You are here: Home > Medical Countermeasures Database > Aminophylline
Aminophylline - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Aminophylline
2. Chemical Defense therapeutic area(s)
— including key possible usesAminophylline can be used to treat lung edema caused by pulmonary irritants such as phosgene.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
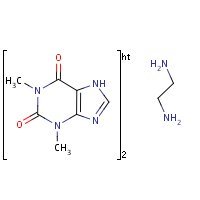
HSDB. Aminophylline
Mechanism of action
Theophylline has two distinct actions in the airways of patients with reversible obstruction; smooth muscle relaxation (i.e., bronchodilation) and suppression of the response of the airways to stimuli (i.e., nonbronchodilator prophylactic effects). While the mechanisms of action of theophylline are not known with certainty, studies in animals suggest that bronchodilation is mediated by the inhibition of two isozymes of phosphodiesterase (PDE III and, to a lesser extent, PDE IV), while nonbronchodilator prophylactic actions are probably mediated through one or more different molecular mechanisms, that do not involve inhibition of PDE III or antagonism of adenosine receptors. Some of the adverse effects associated with theophylline appear to be mediated by inhibition of PDE III (e.g., hypotension, tachycardia, headache, and emesis) and adenosine receptor antagonism (e.g., alterations in cerebral blood flow).
Theophylline increases the force of contraction of diaphragmatic muscles. This action appears to be due to enhancement of calcium uptake through an adenosine-mediated channel.
Serum Concentration-Effect Relationship:
Bronchodilation occurs over the serum theophylline concentration range of 5 - 20 mcg/mL. Clinically important improvement in symptom control and pulmonary function has been found in most studies to require serum theophylline concentrations >10 mcg/mL. At serum theophylline concentrations >20 mcg/mL, both the frequency and severity of adverse reactions increase. In general, maintaining the average serum theophylline concentration between 10 and 15 mcg/mL will achieve most of the drug's potential therapeutic benefit while minimizing the risk of serious adverse events.
Aminophylline is a 2:1 complex of theophylline and ethylenediamine.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Summary of clinical and non-clinical studies
Phosgene is a volatile, colorless, and highly toxic chemical that poses a potential bioterrorism threat due to the ease and low expense of production (Sciuto and Hurt, 2004). It is used in the production of plastics and other substances, and exposure may occur as a result of occupational accidents. It is a highly reactive tissue oxidant that destroys a wide range of macromolecules and precipitates the release of inflammatory cytokines, eventually resulting in the weakening and collapse of the blood-air barrier and a subsequent flooding of the lungs (Borak and Diller, 2001). Mortality therefore occurs as a result of a delayed, but serious, fulminating pulmonary edema arising 2-24 hours after exposure. Male rabbits exposed to phosgene display reduced levels of the signaling molecule cyclic-3,5-adenosine monophosphate (cAMP), increased levels of inflammatory leukotrienes and peroxidized lipids, a greater degree of protein leakage in the lungs, and increased lung wet weight owing to edema (Kennedy et al., 1989). The bronchodilator aminophylline, used intravenously as an adjunct in the treatment of asthma and other chronic lung conditions, has been known to reduce edema from other pulmonary agents. Live male rabbits treated with phosgene gas and injected with aminophylline (15 mg/kg intravenously 10 minutes after exposure, and 8 mg/kg intraperitoneally 2 and 4 hours after exposure) showed a significantly reduced lung weight gain 4 hours after exposure, compared to phosgene-exposed animals not injected with aminophylline (Kennedy et al., 1989). Subsequent experiments used male rabbits exposed to phosgene and immediately sacrificed, followed by perfusion of the lungs with buffer solution (Sciuto et al., 1997; Sciuto and Hurt, 2004). Rabbits that received 30 mg/kg aminophylline via perfusion 80-90 minutes post-phosgene exposure maintained levels of cAMP, peroxidized lipids, and leukotrienes at or close to the levels in control, phosgene-unexposed animals, and showed reduced lung weight gain (Sciuto et al., 1997; Sciuto and Hurt, 2004). Aminophylline is thought to have a multifactorial mechanism of action; the possibilities include acting as an antioxidant (whether directly or indirectly), inhibiting the production of permeability-promoting peptide leukotrienes, and maintaining a concentration of cAMP sufficient for signaling pathways that maintain tight junctions between smooth muscle cells, thereby preventing leakage (edema) (Sciuto and Hurt, 2004).
B. Link to clinical studies
Pregnancy, breastfeeding studies
The concentration of theophylline in breast milk is about equivalent to the maternal serum concentration. An infant ingesting a liter of breast milk containing 10 - 20 mcg/mL of theophylline per day is likely to receive 10 - 20 mg of theophylline per day. Serious adverse effects in the infant are unlikely unless the mother has toxic serum theophylline concentrations (Class IV).
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Clinical reviews
-
Phosgene (carbonyl chloride, CAS 75-44-5) is a highly reactive gas of historical interest and current industrial importance. Phosgene has also proved to be a useful model for the study of those biochemical mechanisms that lead to permeability-type pulmonary edema (adult respiratory distress syndrome). In turn, the study of phosgene-induced adult respiratory distress syndrome has provided insights leading to revised treatment strategies for exposure victims. The authors summarized findings on the mechanisms of phosgene-induced pulmonary edema and their implications for victim management. In light of that research, the authors also provide a comprehensive approach to the management and treatment of phosgene exposure victims (Class IV).
Borak J, Diller WF; Phosgene exposure: mechanisms of injury and treatment strategies. J Occup Environ Med. 2001 Feb;43(2):110-9. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
Pretreatment with aminophylline has been shown to protect against various types of acute lung injury. Mechanisms responsible for protection are multifactorial but are thought to involve upregulation of cAMP. While previous studies focused on pretreatment, the present investigation examined post-treatment in rabbits following exposure to a lethal dose of the oxidant gas phosgene. Rabbits, 2-3 kg, were exposed to a cumulative dose of phosgene to attain a c x t exposure effect of 1500 ppm.min. Lungs were isolated in situ and perfused for 90-100 min after exposure with Krebs-Henseleit buffer at 40 mL/min. Pulmonary artery pressure (Ppa), tracheal pressure (Pt), and lung weight gain (lwg) were measured continuously. Leukotrienes C4/D4/E4 were measured in the perfusate every 20 min during perfusion. At the immediate conclusion of the experiment, lung tissue was frozen in liquid nitrogen and analyzed for reduced GSH, GSSG, cAMP, and lipid peroxidation (TBARS). Post-treatment with aminophylline 80-90 min after exposure significantly lowered Ppa, Pt, and lwg. Aminophylline significantly reduced TBARS and perfusate LTC4/D4/E4, and prevented phosgene-induced decreases in lung tissue cAMP. These data suggest that protective mechanisms observed with aminophylline involve decreased LTC4/D4/E4-mediated pulmonary capillary permeability and attenuated lipid peroxidation. Direct antipermeability effects of cAMP on cellular contraction may also be important in protection against phosgene-induced lung injury.
Sciuto AM, Strickland PT, Kennedy TP, Gurtner GH. Postexposure treatment with aminophylline protects against phosgene-induced acute lung injury. Exp Lung Res. 1997 Jul-Aug;23(4):317-32. [PubMed Citation]
-
Phosgene is a toxic oxidant gas that causes the adult respiratory distress syndrome in exposed workers. Phosgene exposure markedly increased lung weight gain in buffer-perfused isolated rabbit lungs (31 +/- 5 g over 60 min after phosgene vs. 7.7 +/- 1.2 in control lungs, P less than 0.01) and markedly increased the lung leak index for 125I-albumin (0.28 +/- 0.03 after phosgene vs. 0.02 +/- 0.01 in control lungs, P less than 0.01). Pretreatment with dibutyryl adenosine 3',5' -cyclic monophosphate (DBcAMP), aminophylline, or terbutaline plus isoproterenol prevented the increase in lung weight caused by phosgene (31 +/- 5 g phosgene, 11.7 +/- 2.8 DBcAMP, 7.5 +/- 2.5 aminophylline, 6.1 +/- 1 terbutaline and isoproterenol, 6.1 +/- 1.2 control + aminophylline, and 7.7 +/- 1.2 control; all treatments were P less than 0.01 vs. the untreated phosgene group and not significantly different from control lungs). Pretreatment with aminophylline prevented the increase in lung leak index for 125I-albumin (0.28 +/- 0.03 after phosgene vs. 0.06 +/- 0.02 in aminophylline-treated lungs, P less than 0.01). Posttreatment with aminophylline and terbutaline also prevented the increase in lung weight caused by phosgene. These results indicate that phosgene dramatically increases the movement of fluid and protein across the pulmonary vasculature and that treatment with DBcAMP, aminophylline, terbutaline, or isoproterenol markedly reduces the pulmonary edema caused by phosgene.
Kennedy TP, Michael JR, Hoidal JR, Hasty D, Sciuto AM, Hopkins C, Lazar R, Bysani GK, Tolley E, Gurtner GH. Dibutyryl cAMP, aminophylline, and beta-adrenergic agonists protect against pulmonary edema caused by phosgene. J Appl Physiol. 1989 Dec;67(6):2542-52. [PubMed Citation]
-
A series of studies was performed to address treatment against the former chemical warfare edemagenic gas phosgene. Both in situ and in vivo models were used to assess the efficacy of postexposure treatment of phosgene-induced lung injury using clinically existing drugs. The degree of efficacy was judged by examining treatment effects on pulmonary edema formation (PEF) as measured by wet/dry weight (WW/DW) ratios, real-time (in situ) lung weight gain (LWG), survival rates (SR), odds ratios, and glutathione (GSH) redox states. Drugs included N-acetylcysteine (NAC), ibuprofen (IBU), aminophylline (AMIN), and isoproterenol (ISO). Using the in situ isolated perfused rabbit lung model (IPRLM), intratracheal (IT) NAC (40 mg/kg bolus) delivered 45-60 min after phosgene exposure (650 mg/m(3)) for10 min lowered pulmonary artery pressure, LWG, leukotrienes (LT) C(4)/D(4)/E(4), lipid peroxidation, and oxidized GSH. The authors concluded that NAC protected against phosgene-induced lung injury by acting as an antioxidant by maintaining protective levels of GSH, reducing both lipid peroxidation and production of arachidonic acid metabolites. Also in IPRLM, administration of AMIN (30 mg/kg) 80-90 min after phosgene exposure significantly reduced lipid peroxidation and perfusate LTC(4)/D(4)/E(4), reduced LWG, and prevented phosgene-induced decreases in lung tissue cAMP. These data suggest that protective mechanisms observed with AMIN involve decreased LTC(4)/D(4)/E(4) mediated pulmonary capillary permeability and attenuated lipid peroxidation. Direct antipermeability effects of AMIN-induced upregulation of cAMP on cellular contraction may also be important in protection against phosgene-induced lung injury. Posttreatment with ISO in the IPRLM by either combined intravascular (iv; infused into pulmonary artery at 24 microg/min infused) + IT (24 microg bolus) or IT route alone 50-60 min after phosgene exposure significantly lowered pulmonary artery pressure, tracheal pressure, and LWG. ISO treatment significantly enhanced GSH products or maintained protective levels when compared with results from phosgene-exposed only rabbits. These data suggest that protective mechanisms for ISO involve reduction in vascular pressure, decreased LTC(4)/D(4)/E(4)-mediated pulmonary capillary permeability, and favorably maintained lung tissue GSH redox states. For in vivo male mouse (CD-1, 25-30 g) studies IBU was administered ip within 20 min after a lethal dose of phosgene (32 mg/m(3) for 20 min) at 0 (saline), 3, 9, or 15 mg/mouse. Five hours later, a second IBU injection was given but at half the original doses (0, 1.5, 4.5, and 7.5 mg/mouse); therefore, these treatment groups are now referred to as the 0/0, 3/1.5, 9/4.5, and 15/7.5 mg IBU/mouse groups. SRs and odds ratios were calculated for each dose at 12 and 24 h. The 12-h survival was 63% for 9/4.5 mg IBU and 82% for the 15/7.5 mg IBU groups, compared with 25% for saline-treated phosgene-exposed mice. At 24 h, those survival rates were reduced to 19%, 19%, and 6%, respectively. In the 15/7.5 mg IBU group, lung WW/DW ratios were significantly lower than in saline-treated mice at 12 h. Lipid peroxidation was lower only for the 9/4.5 mg IBU dose; however, nonprotein sulfhydryls (a measure of GSH) were greater across all IBU doses. The odds ratio was 5 for the 9/4.5 IBU group at 12 h and 13 for the 15/7.5 mg IBU group, compared with 3.5 for both groups at 24 h. IBU posttreatment increased the survival of mice at 12 h by reducing PEF, lipid peroxidation, and GSH depletion. In conclusion, effective treatment of phosgene-induced lung injury involves early postexposure intervention that could reduce free radical species responsible for lipid peroxidation, correct the imbalance in the GSH redox state, and prevent the release of biological mediators such as leukotrienes, which are accountable for increased permeability.
Sciuto AM, Hurt HH. Therapeutic treatments of phosgene-induced lung injury. Inhalation Toxicology 2004 Jul;16(8):565-80. [PubMed Citation]
Pregnant animal studies
This study investigated the teratogenic and fetal toxicity of i.v. theophylline and its relationship to maternal plasma levels in pregnant rabbits. From days 6-18 of gestation, each dose of theophylline (15, 30 and 60 mg/kg/day at a rate of 0.5 ml/kg/min) was administered i.v. to pregnant rabbits using an automatic infusion pump. Theophylline showed reversible toxicity: accelerated respiration, sluggish startle reactions, dilation of the auricular vessels and polyuria were observed in dams treated with 60 mg/kg/day but not in animals given 15 or 30 mg/kg/day. Fetuses from the dam group treated with 60 mg/kg/day exhibited teratogenic toxicity such as cleft palate and skeletal variation of the 13th rib. Fetal toxicity was also observed including abortion, increased number of late deaths and decreased body weight appearing on day 29 of gestation. No toxicity was observed in fetuses from the dam group treated with 15 or 30 mg/kg/day. However, in the 30 and 60 mg/kg/day theophylline-treated groups, maternal plasma concentrations (Cmax) during the treatment period were approximately 56 and 106 micrograms/ml, respectively. It is therefore suggested that the risk of teratogenic and fetal toxicity caused by theophylline is dependent on its dosage. In conclusion, caution should be taken when administering theophylline or aminophylline to pregnant individuals at doses that could result in high neonate peak blood levels.
Shibata M, Wachi M, Kawagushi M, Kohima J, Onodera K. Teratogenic and fetal toxicity following intravenous theophylline administration in pregnant rabbits is related to maternal drug plasma levels. Methods Find Exp Clin Pharmacol. 2000 Mar;22(2):101-7. [PubMed Citation]
...there are no teratogenicity studies in nonrodents (e.g., rabbits). Theophylline was not shown to be teratogenic in CD-1 mice at oral doses up to 400 mg/kg, approximately 2.0 times the human dose on a mg/m2 basis or in CD-1 rats at oral doses up to 260 mg/kg, approximately 3.0 times the recommended human dose on a mg/m2 basis. At a dose of 220 mg/kg, embryotoxicity was observed in rats in the absence of maternal toxicity.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
Tobacco and marijuana smoking appears to increase the clearance of theophylline by induction of metabolic pathways. Theophylline clearance has been shown to increase by approximately 50% in young adult tobacco smokers and by approximately 80% in elderly tobacco smokers compared to nonsmoking subjects. Passive smoke exposure has also been shown to increase theophylline clearance by up to 50%. Abstinence from tobacco smoking for one week causes a reduction of approximately 40% in theophylline clearance. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients who stop smoking. Use of nicotine gum has been shown to have no effect on theophylline clearance.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Fever, regardless of its underlying cause, can decrease the clearance of theophylline. The magnitude and duration of the fever appear to be directly correlated to the degree of decrease of theophylline clearance. Precise data are lacking, but a temperature of 39°C (102°F) for at least 24 hours is probably required to produce a clinically significant increase in serum theophylline concentrations. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with sustained fever.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Children
The clearance of theophylline is very low in neonates. Theophylline clearance reaches maximal values by one year of age, remains relatively constant until about 9 years of age and then slowly decreases by approximately 50% to adult values at about age 16. Renal excretion of unchanged theophylline in neonates amounts to about 50% of the dose, compared to about 10% in children older than three months and in adults. Careful attention to dosage selection and monitoring of serum theophylline concentrations are required in children.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Pregnancy
Other factors associated with decreased theophylline clearance include the third trimester of pregnancy, sepsis with multiple organ failure, and hypothyroidism. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with any of these conditions. Other factors associated with increased theophylline clearance include hyperthyroidism and cystic fibrosis.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Geriatric
The clearance of theophylline is decreased by an average of 30% in healthy elderly adults (>60 yrs.) compared to healthy young adults. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in elderly patients.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Renal Impairment
Only a small fraction, e.g., about 10%, of the administered theophylline dose is excreted unchanged in the urine of children greater than three months of age and adults. Since little theophylline is excreted unchanged in the urine and since active metabolites of theophylline (i.e., caffeine, 3-methylxanthine) do not accumulate to clinically significant levels even in the face of end-stage renal disease, no dosage adjustment for renal insufficiency is necessary in adults and children >3 months of age. In contrast, approximately 50% of the administered theophylline dose is excreted unchanged in the urine in neonates. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in neonates with decreased renal function.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Hepatic Impairment
Theophylline clearance is decreased by 50% or more in patients with hepatic insufficiency (e.g., cirrhosis, acute hepatitis, cholestasis). Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with reduced hepatic function.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Theophylline clearance is decreased by 50% or more in patients with CHF. The extent of reduction in theophylline clearance in patients with CHF appears to be directly correlated to the severity of the cardiac disease. Since theophylline clearance is independent of liver blood flow, the reduction in clearance appears to be due to impaired hepatocyte function rather than reduced perfusion. Careful attention to dose reduction and frequent monitoring of serum theophylline concentrations are required in patients with CHF.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults
Reversible airflow obstruction: maximum oral dose of theophylline 400 mg/day (equivalent to aminophylline 507 mg) in the presence of risk factors for reduced theophylline clearance or if it is not feasible to monitor serum theophylline concentration. Initial dosage: 300 mg/day (equivalent to aminophylline 380 mg) divided every 6 to 8 h. After 3 days, if tolerated, increase dosage to 400 mg/day divided every 6 to 8 h.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1111-2
Reversible airflow obstruction (loading dose): in a patient who has received no theophylline in the previous 24 hours, a loading dose of intravenous theophylline of 4.6 mg/kg (5.7 mg/kg as aminophylline), calculated on the basis of ideal body weight and administered over 30 minutes, on average, will produce a maximum post-distribution serum concentration of 10 mcg/mL with a range of 6-16 mcg/mL. When a loading dose becomes necessary in the patient who has already received theophylline, estimation of the serum concentration based upon the history is unreliable, and an immediate serum level determination is indicated.
A serum concentration obtained 30 minutes after an intravenous loading dose, when distribution is complete, can be used to assess the need for and size of subsequent loading doses, if clinically indicated, and for guidance of continuing therapy. Once a serum concentration of 10 to 15 mcg/mL has been achieved with the use of a loading dose(s), a constant intravenous infusion is started. The rate of administration is based upon mean pharmacokinetic parameters for the population and calculated to achieve a target serum concentration of 10 mcg/mL
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Reversible airflow obstruction (dosage adjustment): theophylline has a low therapeutic index; therefore, cautious dosage determination is essential. Because individuals metabolize theophylline at different rates, appropriate dosages must be determined for each patient by carefully monitoring patient response and tolerance, pulmonary function, and serum theophylline concentrations. Dosages required to achieve a therapeutic serum theophylline concentration vary fourfold among otherwise similar patients in the absence of factors known to alter theophylline clearance. Although extended-release preparations have been formulated to release the drug at various rates suitable for dosing every 8-12, 12, or 24 hours, the actual dosing frequency for a given patient and preparation depends on the patient's individual pharmacokinetic parameters. Dosage should be calculated on the basis of lean body weight.
Reversible airflow obstruction (maintenance therapy): serum theophylline concentrations should be obtained after a patient has received a given dosage for 3 days. Peak serum concentrations can be estimated by obtaining blood samples 30 minutes after administration of an IV loading dose, 1-2 hours after administration of an oral solution or uncoated tablet, or 3-12 (usually 3-8) hours (depending on the specific formulation) after administration of an extended-release preparation. Trough concentrations of theophylline can be determined by taking blood samples just before the next dose. When the recommended maximum dosage is exceeded, dosage adjustment should be based on measurement of peak serum theophylline concentrations. For dosage adjustments based on serum theophylline concentrations determined in such circumstances, it is important that dosage in the previous 48 hours be reasonably typical of the prescribed regimen and that the patient not have missed a dose nor taken an additional dose in this time period. Dosage adjustments based on serum theophylline concentrations when these conditions have not been fulfilled may result in dosages that present risk of toxicity to the patient. Therapeutic serum concentrations for bronchospastic disease generally range from 5-15 mcg/mL at steady state. When serum theophylline concentrations exceed 20 mcg/mL, toxicity often becomes apparent.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3605-11
Children (FDA)
Reversible airflow obstruction: maximum dose of oral theophylline in children 1 to 15 years of age 16 mg/kg/day (equivalent to aminophylline 20.3 mg) up to a maximum of 400 mg/day (equivalent to aminophylline 507 mg) in the presence of risk factors for reduced theophylline clearance or if it is not feasible to monitor serum theophylline concentrations.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1111-2
Pregnancy (FDA)
Category C: There are no adequate and well controlled studies in pregnant women.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Nursing Mothers (FDA)
Theophylline is excreted into breast milk and may cause irritability or other signs of mild toxicity in nursing human infants.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Geriatric (FDA)
The maximum daily dose of theophylline in patients older than 60 years of age ordinarily should not exceed 400 mg/day unless the patient continues to be symptomatic and the peak steady-state serum theophylline concentration is less than 10 mcg/mL.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1111-2
Obesity (FDA)
Theophylline distributes poorly into body fat; therefore, mg-per-kg doses should be calculated on the basis of ideal body weight.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1111-2
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Aminophylline has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (public Law 108-276).
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
6. Current available formulations/shelf life
Formulation
Aminophylline (Hydrous)
Oral Tablets 100 mg (78.9 mg of anhydrous theophylline)*; 200 mg (157.8 mg of anhydrous theophylline)* Generic Name: Aminophylline Tablets
Parenteral Injection 25 mg (19.7 mg of anhydrous theophylline) per mL* Generic Name: Aminophyllone Injection
Aminophylline (Anhydrous)
Oral Solution 105 mg (90 mg of anhydrous theophylline) per 5 mL*; Generic Name: Aminophylline Oral Solution
* available from one or more manufacturer, distributor, and/or repackager by generic (nonproprietary) name
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3605-11
Shelf life
Shelf life
Unopened : 3 years (36 months)
eMC. Mercury Pharma Group. Aminophylline injection. Last Updated: March 2009
Stability
-
The authors reported the stability of aminophylline solution 5mg per mL prepared by diluting the injection (Abbott Laboratories) with bacteriostatic water for injection containing 0.9% benzyl alcohol. Solutions were chemically stable for at least 90 days at 4°C and 22°C packed in plastic syringes. Bateriostatic water for injections is not available in many countries and many pediatricians would consider the use of even small amounts of benzyl alcohol undesirable. The injection can be diluted with plain water for injections and packed aseptically into plastic oral syringes. Solutions should be chemically stable for at least 30 days, but the expiry date assigned will depend on microbiological validation of the process. These solutions should be refrigerated. The solutions should be protected from light and packed in airtight containers. Exposure to air can cause the breakdown of theophylline/ethylenediamine complex causing a cloudy appearance. The effect of diluting other brands of aminophylline injection may need validation. All diluted aminophylline preparations should be periodically checked for signs of precipitation.
Nahata MC, Morosco RS, Hipple TF. Stability of aminophylline in bacteriostatic water for injection stored in plastic syringes at two temperatures. Am. J. Hosp. Pharm. 1992 Dec;49:2962-3. [PubMed Citation]
-
The authors evaluated the stability of 3mg/mL and 21mg/mL oral liquids prepared by diluting the commercially available injection (25mg/mL) in a 1:1 mixture of Ora Sweet:Ora Plus. The 3mg/mL liquid was chemically and physically stable for up to 90 days at 4 and 25°C. The 21mg/mL liquid was also stable for 90 days at 25°C but failed to maintain 90% of initial concentration when stored at 4°C.
Chong E, Dumont R.J., Hamilton D.P., Koke P.M., Ensom MHH. Stability of aminophylline in extemporaneously-prepared oral suspensions. J. Inform. Pharmacother. 2000;2:100-6.
-
The stability of aminophyline was studied in the presence of four tablet excipients (cellulose starch, glucose and lactose) and nine intravenous fluids (water for injection, dextrose. 10% normal saline, dextrose normal saline 5%, Ringer lactate, Ringer, aminoplasmal 5%, lipofundin 10%, total parenteral nutrition solution TPNS). Ten percent mixtures of the powder drug with the excipients were kept at 5 degrees C, room temperature (27+/-3 degrees C), 45 degrees C and in direct sunlight for 30 days. The degradation of the drug increases with increase in temperature and on exposure to sunlight. The degradation is particularly intense in the presence of glucose and lactose and change in color from white to yellow occurs in such mixtures. At 45 degrees C and in sunlight both pure drug and its mixtures change color during the storage period. In intravenous fluids, the drug (1 g/L) is stable and physically compatible for 2 days at 5 degrees C, 27+/-3 degrees C and 45 degrees C except in lipofundin 10% and TPNS where the drug is stable for only 12 hours at 45 degrees C. Yellow tinge appears in the admixtures containing dextrose after 2 days at room temperature and at 45 degrees C.
Riaz M, Ami KH. Stability of aminophylline. Pak J Pharm Sci. 1993 Jan;6(1):35-44. [PubMed Citation]
-
Aminophylline stability (dose 0.5 and 0.75 g) was estimated in typical AIO nutrient admixtures [Salviamin 12.5% 1000.0 cm3; Glucose 500 g; Intralipid 20% 500.0 cm3; Soluvit N; Vitalipid N Adult; Addamel N; total volume 3000.0 cm3] prepared in DIMIX bags [Diffuplast srl, Italy]. The pH, particle size distribution [Coulter Counter ZM] and theophylline concentration [Spectrophotometer Pye Unicam] were measured immediately after preparation and after 24, 48, 72 hours of storage in low temperature and then after 24 hours in room temperature. Aminophylline proved to be stable in above mentioned doses and now is commonly added into AIO in practice.
Ciszewska-Jedrasik M, Knyt A, Pertkiewicz M. Aminophylline stability in total parenteral nutrition admixtures. Acta Pol Pharm. 1995 Nov-Dec;52(6):487-90. [PubMed Citation]
SLEP (DOD/FDA)
Storage
Protect from light. Store below 25°C.
eMC. Mercury Pharma Group. Aminophylline injection. Last Updated: March 2009
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
This study is to evaluate the effects of aminophylline on systemic inflammatory response after cardiopulmonary bypass in patients undergoing valve replacement. Thirty patients undergoing elective valve replacement were randomized to receive either aminophylline treatment (aminophylline, n = 15) or no aminophylline (control, n = 15). Administration of aminophylline (5 mg/kg) was injected intravenously after induction of anesthesia and maintained with 0.5 mg/kg per h until the end of cardiopulmonary bypass. Perioperative cytokines (interleukin-8 and interleukin-10, tumor necrosis factor-alpha) and respiratory function, blood neutrophil count ratio of right atrium to that of left atrium, plasma malondialdehyde were measured during the experiment. Interleukin-8 and tumor necrosis factor-alpha levels after cardiopulmonary bypass were significantly lower in the aminophylline group than that in the control group (P < 0.05, for each group), and interleukin-10 level in aminophylline group was significantly higher than in control (P = 0.001). The respiratory index was greater in the control than in aminophylline group (P < 0.05). Neutrophil count ratio of right atrium blood to left atrium blood and plasma malondialdehyde level in aminophylline group were much lower (P = 0.02 and 0.001, respectively) than in the control 30 min after aortic declamping. Compared with control group, the duration of ventilation and intensive care unit stays were shorter in aminophylline group (P = 0.032 and 0.013, respectively). Intraoperative administration of aminophylline had anti-inflammatory effect and improved pulmonary oxygenation in patients undergoing valve replacement.
Luo WJ, Ling Z, Huang RM. Effects of aminophylline on cytokines and pulmonary function in patients undergoing valve replacement. Eur J Cardiothorac Surg. 2004 May;25(5):766-71. [PubMed Citation]
-
This study investigated whether aminophylline has an acute effect on the muscle performance of patients with amyotrophic lateral sclerosis (ALS). The study was a randomized, double-blind, crossover against placebo. Twenty-five patients (48.5 +/- 14.1 years) with ALS were evaluated by means of forced vital capacity (FVC), maximal mouth inspiratory and expiratory pressures (P(Imax)/P(Emax)) and endurance, maximum voluntary ventilation (MVV) and handgrip strength (HS); variables were measured before and after the patients received an intravenous infusion of aminophylline or placebo. MVV (P<0.02) and HS of the right and left hands (P=0.05) increased after aminophylline infusion. There was a positive correlation between FVC and P(Imax) (r=0.80; P<0.05); between MVV and P(Imax) post-aminophylline, respectively (r=0.77; P<0.05). Serum aminophylline levels ranged from 5.3 to 10.5 microg/mL (mean 7.30). The acute administration of aminophylline improves the endurance of respiratory muscles and increases handgrip strength in patients with ALS.
Berto MC, Filha SC, Camelier A, Rosa FW, de Souza Bulle Oliveira A, Jardim JR. Acute action of aminophylline in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2007 May;115(5):301-5. [PubMed Citation]
-
Theophylline has been shown to have beneficial effects on phrenic nerve and diaphragm activation. This case report involves a C5-C6 chronic tetraplegic patient with acute respiratory failure and ventilator dependence. IV aminophylline was administered in increasing doses (2 mg/kg, 4 mg/kg, and 6 mg/kg) over the course of 1 day. Diaphragm surface electromyography (sEMG), measures of respiration (tidal volume, minute ventilation, and frequency), and serum theophylline levels were captured. Diaphragm sEMG activity increased by a maximum of 50% at therapeutic levels. The rapid shallow breathing index dropped from 112 to 86. The subject was successfully weaned from ventilatory support. The authors conclude that administration of aminophylline facilitated weaning from ventilatory support in this tetraplegic patient.
Bascom AT, Lattin CD, Aboussouan LS, Goshgarian HG. Effect of acute aminophylline administration on diaphragm function in high cervical tetraplegia: a case report. Chest. 2005 Feb;127(2):658-61. [PubMed Citation]
8. Route of Administration/Monitoring
Intravenous injection, or oral administration
For maintenance therapy, serum theophylline concentrations should be obtained after a patient has received a given dosage for 3 days. Peak serum concentrations can be estimated by obtaining blood samples 30 minutes after administration of an IV loading dose, 1-2 hours after administration of an oral solution or uncoated tablet, or 3-12 (usually 3-8) hours (depending on the specific formulation) after administration of an extended-release preparation. Trough concentrations of theophylline can be determined by taking blood samples just before the next dose. When the recommended maximum dosage is exceeded, dosage adjustment should be based on measurement of peak serum theophylline concentrations. For dosage adjustments based on serum theophylline concentrations determined in such circumstances, it is important that dosage in the previous 48 hours be reasonably typical of the prescribed regimen and that the patient not have missed a dose nor taken an additional dose in this time period. Dosage adjustments based on serum theophylline concentrations when these conditions have not been fulfilled may result in dosages that present risk of toxicity to the patient. Therapeutic serum concentrations for bronchospastic disease generally range from 5-15 mcg/mL at steady state. When serum theophylline concentrations exceed 20 mcg/mL, toxicity often becomes apparent.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3605-11
Aminophylline is a 2:1 complex of theophylline and ethylenediamine.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
9. Adverse effects
-
Hypersensitivity reactions characterized by urticaria, generalized pruritus, and angioedema have been reported with aminophylline administration.
-
A contact-type dermatitis, caused by hypersensitivity to the ethylenediamine component of aminophylline, has also been reported.
-
Rapid IV injection of aminophylline may produce dizziness, faintness, lightheadedness, palpitation, syncope, precordial pain, flushing, profound bradycardia, ventricular premature complexes (VPCs, PVCs), severe hypotension, or cardiac arrest. IM injection of aminophylline produces intense local pain and sloughing of tissue.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3605-11
-
Products containing aminophylline may rarely produce severe allergic reactions of the skin, including exfoliative dermatitis, after systemic administration in a patient who has been previously sensitized by topical application of a substance containing ethylenediamine.
-
In such patients skin patch tests are positive for ethylenediamine, a component of aminophylline, and negative for theophylline. Pharmacists and other individuals who experience repeated skin exposure while physically handling aminophylline may develop a contact dermatitis due to the ethylenediamine component.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
-
Fatalities in adults have generally occurred during or following IV administration of large doses of aminophylline in patients with renal, hepatic, or cardiovascular complications. In other patients, the rapidity of the injection, rather than the dose used, appears to be the more important factor precipitating acute hypotension, seizures, coma, cardiac standstill, ventricular fibrillation, and death. IV aminophylline or theophylline should therefore be given slowly. In children, fatalities usually are a result of over dosage and marked sensitivity to the CNS stimulation of theophylline.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
10. Contraindication(s)
-
When therapeutic doses of theophylline are administered simultaneously by more than one route or in more than one preparation, the hazard of serious toxicity is increased; theophyllines should not be administered concomitantly with other xanthine drugs.
-
Theophyllines are contraindicated in patients who are allergic to any of the theophyllines, caffeine, or theobromine; aminophylline should not be used in patients hypersensitive to ethylenediamine. At least one manufacturer states that theophyllines also are contraindicated in patients with active peptic ulcer disease and in those with underlying seizure disorders, unless the latter patients are receiving adequate anticonvulsant therapy.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3605-11
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesFunctional genomics of chemical-induced acute lung injury
Chemical-induced acute lung injury (CIALI) can result from numerous chemical threats that avail themselves to terrorist attacks. Therapies are needed to treat the acute effects and pathologies that are common to several chemical threat agents. Despite intensive effort, much remains to be understood regarding pathological events linking Inhalation exposures to delayed pulmonary edema, respiratory failure, and ultimately death. In past funding period the investigators developed and validated mouse models of acute lung injury to 5 common chemicals (acrolein, ammonia, chlorine, phosgene, and sulfuric acid). Using a functional genomics approach, 40 mouse strains were used to identify candidate genes associated with survival time following exposure. Investigators combined the results to build a protein interaction network (interactome). Within this network, a cell signaling hub (i.e. a protein with several protein-protein interactions) was uncovered that implicated v- AKT1 thymoma viral oncogene homolog 1 (AKT1). When phosphorylated, AKT1 enhances survival by inactivating components of the cell death machinery. Although undesirable in cancer, the authors reasoned that short-term, reversible enhancement of the cell survival AKT1 activity could be beneficial in CIALI treatment. The authors subsequently found that inhibition of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), a phosphatase that limits AKT1 activity, enhances epithelial repair in vitro and protect against CIALI in vivo. Hypothesis: Inhibition of PTEN activity will impart resistance to CIALI by activating signaling events that promote cell survival. Approach: Using a high content screening method, lead compounds [including a PTEN inhibitor] will be administered in vitro to test reverse of lethality in target cells. Lead compounds will be tested in mouse models of lethality from CIALI with 5 chemicals. Overall Objective: To develop a therapy that improves survival during lung injury induced by multiple chemicals. Public Health Relevance: Acute lung injury can result from numerous chemical threats that avail themselves to terrorist attacks. Current therapy remains limited to supportive care with no approved therapeutic for post insult treatment. The overall objective is to develop a therapy that will improve survival during acute lung injury induced by more than one chemical.
RePORTER. NIH Functional genomics of chemical-induced acute lung injury
Targeting injury pathways to counteract pulmonary agent and vesicant toxicity
Chlorine (CI2), phosgene, vesicants and electrophilic reactive chemicals (industrial and riot control agents) are considered among the most imminent chemical threats to be diverted for terrorism attacks, or released during accidents. In the last four years research in the Jordt laboratory has identified TRPA1, a Transient Receptor Potential ion channel expressed in sensory neurons, as the major neuronal target of chlorine, riot control agents and industrial chemicals such as acrolein and isocyanates. Post-exposure treatment of chlorine-exposed mice with a TRPA1 antagonist strongly reduced lung inflammation and injury parameters. The same TRPA1 antagonist increased survival rates of phosgene-exposed mice, and also inhibited vesicant injury induced by the sulfur mustard analog, CEES. In the laboratory's recent work the authors identified TRPV4, an ion channel expressed in the lung epithelium and vasculature, as an additional mediator of oxidant-induced pulmonary injury. Activation of TRPV4 leads to severe lung injury and cardiovascular depression, and the investigators show that a TRPV4 antagonist inhibits ozone induced oxidative lung edema. TRPV3, a TRP ion channel in keratinocytes, is a candidate mediator of cutaneous injury by vesicants and corrosive electrophiles. TRP channel, through influx of calcium, activate p38 MAP kinase, a major transducer and activator of inflammation and cell death in injured tissue. In summary, the investigators hypothesize that TRP channels are major targets of chemical warfare agents, mediating local and systemic injury and inflammation through neuronal and local cellular signaling. In this proposal we aim to 1: Develop advanced intramuscular formulations of TRPA1 antagonists for immediate and sustained release to counteract chlorine and vesicant injury, 2: Examine the role of pulmonary and cutaneous TRP ion channels in chemical injury, and 3: Investigate the effects of a p38 kinase antagonist in pulmonary and cutaneous chemical injury. Public Health Relevance: Our research is aimed to further develop treatments for lung and skin injury by chemical warfare agents and industrial chemicals that can be diverted for terrorism attacks. We identified a new group of drugs that we found to effectively diminish injury by chlorine gas and skin blistering agents. The goal of this proposal is to improve these drugs and investigate how they counteract injury.
RePORTER. NIH. Targeting injury pathways to counteract pulmonary agent and vesicant toxicity
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations-
A better understanding of the mechanisms of injury both during and after exposure to phosgene, including the genetic, molecular and biochemical changes occurring in cells and tissues, is needed.
-
A non-human primate model for phosgene inhalation mimicking the real-time conditions expected in a mass exposure incident is needed.
-
Licensed drugs that can be used in the prevention or treatment of chemically induced pulmonary edema should be identified.
-
Drugs that can limit the inflammatory cascade of events produced by phosgene and other choking agents should be developed.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
A better understanding of the mechanisms of injury both during and after exposure to phosgene, including the genetic, molecular and biochemical changes occurring in cells and tissues, is needed.
-
A non-human primate model for phosgene inhalation mimicking the real-time conditions expected in a mass exposure incident is needed.
-
Licensed drugs that can be used in the prevention or treatment of chemically induced pulmonary edema should be identified.
-
Drugs that can limit the inflammatory cascade of events produced by phosgene and other choking agents should be developed.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
-
Needed studies for nonbiodefense clinical indications: Bronchiolitis; animal studies of standardized exposure; systematic data collection in the event of a disaster; use of a systematic treatment approach
-
Primate studies
Will produce more information on ventilation
-
Will produce more information on respiratory failure
Will permit exploration of 3-chlorotyrosine
-
Prospective, acute human studies
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
15. Study-related ethical concerns
— including review panel recommendations-
Informed consent for data collection in disaster/mass casualty situation
-
Central institutional review boards (IRBs)
-
How to conduct multicenter studies
Poison centers
-
Pediatric Emergency Care Applied Research Network
Hospital consortia.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
16. Global regulatory status
U.S.
AMINOPHYLLINE (aminophylline dihydrate) injection, solution:
Intravenous theophylline is indicated as an adjunct to inhaled beta-2 selective agonists and systemically administered corticosteroids for the treatment of acute exacerbations of the symptoms and reversible airflow obstruction associated with asthma and other chronic lung diseases, e.g., emphysema and chronic bronchitis.
Aminophylline is a 2:1 complex of theophylline and ethylenediamine.
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
AMINOPHYLLINE (aminophylline dihydrate) tablet
Theophylline is indicated for the treatment of the symptoms and reversible airflow obstruction associated with chronic asthma and other chronic lung diseases, e.g., emphysema and chronic bronchitis.
Product label: AMINOPHYLLINE (aminophylline dehydrate) tablet. [West-ward Pharmaceutical Corp] Last revised March 2012 [DailyMed]
U.K.
-
Reversible airways obstruction, acute severe asthma.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 161
17. Other potentially useful information
-
1 g dissolves in about 5 mL water; insoluble in alcohol, ether
HSDB. Aminophylline
-
Octanol/Water Partition Coefficient: log Kow= - 0.02
HSDB. Theophylline
18. Publications
Bascom AT, Lattin CD, Aboussouan LS, Goshgarian HG. Effect of acute aminophylline administration on diaphragm function in high cervical tetraplegia: a case report. Chest. 2005 Feb;127(2):658-61. [PubMed Citation]
Berto MC, Filha SC, Camelier A, Rosa FW, de Souza Bulle Oliveira A, Jardim JR. Acute action of aminophylline in patients with amyotrophic lateral sclerosis. Acta Neurol Scand. 2007 May;115(5):301-5. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Borak J, Diller WF; Phosgene exposure: mechanisms of injury and treatment strategies. Occup Environ Med. 2001 Feb;43(2):110-9. [PubMed Citation]
Chong E, Dumont R.J., Hamilton D.P., Koke P.M., Ensom MHH. Stability of aminophylline in extemporaneously-prepared oral suspensions. J. Inform. Pharmacother. 2000;2:100-6.
Ciszewska-Jedrasik M, Knyt A, Pertkiewicz M. Aminophylline stability in total parenteral nutrition admixtures. Acta Pol Pharm. 1995 Nov-Dec;52(6):487-90. [PubMed Citation]
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
eMC. Mercury Pharma Group. Aminophylline injection. Last Updated: March 2009
HSDB. Aminophylline
HSDB. Theophylline
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1111-2
Kennedy TP, Michael JR, Hoidal JR, Hasty D, Sciuto AM, Hopkins C, Lazar R, Bysani GK, Tolley E, Gurtner GH. Dibutyryl cAMP, aminophylline, and beta-adrenergic agonists protect against pulmonary edema caused by phosgene. J Appl Physiol. 1989 Dec;67(6):2542-52. [PubMed Citation]
Luo WJ, Ling Z, Huang RM. Effects of aminophylline on cytokines and pulmonary function in patients undergoing valve replacement. Eur J Cardiothorac Surg. 2004 May;25(5):766-71. [PubMed Citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 161
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3605-11
Nahata MC, Morosco RS, Hipple TF. Stability of aminophylline in bacteriostatic water for injection stored in plastic syringes at two temperatures. Am. J. Hosp. Pharm. 1992 Dec;49:2962-3. [PubMed Citation]
Product label: AMINOPHYLLINE (aminophylline dehydrate) injection solution. [Hospira, Inc.] Last revised: June 2011 [DailyMed]
Product label: AMINOPHYLLINE (aminophylline dehydrate) tablet. [West-ward Pharmaceutical Corp] Last revised March 2012 [DailyMed]
RePORTER. NIH Functional genomics of chemical-induced acute lung injury
RePORTER. NIH. Targeting injury pathways to counteract pulmonary agent and vesicant toxicity
Riaz M, Ami KH. Stability of aminophylline. Pak J Pharm Sci. 1993 Jan;6(1):35-44. [PubMed Citation]
Sciuto AM, Hurt HH. Therapeutic treatments of phosgene-induced lung injury. Inhalation Toxicology 2004 Jul;16(8):565-80. [PubMed Citation]
Sciuto AM, Strickland PT, Kennedy TP, Gurtner GH. Postexposure treatment with aminophylline protects against phosgene-induced acute lung injury. Exp Lung Res. 1997 Jul-Aug;23(4):317-32. [PubMed Citation]
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
