbutamol resulted in plasma levels comparable to those achieved after intravenous or oral administration. Methods: Twenty-nine women with preterm labor received subcutaneous infusion of salbutamol through a portable pump. We used three different rates of continuous infusion: a low rate of 3.33 micrograms/minute (20 subjects), an intermediate rate of 6.66 micrograms/minute (four subjects), and a high rate of 9.99 micrograms/minute (five subjects). Plasma salbutamol concentrations were assayed by high-performance liquid chromatography after 48 hours of continuous infusion in the subcutaneous tissue and after bolus injections (184 micrograms in the low-rate group and 368 micrograms in the intermediate- and high-rate groups). Results: Plasma salbutamol concentrations after 48 hours of subcutaneous infusion increased almost linearly with the rate of infusion: 6.29 ± 1.58, 15.5 ± 1.0, and 21.7 ±4.26 ng/mL in the low-, intermediate-, and high-rate groups, respectively (P < .001 between the three groups). After bolus injection, maximum plasma concentrations were significantly different between the three groups (P < .001) and from their respective baseline values (P < .001): 8.33 ± 1.9, 18.85 ± 2.0, and 25.86 ± 4.8 ng/mL in the low-, intermediate-, and high-rate groups, respectively. Conclusion: Subcutaneous tocolysis can provide plasma salbutamol levels similar to the levels obtained orally or intravenously.
Milliez JM, Flouvat B, Jannet D. Pharmacokinetics of salbutamol in the pregnant woman after subcutaneous administration with a portable pump. Obstet Gynecol. 1992 Aug; 80 (2): 182-185. [PubMed Citation]
-
The pharmacokinetics of salbutamol and its sulphate conjugate were examined during intravenous and steady-state oral administration in nine patients receiving the drug for the prevention or treatment of premature labor. Uterine contractions were inhibited by plasma salbutamol concentrations in the range 8-33 ng ml-1 in six of the seven patients who were receiving intravenous drug. By comparison with our previous study in a control group of healthy males and nonpregnant females, the total clearance of salbutamol in premature labor (501; s.d. 185 ml min-1) was similar to that in the control group (480; s.d. 123 ml min-1). Renal salbutamol clearance (208; s.d. 51 ml min-1), however, was significantly less than that in the control group (283; s.d. 51 ml min-1). The systemic availability (n = 3), urinary recovery of unchanged oral salbutamol and area under the plasma curve during the oral dosage interval (n = 5) were all only slightly lower (10-20%) than control values, suggesting slightly lower oral absorption. Formation and elimination of the sulphate conjugate were similar to those observed in control subjects. suggesting that first-pass sulphation in the gut wall is unchanged in pregnancy. Overall there were only minor differences in salbutamol pharmacokinetics between control subjects and patients in premature labor.
Hutchings MJ, Paull JD, Wilson-Evered E, Morgan DJ. Pharmacokinetics and metabolism of salbutamol in premature labor. Br J Clin Pharmacol. 1987 Jul; 24 (1): 69-75. [PubMed Citation]
Renal Impairment
-
Salbutamol was administered intravenously to 5 patients with renal function impairment for estimation its pharmacokinetic parameters. The mean terminal half-life was 256 min, similar to previously reported values in healthy adults. The mean clearance (167 ml/min) and the mean volume of distribution (551) were decreased. These parameters were not correlated with the creatinine clearance. A slight but significant decrease was observed in the plasma potassium level up to 125 min after the salbutamol infusion. The heart rate was significantly increased, and the increase in 3 patients was correlated with the salbutamol concentration. The biological effects of the drug were less marked than expected.
Rey E, Luquel L, Richard MO, Mory B, Offenstadt G, Olive G. Pharmacokinetics of intravenous salbutamol in renal insufficiency and its biological effects. Eur J Clin Pharmacol. 1989; 37 (4): 387-9. [PubMed Citation]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults
Oral Inhalation via Metered-Dose Aerosol
-
For the symptomatic relief of acute episodes of bronchospasm or prevention of asthmatic symptoms, the usual dosage of orally inhaled albuterol administered via a metered-dose aerosol for adults and children 4 years of age or older (Ventolin® HFA, Proventil® HFA, ProAir® HFA) is 180 mcg (2 inhalations) every 4-6 hours. In some patients, 90 mcg (1 inhalation) every 4 hours may be sufficient.
-
For treatment of acute mild to moderate asthma exacerbations (forced expiratory volume in 1 second [FEV1] or PEF at least 40% of predicted value or personal best) in the emergency department... In adults and adolescents older than 12 years of age, some experts suggest 720 mcg of albuterol every 20 minutes for the first hour, followed by 720 mcg hourly for 1-4 hours.
-
For the prevention of exercise-induced bronchospasm, the usual dosage of albuterol inhalation aerosol with HFA propellant (Ventolin® HFA, Proventil® HFA, Proair® HFA) is 180 mcg (2 inhalations) given 15-30 minutes before exercise.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
Oral Inhalation via Nebulization
-
For administration via a nebulizer, the initial dosage of albuterol for adults and for children 2-12 years of age who weigh at least 15 kg is 2.5 mg 3 or 4 times daily.
-
The usual initial dosage of single-use albuterol inhalation solution via nebulization for the symptomatic relief of acute episodes of bronchospasm in adults and adolescents 12 years of age or older is 2.5 mg 3 or 4 times daily. In patients receiving nebulized albuterol, the flow rate of the nebulizer should be adjusted so that the albuterol is delivered over a period of approximately 5-15 minutes.
-
For acute asthma exacerbations in adults and adolescents 12 years of age or older that are managed initially in the patient's home, some experts state that up to 2 doses (2.5-5 mg per dose) may be administered via nebulization 20 minutes apart.
-
For treatment of acute mild to moderate asthma exacerbations in the emergency department In adults and adolescents 12 years of age and older, some experts suggest 2.5-5 mg of albuterol every 20 minutes for the first hour, followed by 2.5-10 mg every 1-4 hours as needed.
-
For treatment of acute severe asthma exacerbations in the emergency department In adults or adolescents 12 years of age or older, albuterol inhalation solution may be given continuously at a rate of 10-15 mg per hour for the first hour or intermittently at 2.5-5 mg of albuterol every 20 minutes for the first hour. If the exacerbation continues to be severe after the first hour, albuterol inhalation solution is given intermittently at 2.5-10 mg every hour or continuously at 10-15 mg per hour.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
Oral Dosage
-
The usual initial oral dosage of albuterol for adults and children 12 years of age or older is 2 or 4 mg 3 or 4 times daily as conventional tablets. Alternatively, adults and children 12 years of age or older may receive an initial oral dosage of 8 mg every 12 hours as extended-release tablets; in some patients (e.g., those with low body weight), 4 mg every 12 hours may be sufficient.
-
The usual initial oral dosage of albuterol for adults or children older than 14 years of age is 2 or 4 mg 3 or 4 times daily as the oral solution. Subsequent dosage is adjusted according to the patient's tolerance and response. Dosages exceeding 4 mg 4 times daily as the oral solution should be used only when the patient fails to respond to the usual initial dosage. If necessary, dosage of albuterol oral solution may be cautiously and gradually increased to a maximum of 8 mg 4 times daily in adults and children older than 14 years of age.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
Children
Oral Inhalation via Metered-Dose Aerosol
-
For the symptomatic relief of acute episodes of bronchospasm or prevention of asthmatic symptoms, Some experts suggest 180 mcg of albuterol every 4-6 hours for the treatment of acute episodes of bronchospasm in infants and children younger than 4 years of age. (Use not currently included in product labeling approved by US FDA)
-
For treatment of acute mild to moderate asthma exacerbations (forced expiratory volume in 1 second [FEV1] or PEF at least 40% of predicted value or personal best) in the emergency department in children 12 years of age or younger, some experts suggest 360-720 mcg (4-8 inhalations) of albuterol every 20 minutes for the first hour, followed by 360-720 mcg hourly for 1-4 hours.
-
For the prevention of exercise-induced bronchospasm, the usual dosage of albuterol inhalation aerosol with HFA propellant (Ventolin® HFA, Proventil® HFA, Proair® HFA) is 180 mcg (2 inhalations) given 15-30 minutes before exercise.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
Oral Inhalation via Nebulization
-
For administration via a nebulizer, children 2-12 years of age may receive a lower initial albuterol dosage of 0.63 or 1.25 mg 3 or 4 times daily.
-
In children 2-12 years of age weighing less than 15 kg who require less than 2.5 mg of albuterol per dose, albuterol 0.5% inhalation solution should be used to prepare the appropriate dose. Alternatively, in children 2-12 years of age in whom an albuterol dose of less than 2.5 mg per dose is desired, a single-use pediatric formulation containing 0.63 or 1.25 mg of albuterol per 3 mL may be used.
-
Children 6-12 years of age with more severe asthma (baseline FEV1 less than 60% predicted) or weight more than 40 kg, or children 11-12 years of age may achieve a better initial response with an albuterol dosage of 1.25 mg. The manufacturer states that the single-use pediatric formulation containing 0.63 or 1.25 mg of albuterol per 3 mL (Accuneb) has not been studied in patients with acute exacerbations of asthma; use of a 2.5-mg dose of albuterol administered using a more concentrated inhalation solution (0.083% solution containing 2.5 mg of albuterol per 3 mL) may be more appropriate for treating acute exacerbations, particularly in children 6 years of age or older.
-
For treatment of acute mild to moderate asthma exacerbations in the emergency department in children younger than 12 years of age, some experts suggest 0.15 mg/kg of albuterol inhalation solution every 20 minutes for the first hour, followed by 0.15-0.3 mg/kg (not exceeding 10 mg) every 1-4 hours as needed.
-
For treatment of acute severe asthma exacerbations in the emergency department in children younger than 12 years of age, albuterol inhalation solution may be given continuously at a rate of 0.5 mg/kg per hour or intermittently at 0.15 mg/kg every 20 minutes for the first hour. If the exacerbation continues to be severe after the first hour, albuterol inhalation solution is given intermittently at 0.15-0.3 mg/kg (not exceeding 10 mg) every hour or continuously at a rate 0.5 mg/kg per hour.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
Oral Dosage
-
The usual initial oral dosage of albuterol for children 6 to younger than 12 years of age is 2 mg 3 or 4 times daily as conventional tablets. Dosages exceeding 2 mg 4 times daily as conventional tablets should be used only when the patient fails to respond to the usual initial dosage. If necessary, dosage may be cautiously and gradually increased to a maximum of 24 mg daily (in divided doses) as conventional tablets.
-
In children 6-12 years of age, the usual initial dosage of albuterol extended-release tablets (VoSpire ER®) is 4 mg every 12 hours. Dosage of albuterol given as extended-release tablets may be increased cautiously stepwise as tolerated to a maximum of 12 mg twice daily.
-
The usual initial oral dosage of albuterol as the oral solution (syrup) for children 6-14 years of age is 2 mg 3 or 4 times daily. In patients older than 14 years of age receiving the oral solution who fail to respond to the usual initial dosage, dosage may be cautiously and gradually increased to a maximum of 24 mg daily given in 4 divided doses.
-
For children 2 to 6 years of age, the usual initial oral dosage is 0.1 mg/kg 3 times daily (not to exceed 2 mg 3 times daily) as the oral solution; in patients who fail to respond to the usual initial dosage, dosage may be gradually increased to 0.2 mg/kg 3 times daily (not to exceed 4 mg 3 times daily).
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
Pregnancy
FDA Pregnancy Risk Category:
-
C; RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk.
Product label AccuNeb (Albuterol sulfate) solution [Dey Pharma, LP] Last revised: March 2011 [DailyMed]
Nursing Mothers
Lactation Risk Category:
-
L1 Safest; Drug which has been taken by a large number of breastfeeding mothers without any observed increase in adverse effects in the infant. Controlled studies in breastfeeding women fail to demonstrate a risk to the infant and the possibility of harm to the breastfeeding infant is remote; or the product is not orally bioavailable in an infant.
Hale TW, Medications and Mother's Milk. Amarillo, TX: Hale Publishing L.P., 2006 p.34-35
Geriatric
Oral Inhalation via Metered-Dose Aerosol
-
Because of the greater frequency of decreased hepatic, renal, and/or cardiac function and of concomitant disease and drug therapy in geriatric patients, the manufacturers of Ventolin® HFA or ProAir® HFA inhalation aerosol suggest that patients in this age group receive initial dosages of the drug at the lower end of the usual range (90-180 mcg every 4-6 hours).
-
For the prevention of exercise-induced bronchospasm, the usual dosage of albuterol inhalation aerosol with HFA propellant is 180 mcg (2 inhalations) given 15-30 minutes before exercise.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
Oral Dosage
-
In geriatric patients and those sensitive to sympathomimetic amines, the initial oral albuterol dosage is 2 mg 3 or 4 times daily as conventional tablets or oral solution; if necessary, dosage may be gradually increased to as much as 8 mg 3 or 4 times daily.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
Emergency Use Authorization (FDA/CDC)
-
No Emergency Use Authorization for Albuterol has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (Public Law 108-276).
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
6. Current available formulations/shelf life
Formulations
-
Oral Solution 2 mg/5 mL (of albuterol)
-
Oral Tablets 2 mg, 4 mg (of albuterol)
-
Oral Tablets, extended release 4 mg, 8 mg (of albuterol)
-
Oral Inhalation, aerosol 90 mcg (of albuterol) per metered spray
-
Oral Inhalation, solution for nebulization 0.021% (of albuterol), 0.042% (of albuterol), 0.083% (of albuterol)
-
Oral Inhalation, solution, concentrate, for nebulization 0.5% (of albuterol)
FDA; Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Patent and Generic Drug Product Data Last Updated: September 07, 2012
Shelf life
Stability
-
New albuterol-containing metered-dose inhaler (MDI) formulations were under development to replace chlorofluorocarbon (CFC) propellants with more environmentally friendly hydrofluoroalkane (HFA) propellants. To achieve good chemical and physical stability of MDI formulations with HFA propellants, different drug forms were evaluated in model formulations (drug, oleic acid, and one of the following: P12/P11, P12/ethanol, P12, P134a/ethanol, P134a). The effects of drug form (base versus sulfate), propellant type (P12 versus P134a), and cosolvent type (P11 or ethanol versus none) on the chemical and physical stability were examined. The chemical stability of the formulations was determined by monitoring the percent drug remaining in the formulations using HPLC. The physical stability of the formulations was followed by visually assessing the suspension appearance, and by determining the mass median diameter (MMD) of the suspended particles using laser diffraction analysis. The drug form has a great impact on the chemical and physical stability of the formulations. The sulfate formulations were chemically stable up to 12 months when stored at 30 degrees C and 40 degrees C/85% relative humidity (RH). Poor chemical stability was observed for the base formulations, except for ethanol-free formulations (P12/P11, P12, and P134a) at 30 degrees C and a P134a formulation at 40 degrees C/85% RH. The chemical instability of albuterol base formulations at 30 degrees C correlates with its solubility. The presence of a cosolvent greatly improved the dispersion characteristics of both sulfate and base formulations. The sulfate formulations in the presence of a cosolvent (P12/P11, P12/ethanol, and P134a/ethanol) showed good physical stability when stored for up to 12 months at 30 degrees C and 40 degrees C/85% RH. The physical stability of the base formulations was not acceptable due to crystal growth/agglomeration in all formulations, except for the P12/P11 formulation. The physical instability of both sulfate and base formulations not only correlates with the drug solubility, but also with particle agglomeration. In conclusion, good chemical and physical stability of albuterol-containing suspension formulations can be achieved with the appropriate choice of drug form and formulation constituents.
Tzou TZ, Pachuta RR, Coy RB, Schultz RK. Drug form selection in albuterol-containing metered-dose inhaler formulations and its impact on chemical and physical stability. J Pharm Sci. 1997 Dec; 86 (12): 1352-7. [PubMed Citation]
SLEP (DOD/FDA)
Storage
-
Preparations containing albuterol sulfate should be stored in well-closed, light-resistant containers. Albuterol sulfate inhalation aerosol with hydrofluoroalkane propellant should be stored at 15-25°C. Failure to use these inhalers within these respective temperature ranges could result in delivery of improper doses. At temperatures below these ranges, cooling of the propellants can decrease internal pressure of the canister, which may result in delivery of particles too large to provide full therapeutic effect. A 5-minute cooling of inhalers to 0°C produced a mean decrease in peak propellant pressure of 28% (range: 20-38%); however, this effect was reversed when the canisters were warmed to room temperature. For best results, the canisters should be at room temperature before use. Because the contents of albuterol sulfate oral inhalers are under pressure, the aerosol containers should not be punctured, used or stored near heat or an open flame, or placed into a fire or incinerator for disposal. Exposure of the canisters to temperatures exceeding 49°C may cause them to burst.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
-
Albuterol sulfate inhalation solutions for nebulization should be stored at 2-25°C or 15-30°C depending on the manufacturer.
-
Albuterol sulfate tablets should be stored at 15-30°C and protected from light.
-
Albuterol sulfate extended-release tablets should be stored at 20-25 °C in a well-closed, light resistant container.
-
Albuterol sulfate oral solution should be stored at 20-25°C or 2-30°C, depending on the manufacturer, in a tight, light-resistant container.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
The following dosing is from the National Asthma Education and Prevention guidelines.
Asthma (quick relief): Metered-dose inhaler- 2 inhalations every 4 to 6 hours as needed; Nebulizer - 1.25 to 5 mg (in 3 mL of saline) every 4 to 6 hours. Dose may be doubled in severe exacerbations.
Asthma exacerbations (older than 12 years of age): Metered-dose inhaler - 4 to 8 inhalations every 20 minutes up to 4 hours, then every 1 to 4 hours as needed.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1100-1
-
Drugs to prevent inflammation and pulmonary edema as a result of chemically induced lung injury are needed. Several drugs that have been approved by the FDA for other indications hold promise for treating chemically induced pulmonary edema. These include β2-agonists, dopamine, insulin, allopurinol, and non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen. Inhaled and systemic forms of β2-agonists used in the treatment of asthma have been effective in reducing pulmonary edema in animal models but require further study.
DHHS/NIH; National Institute of Allergy and Infectious Diseases: Medical Countermeasures Against Chemical Threats-Respiratory Tract (NIH/NIAID)
-
Beta 2-agonist bronchodilators delivered by metered-dose inhalers (MDI) are commonly used in the treatment of bronchospasm in both intubated and nonintubated patients. Substantial data support the effectiveness of MDI delivery systems in nonintubated patients. However, few studies have examined the effectiveness of MDIs in intubated, mechanically ventilated patients. MDIs are often used in conjunction with a spacing device that may enhance delivery of drug to the airways, but few in vivo data have demonstrated efficacy of this delivery method in ventilated patients. We studied ten critically ill patients who had a peak (Ppeak) to pause (Ppause) gradient of more than 15 cm H2O during sedated, quiet breathing on assist control ventilation. We administered 5, 10, and 15 puffs (90 micrograms per puff) of MDI albuterol through a specific spacer (Aerovent) at 30-min intervals, while measuring resistive pressure (defined as Ppeak-Ppause) before and after treatments. Resistive airway pressure after 5 puffs decreased in nine of ten patients, from 25.1 +/- 7.2 to 20.8 +/- 5.6 cm H2O (p < 0.12). The addition of 10 more puffs further reduced resistive pressure in nine of nine patients from 20.8 +/- 5.6 to 19.0 +/- 4.4 (p < 0.01). Fifteen more puffs (30 cumulative puffs) did not result in further improvement (p > 0.5). A toxic reaction occurred in one patient (systolic blood pressure decreased 20 mm Hg) after 5 puffs of albuterol. We conclude that MDI administered through this specific spacer is effective in mechanically ventilated patients in doses up to 15 puffs, and that therapy should be titrated to effectiveness and toxicity.
Manthous CA, Chatila W, Schmidt GA, Hall JB. Treatment of bronchospasm by metered-dose inhaler albuterol in mechanically ventilated patients. Chest. 1995 Jan; 107 (1): 210-3. [PubMed citation]
-
103 patients with acute airflow obstruction (56 asthma, 47 chronic obstructive pulmonary disease [COPD]) completed a double-blind trial of nebulized bronchodilator treatment in a hospital accident and emergency department. Each patient was randomized to receive either 10 mg of salbutamol nebulizer solution in 2 ml of saline or 10 mg of salbutamol in 2 ml (0.5 mg) of preservative-free ipratropium bromide. Peak flow rate (PFR) was recorded before treatment and 1 hour after beginning nebulised treatment. In 23 asthmatic patients given salbutamol alone PFR rose by a mean 31% 1 hour after treatment whereas in 33 such patients given combined treatment it rose by a mean 77% (95% confidence interval for the difference 8-84%). Patients whose PFR was below 140 l/min at entry gained maximum benefit from the combined treatment. For COPD patients the PFR rise was almost identical for both treatments. In acute asthma the immediate PFR response to a mixture of salbutamol and ipratropium bromide was better than the response to nebulised salbutamol alone. For COPD patients, the two treatments were of equal benefit.
O'Driscoll BR, Taylor RJ, Horsley MG, Chambers DK, Bernstein A. Nebulised salbutamol with and without ipratropium bromide in acute airflow obstruction. Lancet. 1989 Jun 24;1(8652):1418-20. [PubMed citation]
-
The effects of inhaling 400 micrograms of salbutamol were compared with identical placebo inhaler in a double blind cross-over study of 20 patients with radiological evidence of pulmonary emphysema. There was a small but significant rise in the forced expiratory volume in one second (FEV1), but there was a much greater increase in vital capacity (VC), which was accompanied by a reduction in residual volume. Symptomatic improvement after salbutamol was reported by 14 of the patients and VC increased significantly in this group; there was no significant increase in VC in patients who did not prefer the active inhaler. There was no consistent change in the FEV1/VC ratio after salbutamol administration and this ratio is misleading as an indicator of bronchodilator responsiveness in patients with emphysema. Salbutamol is thus a valuable addition to treatment in patients with pulmonary emphysema and the response to treatment is best judged by measuring the improvement in VC.
Bellamy D, Hutchison DC. The effects of salbutamol aerosol on lung function in patients with pulmonary emphysema. Br J Dis Chest. 1981 Apr; 75 (2): 190-6. [PubMed Citation]
-
Nine patients with severe chronic airway obstruction secondary to chronic bronchitis and emphysema all preferred nebulised salbutamol solution to placebo in a double-blind controlled trial. Four of the patients who had previously received domiciliary nebulised salbutamol failed to complete the placebo period, though all completed the active period. Five others improved subjectively on active therapy, and showed a significant improvement in morning and evening peak flows. Symptom scores for breathlessness, wheezing, and sputum production were lower in the active treatment period and standard aerosol usage fell, although these changes might have been due to chance. Patients with severe chronic airway obstruction who do not respond to conventional bronchodilator therapy should be considered for this form of treatment.
Wilson RS, Connellan SJ. Domiciliary nebulised salbutamol solution in severe chronic airway obstruction. Thorax. 1980 Nov; 35 (11): 873-6. [PubMed Citation]
Children
-
The following dosing is from the National Asthma Education and Prevention guidelines.
Asthma (quick relief): Metered-dose inhaler- 2 inhalations every 4 to 6 hours as needed; Nebulizer (1 year of age and older) - 1.25 to 5 mg (in 3 mL of saline) every 4 to 8 hours as needed. Dose may be doubled in severe exacerbations.
Asthma (quick relief): Nebulizer (4 years of age and younger) - 0.63 to 2.5 mg (in 3 mL of saline) every 4 to 6 hours as needed. For children younger than 1 year of age, another reference suggests 0.05 to 0.15 mg/kg every 4 to 6 hours as needed.
Asthma exacerbations (older than 12 years of age): Metered-dose inhaler - 4 to 8 inhalations every 20 minutes up to 4 hours, then every 1 to 4 hours as needed: Asthma exacerbations (older than 12 years of age): Nebulizer - 2.5 to 5 mg every 20 minutes for 3 doses, then 2.5 to 10 mg every 1 to 4 hours as needed, or 10 to 15 mg/h continuously.
Asthma exacerbations (12 years of age and younger): Metered-dose inhaler - 4 to 8 inhalations every 20 minutes for 3 doses, then every 1 to 4 hours as needed. Use valved holding chamber; add mask in children younger than 4 years of age.
Asthma exacerbations (12 years of age and younger): Nebulizer- 0.15 mg/kg (minimum dose 2.5 mg) every 20 minutes for 3 doses, then 0.15 to 0.3 mg/kg up to 10 mg every 1 to 4 hours as needed, or 0.5 mg/kg/h by continuous nebulization.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1100-1
-
Bronchospasm: oral dose in neonates - 0.1 to 0.3 mg/kg every 6 to 8 hours
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1100-1
8. Route of Administration/Monitoring
-
Oral
-
Oral Inhalation via Metered-Dose Aerosol
-
Oral Inhalation via Nebulization
-
Dosage of albuterol sulfate and levalbuterol must be carefully adjusted according to individual requirements and response.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
9. Adverse effects
-
Cardiovascular Effects
Albuterol Sulfate Syrup, like all other beta-adrenergic agonists, can produce a clinically significant cardiovascular effect in some patients as measured by pulse rate, blood pressure, and/or symptoms. Although such effects are uncommon after administration of albuterol sulfate syrup at recommended doses, if they occur, the drug may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiogram (ECG) changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression. The clinical significance of these findings is unknown. Therefore, Albuterol Sulfate Syrup, like all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Product label Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
-
Deterioration of Asthma
Asthma may deteriorate acutely over a period of hours or chronically over several days or longer. If the patient needs more doses of albuterol sulfate syrup than usual, this may be a marker of destabilization of asthma and requires reevaluation of the patient and treatment regimen, giving special consideration to the possible need for anti-inflammatory treatment, e.g., corticosteroids.
Product label Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
-
Paradoxical Bronchospasm
Albuterol Sulfate Syrup can produce paradoxical bronchospasm, which may be life threatening. If paradoxical bronchospasm occurs, albuterol sulfate syrup should be discontinued immediately and alternative therapy instituted.
Product label Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
-
Immediate Hypersensitivity Reactions
Immediate hypersensitivity reactions may occur after administration of albuterol, as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm, and oropharyngeal edema. Albuterol, like other beta-adrenergic agonists, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes.
Product label Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
-
Rarely, erythema multiforme and Stevens-Johnson syndrome have been associated with the administration of albuterol sulfate in children.
-
Product label Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
-
Adverse reactions observed in the fetus and mother after albuterol treatment are secondary to the cardiovascular and metabolic effects of the drug. Albuterol may cause maternal and fetal tachycardia with fetal rates >160 beats/minute. Major decreases in maternal blood pressure have been reported with both systolic and diastolic pressures dropping >30 mm Hg. Fetal distress after maternal hypotension was not mentioned. One study observed a maximum decrease in diastolic pressure of 24 mm Hg (34% decrease) but a rise in systolic pressure. Other maternal adverse effects associated with albuterol have been acute congestive heart failure, pulmonary edema, and death.
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.32-34
-
Like all beta-mimetics, albuterol may cause transient fetal and maternal hyperglycemia followed by an increase in serum insulin. Cord blood levels of insulin are about twice those of untreated control infants and are not dependent on the duration of exposure, gestational age, or birth weight. These effects are more pronounced in diabetic patients, especially in juvenile diabetics, with the occurrence of significant increases in glycogenolysis and lipolysis. Maternal blood glucose should be closely monitored and neonatal hypoglycemia prevented with adequate doses of glucose.
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.32-34
10. Contraindication(s)
-
Albuterol tablets are contraindicated in patients with a history of hypersensitivity to albuterol, or any of its components.
Product label Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues-
"Study Terminated/Has Results"
-
Drug Study of Albuterol to Treat Acute Lung Injury
Condition: Respiratory Distress Syndrome, Adult
Interventions: Drug: Albuterol Sulfate USP; Procedure: Mini-Bronchoalveolar Lavage; Drug: Placebo
-
Acute Respiratory Distress Syndrome (ARDS) and a lesser condition that occurs prior to ARDS, Acute Lung Injury (ALI), are medical conditions that occur when there is severe inflammation and increased fluids (edema) in both lungs, making it hard for the lungs to function properly. Patients with these conditions require treatment that includes the use of a breathing machine (ventilator). The purpose of this study is to find out whether giving albuterol (a drug commonly used in asthmatics) or not giving albuterol to patients with ALI or ARDS makes a difference in how long it takes for a patient to be able to breath without the ventilator.
ClinicalTrials.gov; Drug Study of Albuterol to Treat Acute Lung Injury (NIH)
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations- No data available at this time.
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
Nonbiodefense clinical need(s): Treatment of asthma, bronchiolitis.
Needed studies for nonbiodefense clinical indications: Bronchiolitis; animal studies of standardized exposure; systematic data collection in the event of a disaster; use of a systematic treatment approach.
-
Primate studies
-
Will produce more information on ventilation
-
Will produce more information on respiratory failure
-
Will permit exploration of 3-chlorotyrosine
-
-
Prospective, acute human studies
-
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
15. Study-related ethical concerns
— including review panel recommendationsEthical concerns include:
-
Informed consent for data collection in disaster/mass casualty situation
-
Central institutional review boards (IRBs)
-
How to conduct multicenter studies
Poison centers
-
Pediatric Emergency Care Applied Research Network
Hospital consortia.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
16. Global regulatory status
U.S.
-
Albuterol sulfate solution
AccuNeb is indicated for the relief of bronchospasm in patients 2 to 12 years of age with asthma (reversible obstructive airway disease).
Product label AccuNeb (Albuterol sulfate) solution [Dey Pharma, LP] Last revised: March 2011 [DailyMed]
-
Albuterol sulfate tablet
Albuterol tablets are indicated for the relief of bronchospasm in adults and children 6 years of age and older with reversible obstructive airway disease.
Product label Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
-
Albuterol sulfate tablet, film coated, extended release
Albuterol sulfate extended-release tablets are indicated for the relief of bronchospasm in adults and children 6 years of age and older with reversible obstructive airway disease.
Product label Albuterol Sulfate tablet, film coated, extended release [Mylan Pharmaceuticals Inc.] Last revised: November 2006 [DailyMed]
-
Albuterol aerosol, metered
Albuterol Inhalation Aerosol is indicated for the prevention and relief of bronchospasm in patients 4 years of age and older with reversible obstructive airway disease, and for the prevention of exercise induced bronchospasm in patients 4 years of age and older.
Product label Albuterol aerosol, metered [Rebel Distributors Corp] Last revised: July 2011 [DailyMed]
-
Albuterol sulfate Inhalant
Albuterol Sulfate Inhalation Solution 0.083% is indicated for the relief of bronchospasm in patients with reversible obstructive airway disease and acute attacks of bronchospasm.
Product label Albuterol Sulfate (albuterol sulfate) inhalant [Actavis Mid Atlantic LLC] Last revised: November 2007 [DailyMed]
-
Albuterol sulfate syrup
Albuterol Sulfate Syrup is indicated for the relief of bronchospasm in adults and children 2 years of age and older with reversible obstructive airway disease.
Product label Albuterol Sulfate syrup [Physicians Total Care, Inc.] Last revised: April 2012 [DailyMed]
U.K.
-
Asthma and other conditions associated with reversible airways obstruction; premature labor.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.157-158
17. Other potentially useful information
-
Albuterol sulfate has been investigated as a potential medical mitigation agent against both upper and lower chemical-induced pulmonary injury. Upper pulmonary agents include gases, aerosols, and particulates that are readily soluble in water or react with it to form a corrosive environment, or react directly with the linings of the nose, throat, and airways of the upper pulmonary system. These chemicals are almost completely removed by solution and react at the surfaces of the respiratory tract, and thus are very efficiently scrubbed by the upper respiratory tract. Lower pulmonary toxicants include relatively water insoluble gases, aerosols, and particulates up to about 5 um. Toxicity mechanisms include direct damage to tissues from hydrolysis products, inactivation of key enzymes by reaction with biological functional groups, reaction with alveolar surfactants, and organ toxicity from chemicals that may successfully cross the alveolar-capillary boundary.
Whitmire M, Cox J, Salem H. (2011, January). Chemical Segregation by Toxidrome for the Chemical Terrorism Risk Assessment. Presented at: OnSiteAnnual Meeting, Baltimore, MD.
-
Octanol/Water Partition Coefficient: log Kow = 0.64 /Estimated/
HSDB. Albuterol
18. Publications
Advenier C, Qian Y, Koune JD, Molimard M, Candenas ML, Naline E. Formoterol and salbutamol inhibit bradykinin- and histamine-induced airway microvascular leakage in guinea-pig. Br J Pharmacol. 1992 Apr; 105 (4): 792-798. [PubMed Citation]
Anderson PJ, Zhou X, Breen P, Gann L, Logsdon TW, Compadre CM, Hiller FC. Pharmacokinetics of (R,S)-Albuterol after aerosol inhalation in healthy adult volunteers. J Pharm Sci. 1998 Jul; 87 (7): 841-4. [PubMed Citation]
Bellamy D, Hutchison DC. The effects of salbutamol aerosol on lung function in patients with pulmonary emphysema. Br J Dis Chest. 1981 Apr; 75 (2): 190-6. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Boskabady MH, Attaran D, Shaffei MN. Airway responses to salbutamol after exposure to chemical warfare. Respirology. 2008 Mar; 13 (2): 288-93. [PubMed citation]
Boulton DW, Fawcett JP. Enantioselective disposition of salbutamol in man following oral and intravenous administration. Br J Clin Pharmacol. 1996 Jan; 41 (1): 35-40. [PubMed Citation]
Bowring NE, Buckle DR, Clarke GD, Taylor JF, Arch JR. Evaluation of the Potassium Channel Activator BRL 38227 as an Inhaled Bronchodilator in the Guinea-pig: Contrast with Nifedipine and Salbutamol. Pulm Pharmacol. 1991; 4 (2): 99-105. [PubMed Citation]
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.32-34
Brunton, LL, et al. (eds.). (2006). (Goodman & Gilman's) The Pharmacological Basis of Therapeutics (11th Ed.). McGraw-Hill-Medical Publishing Division, New York, NY. 720.
Cevik Y, Onay M, Akmaz I, Sezigen S Mass casualties from acute inhalation of chlorine gas. South Med J. 2009 Dec; 102 (12): 1209-13. [PubMed Citation]
ClinicalTrials.gov. Drug Study of Albuterol to Treat Acute Lung Injury (NIH)
Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, Moss M, Schoenfeld D, Steingrub J, Thompson BT. Randomized, placebo-controlled clinical trial of an aerosolized beta2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011 Sep 1; 184 (5): 561-8. [PubMed Citation]
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
DHHS/NIH; National Institute of Allergy and Infectious Diseases: Medical Countermeasures Against Chemical Threats-Respiratory Tract (NIH/NIAID)
Du XL, Zhu Z, Fu Q, Li DK, Xu WB. Pharmacokinetics and relative bioavailability of salbutamol metered-dose inhaler in healthy volunteers. Acta Pharmacol Sin. 2002 Jul; 23 (7): 663-6. [PubMed Citation]
FDA; Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Patent and Generic Drug Product Data Last Updated: September 07, 2012
Filbert MG, Moore DH, Adler M. Relaxation of soman-induced contracture of airway smooth muscle in vitro. Drug Chem Toxicol. 1992; 15 (3): 203-15. [PubMed Citation]
Grainge C, Rice P. Management of phosgene-induced acute lung injury. Clin Toxicol (Phila). 2010 Jul; 48 (6): 497-508. [PubMed Citation]
Grainge C, Brown R, Jugg BJ, Smith AJ, Mann TM, Jenner J, Rice P, Parkhouse DA; Early Treatment with nebulized salbutamol worsens physiological measures and does not improve survival following phosgene induced acute lung injury. J R Army Med Corps. 2009 Jun; 155 (2): 105-9. [PubMed Citation]
Hale TW, Medications and Mother's Milk. Amarillo, TX: Hale Publishing L.P., 2006 p.34-35
Hardison LS Jr, Wright E, Pizon AF. Phosgene exposure: a case of accidental industrial exposure. J Med Toxicol. 2014; 10(1): 51-6. [PubMed Citation]
Harvey JE, Tattersfield AE. Airway response to salbutamol: effect of regular salbutamol inhalations in normal, atopic, and asthmatic subjects. Thorax. 1982 Apr; 37 (4): 280-7. [PubMed Citation]
HSDB. Albuterol
Hussey EK, Donn KH, Powell JR; Albuterol extended-release products: a comparison of steady-state pharmacokinetics. Pharmacotherapy. 1991; 11 (2): 131-5. [PubMed Citation]
Hutchings MJ, Paull JD, Wilson-Evered E, Morgan DJ. Pharmacokinetics and metabolism of salbutamol in premature labor. Br J Clin Pharmacol. 1987 Jul; 24 (1): 69-75. [PubMed Citation]
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1100-1
Lee JW. Beta2 adrenergic agonists in acute lung injury? The heart of the matter. Crit Care. 2009; 13 (6): 1011. [PubMed Citation]
Libretto SE. A review of the toxicology of salbutamol (albuterol). Arch Toxicol. 1994; 68 (4): 213-6. [PubMed Citation]
Maier G, Rubino C, Hsu R, Grasela T, Baumgartner RA. Population pharmacokinetics of (R)-albuterol and (S)-albuterol in pediatric patients aged 4-11 years with asthma. Pulm Pharmacol Ther. 2007; 20 (5): 534-42. [PubMed Citation]
Manthous CA, Chatila W, Schmidt GA, Hall JB. Treatment of bronchospasm by metered-dose inhaler albuterol in mechanically ventilated patients. Chest. 1995 Jan; 107 (1): 210-3. [PubMed citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.157-158
McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004 Jul; 32 (7): 1470-6. [PubMed Citation]
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1320-1334
Milliez JM, Flouvat B, Jannet D. Pharmacokinetics of salbutamol in the pregnant woman after subcutaneous administration with a portable pump. Obstet Gynecol. 1992 Aug; 80 (2): 182-185. [PubMed Citation]
Mohamed MH, Lima JJ, Eberle LV, Self TH, Johnson JA. Effects of gender and race on albuterol pharmacokinetics. Pharmacotherapy. 1999 Feb; 19 (2): 157-61. [PubMed Citation]
Morgan DJ, Paull JD, Richmond BH, Wilson-Evered E, Ziccone SP. Pharmacokinetics of intravenous and oral salbutamol and its sulphate conjugate. Br J Clin Pharmacol. 1986 Nov; 22 (5): 587-593. [PubMed Citation]
Newman-Taylor AJ, Morris AJ. Experience with mustard gas casualties. Lancent 1991 Jan; 337 (8735): 242. [PubMed Citation]
O'Driscoll BR, Taylor RJ, Horsley MG, Chambers DK, Bernstein A. Nebulised salbutamol with and without ipratropium bromide in acute airflow obstruction. Lancet. 1989 Jun 24; 1 (8652):1418-20. [PubMed citation]
Perkins GD, Gates S, Lamb SE, McCabe C, Young D, Gao F. Beta Agonist Lung Injury Trial (BALT-2) trial protocol: a randomized, double-blind, placebo controlled of intravenous infusion of albutamol in the acute respiratory distress syndrome. Trials. 2011 May 9; 12: 113. [PubMed Citation]
Perkins GD, Nathani N, McAuley DF, Gao F, Thickett DR; In vitro and in vivo effects of salbutamol on neutrophil function in acute lung injury. Thorax. 2007 Jan; 62 (1): 36-42. [PubMed Citation]
Perkins GD, McAuley DF, Richter A, Thickett DR, Gao F. Bench-to-bedside review: beta2-Agonists and the acute respiratory distress syndrome. Crit Care. 2004 Feb; 8 (1): 25-32. [PubMed Citation]
Powell ML, Weisberger M, Gural R, Chung M, Patrick JE, Radwanski E, Symchowicz SS. Comparative bioavailability and pharmacokinetics of three formulations of albuterol. J Pharm Sci. 1985 Feb; 74 (2): 217-9. [PubMed Citation]
Product label: AccuNeb (Albuterol sulfate) solution [Dey Pharma, LP] Last revised: March 2011 [DailyMed]
Product label: Albuterol (aerosol metered) [Rebel Distributors Corp] Last revised: July 2011 [DailyMed]
Product label: Albuterol (albuterol sulfate) tablet [Mylan Pharmaceuticals Inc.] Last revised: January 2010 [DailyMed]
Product label: Albuterol Sulfate syrup [Physicians Total Care, Inc.] Last revised: April 2012 [DailyMed]
Product label: Albuterol Sulfate (albuterol sulfate) inhalant [Actavis Mid Atlantic LLC] Last revised: November 2007 [DailyMed]
Product label: Albuterol Sulfate tablet, film coated, extended release [Mylan Pharmaceuticals Inc.] Last revised: November 2006 [DailyMed]
Rey E, Luquel L, Richard MO, Mory B, Offenstadt G, Olive G. Pharmacokinetics of intravenous salbutamol in renal insufficiency and its biological effects. Eur J Clin Pharmacol. 1989; 37 (4): 387-9. [PubMed Citation]
Russell D, Blain PG, Rice P. Clinical management of casualties exposed to lung damaging agents: a critical review. Emerg Med J. 2006 Jun; 23 (6): 421-4. [PubMed Citation]
Tzou TZ, Pachuta RR, Coy RB, Schultz RK. Drug form selection in albuterol-containing metered-dose inhaler formulations and its impact on chemical and physical stability. J Pharm Sci. 1997 Dec; 86 (12): 1352-7. [PubMed Citation]
van Helden HP, Kuijpers WC, Diemel RV. Asthma-like symptoms following intratracheal exposure of Guinea pigs to sulfur mustard aerosol: therapeutic efficacy of exogenous lung surfactant curosurf and salbutamol. Inhal Toxicol. 2004 Jul; 16 (8): 537-48. [PubMed Citation]
Whitmire M, Cox J, Salem H. (2011, January). Chemical Segregation by Toxidrome for the Chemical Terrorism Risk Assessment. Presented at: OnSiteAnnual Meeting, Baltimore, MD.
Wilson RS, Connellan SJ. Domiciliary nebulised salbutamol solution in severe chronic airway obstruction. Thorax. 1980 Nov; 35 (11): 873-6. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 9/18/2014
