You are here: Home > Medical Countermeasures Database > Amyl Nitrite
Amyl Nitrite - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Amyl nitrite
2. Chemical Defense therapeutic area(s)
— including key possible usesAmyl nitrite is typically used with other agents (sodium nitrite and sodium thiosulfate) as an antidote for acute cyanide poisoning.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
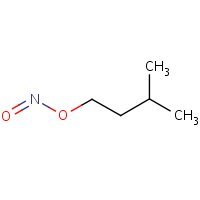
HSDB. Amyl Nitrite
Mechanism of action
-
Cyanide inhibits aerobic metabolism by binding to the binuclear heme center of cytochrome c oxidase (CcOX). Amyl nitrite and sodium nitrite (NaNO(2)) antagonize cyanide toxicity in part by oxidizing hemoglobin to methemoglobin (mHb), which then scavenges cyanide. mHb generation is thought to be a primary mechanism by which the NO(2)(-) ion antagonizes cyanide. On the other hand, NO(2)(-) can undergo biotransformation to generate nitric oxide (NO), which may then directly antagonize cyanide inhibition of CcOX. In this study, nitrite-mediated antagonism of cyanide inhibition of oxidative phosphorylation was examined in rat dopaminergic N27 cells. NaNO(2) produced a time- and concentration-dependent increase in whole-cell and mitochondrial levels of NO. The NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxy 3-oxide (PTIO) reversed this increase in cellular and mitochondrial NO. NO generated from NaNO(2) decreased cellular oxygen consumption and inhibited CcOX activity. PTIO reversed the NO-mediated inhibition, thus providing strong evidence that NO mediates the action of NaNO(2). Under similar conditions, KCN (20muM) inhibited cellular state-3 oxygen consumption and CcOX activity. Pretreatment with NaNO(2) reversed KCN-mediated inhibition of both oxygen consumption and CcOX activity. The NaNO(2) antagonism of cyanide was blocked by pretreatment with the NO scavenger PTIO. It was concluded that NaNO(2) antagonizes cyanide inhibition of CcOX by generating of NO, which then interacts directly with the binding of KCN x CcOX to reverse the toxicity. In vivo antagonism of cyanide by NO(2)(-) appears to be due to both generation of mHb and direct displacement of cyanide from CcOX by NO.
Leavesley HB, Li L, Mukhopadhyay S, Borowitz JL, Isom GE. Nitrite-mediated antagonism of cyanide inhibition of cytochrome c oxidase in dopamine neurons. Toxicol Sci. 2010 Jun; 115(2):569-76. [PubMed Citation]
-
Cyanide quickly and reversibly binds to the ferric iron in cytochrome oxidase, inhibiting effective energy production throughout the body. The ferric iron in methemoglobin preferentially combines with cyanide, producing cyanomethemoglobin. This drives the reaction toward cyanomethemoglobin and liberates cyanide from cytochrome oxidase. Strom-free methemoglobin is effective against four minimum lethal doses of cyanide in rats. Nitrites oxidize the iron in hemoglobin to produce methemoglobin. Because nitrites are accepted antidotes for cyanide poisoning, for many years methemoglobin formation was assumed to be their sole antidotal mechanism of action. Other faster methemoglobin induces, such as 4-dimethyaminophenol and hydroxylamine, also are affective as cyanide antidotes. The production of methemoglobin by nitrite is slow, but when methylene blue is administered to prevent methemoglobin formation, nitrite still is an effective antidote. Reasoning that nitrite-induced vasodilation might be a part of the mechanism of action, investigators considered the antidotal actions of other vasodialators. Only the alpha-adrenergic antagonists and ganglionic blockers demonstrate antidotal activity, and only when administered with sodium thiosulfate. It is possible that the benefits of nitrites given shortly after cyanide result from reversal of cyanide-induced circulatory effects rather than reversal of the effects of cyanide on cytochrome oxidase. Experimental evidence in organ damage induced by hypoxia or hypotension suggest that the benefits of nitrite may be related to its ability to be converted to nitric oxide, a potent vasodilator. The conversion to nitric oxide appears to occur only in tissues or blood with the lowest oxygen concentrations.
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1689-91
-
Amyl nitrite causes a non specific relaxation of smooth muscle with the most prominent actions occurring in vascular smooth muscle. This effect on vascular smooth muscle results in coronary vasodilation and decreased systemic vascular resistance and left ventricular preload and afterload.
Product label: AMYL NITRITE inhalant [James Alexander Corporation] Last revised: July 2010 [DailyMed]
Summary of clinical and non-clinical studies
Cyanide is a potent chemical that has been used as a poison for centuries, even before its isolation and identification (Gracia and Shepherd, 2004). With several attributes that make it an ideal terrorist weapon, including ease in use as a weapon, versatility in means of delivery to intended victims, and widespread availability, cyanide still poses a significant threat to individuals and targeted populations (Keim, 2006). Cyanide can cause death within seconds to minutes and is not only among the most rapidly acting of poisons, but in sufficient concentrations, is also among the most lethal. Cyanide exposure can result from industrial and residential applications, fires, iatrogenic sources, and ingestion of certain foods (Gracia and Shepherd, 2004). Clinical symptoms of cyanide poisoning are variable but frequently include loss of consciousness, metabolic acidosis, and cardiopulmonary failure (Yen et al., 1995). By binding to the ferric iron of mitochondrial cytochrome oxidase, cyanide inhibits cellular respiration (Gracia and Shepherd, 2004). Amyl nitrite, a compound that has vasodilatatory properties and that oxidizes hemoglobin to cyanide-binding methemoglobin, has been used to treat cyanide poisoning (Lavon and Bentur, 2010). It is often, but not always, administered with other agents (sodium nitrite and sodium thiosulfate) in a cyanide antidote kit and with other supportive measures such as oxygen (Hall, Saiers, and Baud, 2009). The reports on the safety and efficacy of amyl nitrite in treating acute cyanide exposure are contradictory. Of 45 beagle dogs that received intravenous injections of cyanide (2.5 mg/kg), 39 had improved cardiovascular and respiratory function and survival after inhalation or intravenous administration of amyl nitrite (Vick and Froehlich, 1985 and 1991). The 30 control animals treated with only cyanide died 5 to 7 minutes post-administration. A retrospective study examining the treatment of cyanide intoxication in industrial workers concluded that amyl nitrite was effective, with no residual adverse effects except headache and transient loss of appetite (Wurzburg, 1996). Other studies and case reports on cyanide poisoning, including one on a 4-year old child who nearly died after ingestion of amygdalin, also noted the therapeutic effects of the cyanide antidote kit, which amyl nitrite comprises (Hall et al., 1986; Johnson et al., 1989; Yen et al., 1995). However, other studies have cautioned about the use of amyl nitrite to treat mass casualty cyanide poisoning due to several limitations: uncontrolled administration of amyl nitrite ampoules with potential inadequate dosing; potential for serious toxicity such as nitrite-induced methemoglobinemia, and in smoke inhalation victims, carboxyhemoglobinemia with a subsequent decrease in oxygen delivery to vital tissues and organs; and distracted management by caregivers of a mass casualty incident (Hall et al., 2009; Lavon and Bentur, 2010). Administration of amyl nitrite using a nebulizer or inhaler may help to minimize its deficiencies (Donoghue, 2003). In summary, studies demonstrating the safety and efficacy of amyl nitrite are inconclusive, and its risk-benefit profile may not be favorable. Hydroxocobalamin, which was approved by the Food and Drug Administration in 2006, has a more positive risk-benefit profile and may be a better antidote for cyanide poisoning (Hall et al., 2009).
B. Link to clinical studies
Adult
-
Poisoning with potassium cyanide is usually fatal because of the inhibition of cytochrome oxidase. The parameters of oxygen metabolism in a patient with cyanide poisoning who was admitted in a coma with seizures was monitored. The administration of amyl nitrite and sodium thiosulfate led to a rapid improvement: the parameters reflecting oxygen metabolism improved and the plasma level of cyanide decreased. The patient revived 1 1/2 hours after treatment. The arterial ketone body ratio (AKBR), which is the ratio of acetoacetate to beta-hydroxybutyrate in arterial blood and which reflects the redox state in liver mitochondria, improved dramatically following treatment. Because the AKBR changes in relation to electron transport in liver mitochondria, it seems to be a logical parameter for evaluating the effect of potassium cyanide poisoning on electron transport. The AKBR also reflects the efficacy of treatment for cyanide poisoning. (Class IV)
Nakatani T, Kosugi Y, Mori A, Tajimi K, Kobayashi K; Changes in the parameters of oxygen metabolism in a clinical course recovering from potassium cyanide. Am J Emerg Med. 1993 May; 213-7. [PubMed Citation]
-
Two cases of acute poisoning by the inhalation of hydrogen cyanide are described. The first patient regained consciousness 40 minutes after his collapse; in the interval he was treated with inhalations of amyl nitrite and intravenous injections of 0.3 gm. of sodium nitrite and 12.5 gm. of sodium thiosulfate. The second patient remained stuporous during the five hours that followed her collapse; during that interval she received inhalations of oxygen and carbon dioxide and of amyl nitrite as well as an intravenous injection of 0.6 gm. of sodium thiosulfate. Much improvement followed the intravenous injection of 50 cc. of a 1% solution of methylene blue. No dramatic effects were obtained from alternating injections of sodium nitrite and sodium thiosulfate in her case. Both patients made complete recoveries. (Class IV)
Chen KK, Rose CL. Treatment of acute cyanide poisoning. J Am Med Assoc. 1956;162(12):1154-5.
-
A 24 year-old woman ingested an unknown amount of potassium cyanide in a suicide attempt. Coma and metabolic acidosis developed.
Administration of the Lilly Cyanide Antidote kit (Eli Lilly and Co, Indianapolis) resulted in prompt resolution of symptoms and full recovery. Whole blood cyanide level was 13 pg/mL approximately one hour after ingestion. The highest measured methemoglobin level after sodium nitrite administration was 9.2%, demonstrating that attaining a "therapeutic methemoglobin level" of 25% is unnecessary to insure a satisfactory clinical outcome. Because severe hypotension or excessive methemoglobinemia can be caused by the sodium nitrite component of the Lilly kit, only enough to produce an acceptable clinical response should be administered. (Class IV)
Johnson WS, Hall AH, Rumack BH. Cyanide poisoning successfully treated without 'therapeutic methemoglobin levels'. Am J Emerg Med. 1989; 7(4):437-440. [PubMed Citation]
Pediatric studies
-
The case of a 4-yr-old child in whom cyanide poisoning developed with extremely high blood cyanide levels after ingestion of oral laetrile (I) tablet, 12 tablets of 500 mg, successfully treated with amyl nitrate perles by intermittent inhalation followed by 5 ml of 3% sodium nitrite, and 25 ml of 25% sodium thiosulfate, given as intravenous injection, Lilly Cyanide Antidote Package, is reported. Treatment with the Lilly cyanide antidote kit resulted in rapid, complete recovery. It was concluded that although the necessity for using the Lilly cyanide antidote kit in serious cyanide poisoning has been questioned, the case demonstrates the benefit from antidotal treatment. (Class IV)
Hall AH , Linden CH, Kulig KW, Rumack BH; Cyanide poisoning from laetrile ingestion: role of nitrite therapy. Pediatrics. 1986 Aug;78:269-272
-
Two episodes of cyanide poisoning occurred in children after ingestion of apricot kernels. The first episode involved eight children who exhibited typical signs and symptoms of cyanide poisoning two hours after having ingested a large amount of apricot kernels. Seven children recovered. One died soon after admission. The second episode involved 16 children who had eaten a sweet prepared from such kernels. The symptoms and signs were identical with those in the first group but appeared one-half hour after the ingestion and were much more severe. Thirteen children recovered, two died shortly after admission, and a third child died two hours later. Cyanide poisoning was diagnosed and appropriate treatment was initiated. This included100% oxygen when indicated, gastric lavage, inhalations of amyl nitrite, and intravenous administration of 3% sodium nitrite followed by a 25% solution of sodium thiosulfate. (Class IV)
Lasch EE and Shawa RE. Multiple cases of cyanide poisoning by apricot kernels in children from Gaza. Pediatrics. 1981; 68(1):5-7. [PubMed Citation]
Pregnancy, breastfeeding studies
-
Amyl nitrite is a smooth muscle relaxant that has been used clinically to facilitate uterine relaxation in difficult deliveries. In this retrospective study, we evaluate the safety of amyl nitrite use during preterm cesarean deliveries, and we assess possible advantageous effects on surgical incision choice. Women who received amyl nitrite cesarean section were compared to a control group matched for gestational age, fetal presentation, and mode of delivery who did not receive amyl nitrite. There were no statistical differences between the groups in the independent variables (maternal age, parity, medical or obstetric history, type of anesthesia, anesthesia or obstetric attending physician, antepartum hematocrit, or neonatal weight). Outcome (dependent) variables (estimated blood loss, Apgar scores, postpartum hematocrit, cord gases, or postpartum complications) were assessed, and there were no significant differences between the groups. Low transverse cesarean section was performed more frequently in the amyl nitrite group (58 of 64) than in the comparison group (48 of 64) (p less than 0.03). Considering the 128 women with and without amyl nitrite together, the decrease in hematocrit observed postpartum was greater after classic section (7%) than after low transverse section (4%) (p less than 0.002). We conclude that the use of amyl nitrite during preterm cesarean section poses no threat to mother or fetus and may facilitate delivery by allowing the performance of a low transverse rather than a classic cesarean section without maternal or neonatal complications (Class III).
Hendricks SK, Ross B, Colvard MA, Cahill D, Shy K, Benedetti TJ. Amyl nitrite: use as a smooth muscle relaxant in difficult preterm cesarean section. Am J Perinatol 1992 Jul;9(4):289-92. [PubMed Citation]
-
It is not known whether this drug is excreted in human milk (Class IV).
Product label: AMYL NITRITE inhalant [James Alexander Corporation] Last revised: July 2010 [DailyMed]
Clinical reviews
-
Cyanide has several antidotes, with differing mechanisms of action and diverse toxicological, clinical, and risk-benefit profiles. The international medical community lacks consensus about the antidote or antidotes with the best risk-benefit ratio. Critical assessment of cyanide antidotes is needed to aid in therapeutic and administrative decisions that will improve care for victims of cyanide poisoning (particularly poisoning from enclosed-space fire-smoke inhalation), and enhance readiness for cyanide toxic terrorism and other mass-casualty incidents. This paper reviews preclinical and clinical data on available cyanide antidotes and considers the profiles of these antidotes relative to properties of a hypothetical ideal cyanide antidote. Each of the antidotes shows evidence of efficacy in animal studies and clinical experience. The data available to date do not suggest obvious differences in efficacy among antidotes, with the exception of a slower onset of action of sodium thiosulfate (administered alone) than of the other antidotes. The potential for serious toxicity limits or prevents the use of the Cyanide Antidote Kit, dicobalt edetate, and 4-dimethylaminophenol in prehospital empiric treatment of suspected cyanide poisoning. Hydroxocobalamin differs from these antidotes in that it has not been associated with clinically significant toxicity in antidotal doses. Hydroxocobalamin is an antidote that seems to have many of the characteristics of the ideal cyanide antidote: rapid onset of action, neutralizes cyanide without interfering with cellular oxygen use, tolerability and safety profiles conducive to prehospital use, safe for use with smoke-inhalation victims, not harmful when administered to non-poisoned patients, easy to administer. (Class IV)
Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol 2009;39(7):541-52. [PubMed Citation]
-
The authors reviewed the clinical manifestations, complications, and the prognosis affected by Lilly Cyanide Antidote in 21 victims of acute cyanide poisoning over a 10-year period. The clinical signs and symptoms in cyanide poisoning are variable. Among 21 cases, loss of consciousness (15), metabolic acidosis (14), and cardiopulmonary failure (9) were the three leading manifestations of cyanide intoxication. Anoxic encephalopathy (6) was not uncommon in the severely intoxicated victims. Diabetes insipidus (1) or clinical signs and symptoms mimicking diabetes insipidus (3) may be an ominous sign to encephalopathy victims. The major cause of fatal cyanide poisoning is the intentional ingestion of cyanide compounds as part of a suicide attempt. Decrease of arteriovenous difference of O2 partial pressure may be a clue for the suspicion of cyanide intoxication. Although the authors cannot show a statistically significant difference (P = 0.47) for the Lilly cyanide antidote kit in terms of improving the survival rate for victims of cyanide poisoning, the antidote kit was always mandatory in our study in the cases of severely intoxicated victims who survived. Early diagnosis, prompt, intensive therapy with antidote, and supportive care are still the golden rules for the treatment of acute cyanide poisoning, whether in the Emergency Department or on the scene. (Class III)
Yen D, Tsai J, Wang LM, Kao WF, Hu SC, Lee CH, Deng JF. The clinical experience of acute cyanide poisoning. Am J Emerg Med. 1995 Sep;13(5):524-8.
-
Cyanide is both widely available and easily accessible throughout the world. Although the compound is not frequently encountered, it has been used as a poison and contaminant in the past and is a potential terrorist agent. Cyanide has the ability to cause significant social disruption and demands special attention to public health preparedness. It can be obtained from a variety of sources, including industrial, medical, and even common household products. Another frequently encountered source of cyanide exposure is residential fires. Exposure to high concentrations of the chemical can result in death within seconds to minutes. Long-term effects from cyanide exposure can cause significant morbidity. The only treatment for cyanide toxicity approved for use in the United States is a kit consisting of amyl nitrite, sodium nitrite, and sodium thiosulfate. Future research aims to find a faster-acting, more effective, and better tolerated treatment for cyanide toxicity. (Class IV)
Garcia R, Sherpherd G; Cyanide poisoning and its treatment. Pharmacotherapy. 2004 Oct;24(10):1358-65. [PubMed Citation]
-
The traditional method of administering amyl nitrite to a victim of cyanide poisoning, is to break an ampoule in a handkerchief and then intermittently hold this under the victim's nose. The author would like to suggest two alternative methods for administering amyl nitrite. The first method is to use a nebuliser. The second method is to use an inhaler similar to the Penthrox device, normally used to administer methoxyflurane for emergency analgesia. With appropriate training, either method could be used by first aid staff. This could be of particular value to remote mine sites where the absence of medical staff may preclude intravenous administration of cyanide antidotes such as dicobalt edetate, sodium thiosulphate, sodium nitrite, or hydroxocobalamin. Both methods offer the following advantages over the traditional method:
Oxygen can be administered during treatment
Rapid delivery of the drug
Accurate dose delivery
-
Less risk of inhalation by first aid or medical staff
Less risk of injury due to glass fragments.
The inhaler device would also be particularly well suited to the treatment of large numbers of victims following industrial disaster or terrorist attack — the risk of which has been recently alluded to. One concern about introducing these methods is the potential for amyl nitrite toxicity. Experimental research is recommended to determine safe dosages and frequencies for each method. (Class IV)
Donoghue AM. Alternative methods of administering amyl nitrite to victims of cyanide poisoning. Occup Environ Med. 2003 Feb;60(2):147. [PubMed Citation]
-
Amyl nitrite has been recommended as a cyanide antidote for several decades. Its antidotal properties were initially attributed to induction of methemoglobin and later to a nitric oxide mediated hemodynamic effect. The ease of administration and alleged rapid clinical effect would recommend its wide use in the pre-hospital management of mass casualty cyanide poisoning; yet there are concerns regarding the use of amyl nitrite for this indication. The authors review the data on amyl nitrite in cyanide poisoning and evaluate its efficacy and safety in mass casualty cyanide poisoning. A literature search utilizing PubMed, Toxnet, textbooks in toxicology and pharmacology, and the bibliographies of the articles retrieved identified 17 experimental studies and human reports on the use of amyl nitrite in cyanide poisoning, and 40 additional articles on amyl nitrite's properties and adverse effects. One paper was excluded as it was a conference abstract with limited data. MECHANISMS OF ACTION: The antidotal properties of amyl nitrite were attributed initially to induction of methemoglobinemia and later to nitric oxide mediated vasodilation. EXPERIMENTAL STUDIES: Animal studies on the use of amyl nitrite in cyanide poisoning are limited, and their results are inconsistent, which makes their extrapolation to humans questionable. HUMAN STUDIES: Clinical reports are limited in number and the part played by amyl nitrite relative to the other treatments administered (e.g. life support, sodium nitrite, and sodium thiosulfate) is unclear. ADVERSE EFFECTS: Amyl nitrite can be associated with potentially serious adverse reactions such as hypotension, syncope, excessive methemoglobinemia, and hemolysis in G6PD deficient patients. These effects are more pronounced in young children, in the elderly, and in patients with cardiac and pulmonary disorders. Dose regimen. The method of administration of amyl nitrite (breaking pearls into gauze or a handkerchief and applying it intermittently to the victim's nose and mouth for a few minutes) is not easily controlled, might result in under- or over-dosing, can prevent the caregiver from administering life support, and possibly expose him/her to amyl nitrite's adverse effects. Administration of amyl nitrite in mass casualty cyanide poisoning can result in unnecessary morbidity and may interfere with the proper management of the incident and the required supportive treatment and rapid evacuation. In the authors' opinion these drawbacks make the use of amyl nitrite in pre-hospital mass casualty cyanide poisoning unwarranted. (Class IV)
Lavon O, Bentur Y. Does amyl nitrite have a role in the management of pre-hospital mass casualty cyanide poisoning? Clin Toxicol (Phila). 2010 Jul;48(6):477-84 (2010). [PubMed Citation]
-
Confirmed cases of childhood exposure to cyanide are rare despite multiple potential sources including inhalation of fire smoke, ingestion of toxic household and workplace substances, and ingestion of cyanogenic foods. Because of its infrequent occurrence, medical professionals may have difficulty recognizing cyanide poisoning, confirming its presence, and treating it in pediatric patients. The sources and manifestations of acute cyanide poisoning seem to be qualitatively similar between children and adults, but children may be more vulnerable than adults to poisoning from some sources. The only currently available antidote in the United States (the cyanide antidote kit) has been used successfully in children but has particular risks associated with its use in pediatric patients. Because hemoglobin kinetics vary with age, methemoglobinemia associated with nitrite-based antidotes may be excessive at standard adult dosing in children. A cyanide antidote with a better risk/benefit ratio than the current agent available in the United States is desirable. The vitamin B12 precursor hydroxocobalamin, which has been used in Europe, may prove to be an attractive alternative to the cyanide antidote kit for pediatric patients. In this article we review the available data on the sources, manifestations, and treatment of acute cyanide poisoning in children and discuss unmet needs in the management of pediatric cyanide poisoning (Class IV).
Geller RJ, Barthold C, Saiers JA, Hall AH. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006 Nov;118(5):2146-58. [PubMed Citation]
-
Cyanide poisoning is uncommon, but generates interest because of the presumed utility of an antidote immediately available in those areas with a high risk of cyanide exposure. As part of its regular review of guidelines, the Australian Resuscitation Council conducted a systematic review of the human evidence for the use of various proposed cyanide antidotes, and a narrative review of the relevant pharmacological and animal studies. There have been no relevant comparative or placebo-controlled human trials. Nine case series were identified. Treatment with hydroxocobalamin was reported in a total of 361 cases. No serious adverse effects of hydroxocobalamin were reported, and many patients with otherwise presumably fatal poisoning survived. Sodium thiosulphate use was reported in two case series, similarly with no adverse effects. Treatment with a combination of sodium nitrite, amyl nitrite and sodium thiosulphate was reported in 74 patients, with results indistinguishable from those of hydroxocobalamin and sodium thiosulphate. No case series using dicobalt edetate or 4-dimethylaminophenol were identified, but successful use in single cases has been reported. Hydroxocobalamin and sodium thiosulphate differ from alternatives in having negligible adverse effects, and on the basis of current evidence are the antidotes of choice. The indications for the use of an antidote, the requirements for supportive care and a recommended approach for workplaces where there is a risk of cyanide poisoning are presented (Class IV).
Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Review article: management of cyanide poisoning. Emerg Med Australas. 2012 Jun;24(3):225-38. [PubMed Citation]
-
Cyanide causes intracellular hypoxia by reversibly binding to mitochondrial cytochrome oxidase a3. Signs and symptoms of cyanide poisoning usually occur less than 1 minute after inhalation and within a few minutes after ingestion. Early manifestations include anxiety, headache, giddiness, inability to focus the eyes, and mydriasis. As hypoxia progresses, progressively lower levels of consciousness, seizures, and coma can occur. Skin may look normal or slightly ashen, and arterial oxygen saturation may be normal. Early respiratory signs include transient rapid and deep respirations. As poisoning progresses, hemodynamic status may become unstable. The key treatment is early administration of 1 of the 2 antidotes currently available in the United States: the well-known cyanide antidote kit and hydroxocobalamin. Hydroxocobalamin detoxifies cyanide by binding with it to form the renally excreted, nontoxic cyanocobalamin. Because it binds with cyanide without forming methemoglobin, hydroxocobalamin can be used to treat patients without compromising the oxygen carrying capacity of hemoglobin (Class IV).
Hamel J; A Review of acute cyanide poisoning with a treatment update. Critical Care Nurse. 2011 Feb;31(1):72-82. [PubMed Citation]
-
The potential for domestic or international terrorism involving cyanide has not diminished and in fact may have increased in recent years. This paper discusses cyanide as a terrorist weapon and the current state of readiness for a cyanide attack in the United States. Many of the factors that render cyanide appealing to terrorists are difficult to modify sufficiently to decrease the probability of a cyanide attack. For example, the relative ease with which cyanide can be used as a weapon without special training, its versatile means of delivery to intended victims, and to a large degree, its ready availability cannot be significantly modified through preparedness efforts. On the other hand, the impact of an attack can be mitigated through preparedness measures designed to minimize the physical, psychological, and social consequences of cyanide exposure. Although the nation remains ill-equipped to manage a cyanide disaster, significant progress is being realized in some aspects of preparedness. Hydroxocobalamin-a cyanide antidote that may be appropriate for use in the prehospital setting for presumptive cases of cyanide poisoning-currently is under development for potential introduction in the US. If it becomes available in the US, hydroxocobalamin could enhance the role of the prehospital emergency responder in providing care to victims of a cyanide disaster. Additional progress is required in the areas of ensuring local and regional availability of antidotal treatment and supportive interventions, educating emergency healthcare providers about cyanide poisoning and its management, and raising public awareness of the potential for a cyanide attack and how to respond (Class IV).
Keim ME. Terrorism involving cyanide: the prospect of improving preparedness in the prehospital setting. Prehosp Disaster Med. 2006 Mar-Apr;21(2):s56-60. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
The effects of intravenously (i.v.) administered or inhaled amyl nitrite (AN) were followed under chloralose anaesthesia in intact and cyanide-poisoned, spontaneously breathing beagles. The i.v. doses of AN were 0.03 and 0.15 mmol/kg and the i.v. dose of KCN was 0.06 mmol/kg. AN was inhaled in a closed system at 0.15 mmol/kg without previous poisoning and, in addition, at 0.074 mmol/kg (two ampoules at 0.3 ml AN) during artificial ventilation after poisoning with 0.045 mmol KCN/kg i.v.. Mean arterial pressure decreased by 15 and 40 mmHg, respectively, after i.v. injection of AN, associated with bradycardia and lowered peripheral blood flow. Respiratory minute volume rose by 65% with the higher dose. Arterial pO2 decreased by 20 mmHg while pCO2 rose by 6 mmHg. Within 30 min of injection, these changes were only partially reversible. Similar results were obtained following inhalation of AN in a closed system. Lactic acidosis and lowering of pH were produced by the i.v. route, but not by inhalation. Total haemoglobin increased. The lethality of KCN was abolished with AN doses that produced 10-30% ferrihaemoglobin. Artificial ventilation and simultaneous inhalation of AN after poisoning with lethal doses of KCN turned out to be ineffective therapeutic measures. The findings are compared with those of other papers dealing with cyanide poisoning and AN. It is pointed out that, for the present, there is no experimental proof for another antidotal mechanism of action of AN than ferrihaemoglobin formation.
Klimmek R, Krettek C. Effects of amyl nitrite on circulation, respiration and blood homoeostasis in cyanide poisoning. Arch Toxicol. 1988 62(2-3):161-6. [PubMed Citation]
-
The use of amyl nitrite and phenoxybenzamine in the treatment of acute cyanide poisoning was evaluated. Sixty anesthetized beagle dogs were injected i.v. with sodium cyanide (2.5 mg/kg) and were followed for changes in the heart rate, electrocardiogram, respiration, blood pressure and methemoglobin concentration. Twenty control dogs died within 5 to 7 min, showing severe bradycardia, a sharp drop in arterial blood pressure, and respiratory paralysis. Pretreatment with phenoxybenzamine (0.5 mg/kg) prevented these changes in 8 of 10 dogs; however, this drug was ineffective if given after the cyanide. In contrast, amyl nitrite given after cyanide administration reversed both the cardiovascular changes and the respiratory paralysis in 24 of the 30 dogs studied. These changes occurred before the formation of significant amounts of methemoglobin and indicate that early death caused by cyanide may be due in part to cardiovascular-respiratory failure in addition to the classic poisoning of the cytochrome oxidase system. These studies indicate that phenoxybenzamine prevents and amyl nitrite reverses the otherwise lethal effects of cyanide.
Vick JA, Froehlich HL. Studies of cyanide poisoning. Arch Int Pharmacodyn Ther 1985 Feb;273(2):314-22. [PubMed Citation]
-
The objective of this study was to evaluate the effectiveness of five regimens in treating cyanide poisoning. A series of anesthetized adult beagle dogs were instrumented to record hemodynamic and respiratory function and given 2.5 mg/kg sodium cyanide intravenously. The 10 control animals given only cyanide died at from 5 to 7 minutes. Therapy, as described below, was given to other groups at from 2 to 3 minutes following the cyanide administration. Artificial respiration did not alter the lethal effects of cyanide nor prolong survival time in any of the 10 animals. Amyl nitrite given by inhalation or by the intravenous route allowed survival of all 15 animals. Sodium nitrite (20 mg/kg), dimethylaminophenol (DMAP) (5 mg/kg), and hydroxylamine hydrochloride (5 mg/kg) given intravenously with no artificial ventilation also allowed for 100% survival (15 animals). Amyl nitrite, sodium nitrite, and sodium thiosulfate were ineffective when given intramuscularly (I.M.) (0 of 12 dogs); however, I.M. DMAP (5 mg/kg) and I.M. hydroxylamine hydrochloride (50 mg/kg) increased heart rate and blood pressure and restored spontaneous breathing. All 15 animals treated with I.M. doses of either of these drugs survived the lethal dose of cyanide. Results of these studies indicate that intravenous sodium nitrite, DMAP, and hydroxylamine hydrochloride, and amyl nitrite by inhalation, are all effective in reversing the lethal effects of cyanide poisoning. Only DMAP and hydroxylamine hydrochloride are effective when given by the intramuscular route. These results provide data to support an approach to therapy that is more practical and applicable where expert medical care may not be available following cyanide exposure.
Vick JA, Froehlich H. Treatment of cyanide poisoning. Mil Med. 1991 Jul;156(7):330-9. [PubMed Citation]
-
An estimated 35% of all fire victims in the United States have toxicologically significant blood levels of CO and CN. However, the treatment of concurrent CO/CN intoxication has been paid scant attention. The suggestion has been made that these victims should be treated for CN poisoning. The current therapeutic management of CN poisoning in this country includes the utilization of two methemoglobin formers: amyl nitrite and sodium nitrite. This study was undertaken to determine if the administration of methemoglobin formers is advisable, as the victim is already suffering from O2 deprivation due to the presence of carboxyhemoglobin. Groups of 28 male ICR mice (22-24 g) were injected i.p. with 5.0 mg/kg of KCN and then were exposed immediately to 0.35% CO for 8.5 min in a dynamic inhalation chamber. Half of the animals were marked randomly for antidotal intervention, the other 14 animals acted as controls. Treatment of survivors with amyl nitrite (12 mg/l of chamber air) for 1 min increased mortality 43%, whereas treatment for 2 min resulted in a 59% increase in mortality. A 25% increase in mortality was noted among those animals treated with sodium nitrite (80 mg/kg i.p.), as compared to the nontreated control survivors. Treatment with dimethylaminophenol (49 mg/kg i.p.) did not statistically affect mortality.
Moore SJ, Norris JC, Walsh DA, Hume AS. Antidotal use of methemoglobin forming cyanide antagonists in concurrent carbon monoxide/cyanide intoxication. J Pharmacol Exp Ther. 1987 Jul;242(1):70-3. [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Amyl nitrite vapors are absorbed rapidly through the pulmonary alveoli, manifesting therapeutic effects within one minute after inhalation. The drug is metabolized rapidly, probably by hydrolytic denitration; approximately one-third of the inhaled amyl nitrite is excreted in the urine.
Product label: AMYL NITRITE inhalant [James Alexander Corporation] Last revised: July 2010 [DailyMed]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Amyl Nitrite has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (Public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Stability
-
Amyl nitrite vapor forms an explosive mixture in air or oxygen at room temperature and may explode if ignited. Amyl nitrite inhalant should be packaged in unit-dose containers, wrapped loosely in gauze or other suitable material, and stored at 2-15 deg C. A stabilizer, such as diphenylamine or epoxolol, is added to the commercially available products. The drug should be protected from light.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
AMYL NITRITE inhalant [James Alexander Corporation]
NOTE: THIS DRUG HAS NOT BEEN FOUND BY FDA TO BE SAFE AND EFFECTIVE, AND THIS LABELING HAS NOT BEEN APPROVED BY FDA.
Angina pectoris: Its effect appears within 30 seconds and lasts for approximately 3 to 5 minutes. James Alexander brand amyl nitrite is furnished in covered glass capsules. Each capsule contains 0.3 mL in boxes of 12. With the patient in recumbent or seated position a capsule of amyl nitrite is held away from the face, crushed between the fingers, and held under the patient's nose. Two to six inhalations of the vapors from the capsule are usually sufficient to promptly produce therapeutic effects. Caution is recommended to avoid inhalation of excessive amounts of the drug when it is administered by someone other than the patient. If necessary, the dose may be repeated in 3 to 5 minutes.
Product label: AMYL NITRITE inhalant [James Alexander Corporation] Last revised: July 2010 [DailyMed]
-
Cyanide Antidote Package (Amyl Nitrite Inhalants: 0.3 mL [12 ampoules], Sodium Nitrite: 200 mg/10 mL [2 ampoules], Sodium Thiosulfate: 12.5 mg/50 mL [2 vials]). Cyanide poisoning (adult dosage): apply 1 ampoule of Amyl Nitrite to a handkerchief and hold in front of patient's mouth for 15 seconds followed by a rest for 15 seconds. Then reapply until Sodium Nitrite can be administered. Discontinue Amyl Nitrite and give Sodium Nitrite IV 300 mg at a rate of 2.5 to 5 mL/minute. Immediately after inject 12.5 g of Sodium Thiosulfate.
Bartlett JG, Greenberg MI, eds. PDR Guide to Terrorism Response. Montvale, NJ: Thomson PDR, 2005 p.321-41
Children
-
Cyanide Antidote Package (Amyl Nitrite Inhalants: 0.3 mL [12 ampoules], Sodium Nitrite: 200 mg/10 mL [2 ampoules], Sodium Thiosulfate: 12.5 mg/50 mL [2 vials]). Cyanide poisoning (children dosage): apply 1 ampoule of Amyl Nitrite to a handkerchief and hold in front of patient's mouth for 15 seconds followed by a rest for 15 seconds. Then reapply until Sodium Nitrite can be administered. Discontinue Amyl Nitrite and give 6-8 mL/sq m of Sodium Nitrite IV, max of 12.5 g. Immediately after inject 7 g/sq m of Sodium Thiosulfate, max of 12.5 g.
Bartlett JG, Greenberg MI, eds. PDR Guide to Terrorism Response. Montvale, NJ: Thomson PDR, 2005 p.321-41
Pregnancy
Pregnancy category C
Product label: AMYL NITRITE inhalant [James Alexander Corporation] Last revised: July 2010 [DailyMed]
8. Route of Administration/Monitoring
-
All patients should be monitored in an emergency or intensive care setting. Monitoring should include regular assessments of metabolic acidosis using serial arterial blood gases, metabolic panels, or serum lactate determination, If hypotension develops, the infusion or inhalation of nitrites should be slowed or stopped accordingly, and the patient should be given supplemental fluids or pressors. If clinical or laboratory signs of cyanide toxicity do not improve within 15 or 30 minutes of Cyanide Antidote Package (CAP) administration, the sodium nitrite and sodium thiosulfate portions should be repeated at half the recommended initial dose. They should also be repeated in patients who develop recurrent toxicity after an initial improvement. Recurrent toxicity may also represent ongoing cyanide absorption, so further decontamination would be considered. Patients who do not respond to repeat doses of the CAP should have the diagnosis of cyanide toxicity reconsidered. A MetHgb level should be checked approximately 30 and 60 minutes after nitrite infusion - earlier if signs and methemoglobinemia develop. The MetHgb level needed for optimal benefit has been been established. Some authors describe a target MetHgb level of 25% to 30%; however, recent evidence suggests that high levels are not necessary for therapeutic benefit. Clinical improvement rather than MetHgb level should be the therapeutic goal.
Dart RC, ed. Medical Toxicology 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2004 p.172-5
9. Adverse effects
-
Traditional treatment of cyanide poisoning involves a two-step process: administration of amyl or sodium nitrite followed by administration of sodium thiosulfate. In a mass casualty scenario, there are issues with administering the traditional antidotes amyl nitrite and sodium nitrite: (1) the degree of methemoglobinemia can be exceeded, which is critical in pediatric and obstetric treatment, and (2) because the nitrites are vasodilators, hypotension may be induced.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Mild transitory headache, dizziness and flushing of the face are common with the use of amyl nitrite. The following reactions may occur in susceptible patients: syncope, involuntary passing of urine and feces, hypotension, pallor, cold sweat, tachycardia, restlessness, weakness, vomiting and nausea.
-
High doses of nitrites may produce methemoglobinemia, especially in individuals with methemoglobin reductase deficiencies or other metabolic abnormality that interferes with the normal conversion of methemoglobin back to hemoglobin.
-
Inhaled doses of 5 to 10 drops of amyl nitrite may cause violent flushing of the face, accompanied by a feeling of imminent bursting of the head and very excessive heart action. The inhalation of larger amounts may produce a feeling of suffocation and muscular weakness. Symptons comparable to shock may be produced (such as weakness, restlessness, sweating, pallor, nausea, vomiting, snycope and incontinence) attributable to pooling of blood in the postarteriolar vessels and failure of the venous blood to return to the heart.
-
Taking amyl nitrite after drinking alcohol may worsen side effects and may cause severe hypotension and cardiovascular collapse.
-
The fetus and young infants have increased fetal hemoglobin which results in increased formation of methemoglobin when exposed to amyl and sodium nitrite - CHEMM Editor
Product label: AMYL NITRITE inhalant [James Alexander Corporation] Last revised: July 2010 [DailyMed]
-
Excessive doses of nitrites or their use in susceptible subgroups of patients (e.g., glucose-6-phosphate dehydrogenase deficiency) can lead to life-threatening methemoglobinemia. Some patients may not tolerate even a modest degree of methemoglobinemia due to underlying pulmonary or cardiac disease. Nitrites must be used with caution in these patients, with close monitoring of clinical condition and MetHgb levels. The clinical sequelae of excessive MetHgb may include a bluish or brownish discoloration of the skin; increased respiratory rate, decreased blood pressure, or increased heart rate; a deterioration in mental status; evidence of cardiac ischemia; or worsening acidosis.
Dart RC, ed. Medical Toxicology 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2004 p. 172-5
-
Amyl nitrite and sodium nitrite work by inducing methemoglobinemia, but excessive methemoglobinemia is potentially lethal. Therefore, nitrite dosages must be carefully calculated and nitrites administered to avoid excessive methemoglobinemia, especially in cases where other coexisting conditions, such as carboxyhemoglobin, sulfhenoglobin, and anemia, might compromise hemoglobin oxygen saturation. Children are particularly at risk for medication errors because of dosage miscalculations.
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1689-91
10. Contraindication(s)
-
Caution - amyl nitrite may cause significant hypotension and if taken with drugs like Viagra, Cialis or Levitra (other nitrite containing drugs). This effect is magnified potentially causing fainting and even death. - CHEMM Editor
-
Since it may increase intraocular and intracranial pressures, amyl nitrite is contraindicated or should be used with great caution in patients with glaucoma, recent head trauma or cerebral hemorrhage.
-
Amyl nitrite can cause harm to the fetus when it is administered to a pregnant woman because it significantly reduces systemic blood pressure and blood flow on the maternal side of the placenta.
Product label: AMYL NITRITE inhalant [James Alexander Corporation] Last revised: July 2010 [DailyMed]
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues-
Title: Development of a field-deployable device to rapidly measure blood cyanide levels
The objective of this proposal is to assess the feasibility of a fully integrated device for the rapid and early diagnosis of cyanide poisoning in whole blood using the spectral shift of the vitamin B12 precursor cobinamide upon binding with cyanide as an indicator. Cyanide is an extremely potent and rapid acting poison with as little as 50 mg fatal to humans. Currently there are no portable rapid tests for the detection of cyanide in whole blood available. The primary goal of this proposal is to demonstrate feasibility of the cobinamide-cyanide chemistry in a rapid test using a whole blood sample from a finger-stick. The total assay time from the sample collection to a valid result will be less than 5 minutes. The innovation in this proposal is to incorporate the cobinamide chemistry with blood separation technology, fluid path designs, and a detection area, all integrated in a compact, disposable device which can be interpreted with a handheld visualization system. Feasibility work of this novel rapid assay for detecting blood cyanide through cobinamide will initially focus on several technical approaches for the device design. Parallel paths to pursue a wet chemistry design, a design where the reagents are dried in the device, and a combination thereof will be considered, compared and evaluated. In all of the proposed methods the whole blood sample will be added to the device, followed by an integrated whole blood processing step to yield a plasma sample. Either prior to or immediately after the whole blood separation and lysis, the sample will be mixed with the on-board reagent, dried or wet, and possibly buffers. The processed sample-reagent mixture then moves through the device through various fluid paths to a detection area. To allow for sufficient mixing time between the sample and the reagents, different fluid paths, mixing chambers, polymeric time-gates, and dissolvable films will be evaluated with the goal of maximizing the interaction time. The spectral shift of cobinamide upon binding of cyanide to cobinamide will be measured in the detection area using a handheld or small, portable reader instrument. Upon successful completion of this Phase 1 project we intend to further develop our concept/prototype assay, complete the device design and integrate the finished test with a reader instrument through a Phase 2 proposal. Specific aims for a Phase 2 of this project include the validation of the cobinamide-based device for measuring cyanide in blood. Results will be compared to two established methods for measuring cyanide in biological samples. The sensitivity, specificity, accuracy, precision, and reproducibility of the devices will be determined. Additional studies will be designed to obtain regulatory approval and subsequently we will commercialize the test. If successful, this test will provide the medical community and first responders with a fast, reliable and economic alternative to determine cyanide poisoning. PUBLIC HEALTH RELEVANCE: Cyanide poisoning has been recognized as a threat from smoke inhalation and potentially through weapons of mass destruction, but there are currently no methods available to rapidly detect cyanide in blood from at-risk patients. The objective of the proposed work is to assess the feasibility of a fully integrated method for the rapid and early detection and diagnosis of cyanide poisoning in whole blood using the spectral shift of the vitamin B12 precursor cobinamide upon binding with cyanide as an indicator. Successful accomplishment of the goals of this project will be the basis for the development of a test to detect and measure cyanide blood poisoning which will allow healthcare providers to treat patients quickly and effectively.
RePORTER. NIH. Development of a field-deployable device to rapidly measure blood cyanide levels.
-
Title: Countermeasures against chemical threats: countermeasures against cyanide
Sources for potential exposure to cyanide of both civilians and military personnel include combustion of nitrogenous materials, commercial accidents and the deliberate release of a cyanogenic chemical. The relative ease in obtaining and releasing cyanide means that the risk of cyanide use in a terrorist attack resulting in a mass casualty situation should not be ignored. Rapid intervention is critical for effective medical intervention in cases of cyanide exposure: treatments require a "three minute solution."
The availability of a rapid, IM injectable antidote should meet this requirement. However, there are no such available treatments - the current cyanide antidotes are administered intravenously. Because intravenous administration is time consuming and requires well trained medical personnel mass exposure to cyanide would likely leave many victims untreated. Because IM administration can be performed via an autoinjector and can therefore be done rapidly by minimally trained personnel there is a critical need for a rapid-acting, IM-injectable antidote for the treatment of mass cyanide casualties. In order to address this need /the authors/ are advancing /a/preclinical lead, sulfanegen to clinical development. /They/ have previously demonstrated efficacy of sulfanegen in murine, swine and rabbit models of cyanide toxicity. In these models sulfanegen is effective in reversal of cyanide toxicity by IM injection and therefore should meet the three minute solution. /The authors/ have recently held a pre-IND meeting with the FDA and /their/ goal is to advance this cyanide antagonist to the clinic by validation of animal models, demonstrate efficacy of sulfanegen in these animal models and perform the required GLP pharmacokinetic and safety evaluation required for clinical trials. /The authors/ will then commence a Phase 1 human safety study while simultaneously performing the required GLP studies for NDA submission under the animal rule. Successful completion of the aims of this project should lead to a clinical countermeasure for cyanide toxicity that will be applicable in all cases of cyanide exposure from individuals to mass casualty settings. PUBLIC HEALTH RELEVANCE: /The authors/ have discovered a novel cyanide antidote that /they/ have named sulfanegen and demonstrated that it is more effective than existing antidotes in models of cyanide toxicity. /They/will perform the necessary studies to translate this antidote from the bench to the bedside. Successful completion of the goals of this project will therefore result in a clinical antidote that could be useful in the case of a terrorist attack involving cyanide.
RePORTER. NIH. Countermeasures against chemical threats: countermeasures against cyanide.
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesNo data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendationsNo data available at this time.
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
A better understanding of the mechanism of action of cyanide at the molecular level is needed.
-
The use of antidotes to cyanide poisoning in patients exposed to other toxic hazards such as smoke or industrial chemicals needs further evaluation.
-
Models should be developed to characterize the long-term effects of acute exposure to cyanide and identify how cyanide-containing compounds are metabolized.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
15. Study-related ethical concerns
— including review panel recommendationsNo data available at this time.
16. Global regulatory status
E.U.
-
In case of non-availability of hydroxocobalamin or dicobalt edentate, alternative antidotes which act by indirect complexation of cyanide to methemoglobin, should be considered. The methemoglobin forming antidotes include sodium nitrite, amyl nitrite and 4-dimethylaminophenol.
-
Amyl nitrite in 0.2 ml vials used as a methaemoglobin inducer followed by sodium thiosulfate acting as a sulfur supplier to promote thiocyanate conversion. One vial of amyl nitrite is inhaled over 0.5 to 3 minutes while an IV line is being established.
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
17. Other potentially useful information
-
Sources of cyanide exposure are many, including combustion of plastic and vinyl, such as in a house fire, laboratory or industrial exposures including exposure in the electroplating industry both of printed circuit boards and in jewelry work. Rapid and definitive diagnosis of cyanide poisoning is unavailable in the emergency department setting. It is desirable to make a definitive diagnosis in order to prevent potential complications of empiric treatment of presumptive cyanide poisoning from the cyanide antidote kit currently approved by the US Food and Drug Administration (FDA). We investigated a technique to detect cyanide currently utilized by water treatment facilities to determine if it can be applied to rapidly detect concentrations of cyanide in the clinically important range. Varying standardized dilutions of KCN ranging from 0.25 microg/mL to 30 microg/mL were acidified with a drop of sulphuric acid in a closed system under a ventilation hood. Cyantesmo test strips were placed into the test tubes above the fluid level where liberated HCN gas interacted with the test strip to effect a color change. Color changes were compared to negative controls and to each other. The test strips demonstrated an incrementally increasing deep blue color change over a progressively longer portion of the test strip in less than 5 minutes for each concentration of KCN including 1, 3, 10, and 30 microg/mL. The concentrations of 0.25, 0.5, and 0.75 required more than 2 hours to begin demonstration of any color change. The Cyantesmo test strips accurately and rapidly detected, in a semi-quantifiable manner, concentrations of CN greater than 1 microg/mL contained in each test sample. Future work to validate this test in blood and in clinical specimens is planned.
Rella J, Marcus S, Wagner BJ. Rapid cyanide detection using the Cyantesmo kit. J Toxicol Clin Toxicol. 2004; 42(6):897-900. [PubMed Citation]
-
Exposure to cyanide can occur in a variety of ways, including exposure to smoke from cigarettes or fires, accidental exposure during industrial processes, and exposure from the use of cyanide as a poison or chemical warfare agent. Confirmation of cyanide exposure is difficult because, in vivo, cyanide quickly breaks down by a number of pathways, including the formation of both free and protein-bound thiocyanate. A simple method was developed to confirm cyanide exposure by extraction of protein-bound thiocyanate moieties from cyanide-exposed plasma proteins. Thiocyanate was successfully extracted and subsequently derivatized with pentafluorobenzyl bromide for GC-MS analysis. Thiocyanate levels as low as 2.5 ng mL(-1) and cyanide exposure levels as low as 175 μg kg(-1) were detected. Samples analyzed from smokers and non-smokers using this method showed significantly different levels of protein-bound thiocyanate (p<0.01). These results demonstrate the potential of this method to positively confirm chronic cyanide exposure through the analysis of protein-bound cyanide in human plasma.
Youso SL, Rockwood GA, Lee JP, Logue BA. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2010 Sep 10;677(1):24-8. [PubMed Citation]
-
Exposure to cyanide can occur in a variety of ways, including exposure to smoke from cigarettes or fires, accidental exposure during industrial processes, and exposure from the use of cyanide as a poison or chemical warfare agent. Confirmation of cyanide exposure is difficult because, in vivo, cyanide quickly breaks down by a number of pathways, including the formation of both free and protein-bound thiocyanate. A simple method was developed to confirm cyanide exposure by extraction of protein-bound thiocyanate moieties from cyanide-exposed plasma proteins. Thiocyanate was successfully extracted and subsequently derivatized with pentafluorobenzyl bromide for GC-MS analysis. Thiocyanate levels as low as 2.5 ng mL(-1) and cyanide exposure levels as low as 175 μg kg(-1) were detected. Samples analyzed from smokers and non-smokers using this method showed significantly different levels of protein-bound thiocyanate (p<0.01). These results demonstrate the potential of this method to positively confirm chronic cyanide exposure through the analysis of protein-bound cyanide in human plasma.
Ma J, Dasgupta PK, Zelder FH, Boss GR. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2012 Jul 29;736:78-84. [PubMed Citation]
-
When cyanide is introduced into the body, it quickly transforms through a variety of chemical reactions, normally involving sulfur donors, to form more stable chemical species. Depending on the nature of the sulfur donor, cyanide may be transformed into free thiocyanate, the major metabolite of cyanide transformation, 2-amino-2-thiazoline-4-carboxylic acid or protein-bound thiocyanate (PB-SCN) adducts. Because protein adducts are generally stable in biological systems, it has been suggested that PB-SCN may have distinct advantages as a marker of cyanide exposure. In this study, plasma was analyzed from 25 smokers (chronic low-level cyanide exposure group) and 25 non-smokers for PB-SCN. The amount of PB-SCN found in the plasma of smokers, 1.35 µM, was significantly elevated (p < 0.0001) when compared to non-smokers, 0.66 µM. Differences in sub-groups of smokers and non-smokers were also evaluated. The results of this study indicate the effectiveness of analyzing PB-SCN in determining instances of chronic cyanide exposure with possible extension to confirmation of acute cyanide exposure.
Youso SL, Rockwood GA, Logue BA. The analysis of protein-bound thiocyanate in plasma of smokers and non-smokers as a marker of cyanide exposure. J Anal Toxicol 2012 May;36(4):265-9. [PubMed Citation]
-
Miscible with ether, alcohol, chloroform.
-
Very slightly soluble in water.
HSDB. Isoamyl nitrite
-
Log P (octanol-water) = 2.770 (est)
US NLM. ChemIDplus lite. Amyl nitrite
18. Publications
Bartlett JG, Greenberg MI, eds. PDR Guide to Terrorism Response. Montvale, NJ: Thomson PDR, 2005 p.321-41
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Chen KK, Rose CL. Treatment of acute cyanide poisoning. J Am Med Assoc. 1956;162(12):1154-5.
Dart RC, ed. Medical Toxicology 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2004 p. 172-5
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
Donoghue AM. Alternative methods of administering amyl nitrite to victims of cyanide poisoning. Occup Environ Med. 2003 Feb;60(2):147. [PubMed Citation]
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
Garcia R, Sherpherd G; Cyanide poisoning and its treatment. Pharmacotherapy. 2004 Oct;24(10):1358-65. [PubMed Citation]
Geller RJ, Barthold C, Saiers JA, Hall AH. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006 Nov;118(5):2146-58. [PubMed Citation]
Hall AH , Linden CH, Kulig KW, Rumack BH; Cyanide poisoning from laetrile ingestion: role of nitrite therapy. Pediatrics. 1986 Aug;78:269-272
Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol 2009;39(7):541-52. [PubMed Citation]
Hamel J; A Review of acute cyanide poisoning with a treatment update. Critical Care Nurse. 2011 Feb;31(1):72-82. [PubMed Citation]
Hendricks SK, Ross B, Colvard MA, Cahill D, Shy K, Benedetti TJ. Amyl nitrite: use as a smooth muscle relaxant in difficult preterm cesarean section. Am J Perinatol 1992 Jul;9(4):289-92. [PubMed Citation]
HSDB. Isoamyl nitrite
Johnson WS, Hall AH, Rumack BH. Cyanide poisoning successfully treated without 'therapeutic methemoglobin levels'. Am J Emerg Med. 1989; 7(4):437-440. [PubMed Citation]
Keim ME. Terrorism involving cyanide: the prospect of improving preparedness in the prehospital setting. Prehosp Disaster Med. 2006 Mar-Apr;21(2):s56-60. [PubMed Citation]
Klimmek R, Krettek C. Effects of amyl nitrite on circulation, respiration and blood homoeostasis in cyanide poisoning. Arch Toxicol. 1988 62(2-3):161-6. [PubMed Citation]
Lasch EE and Shawa RE. Multiple cases of cyanide poisoning by apricot kernels in children from Gaza. Pediatrics. 1981; 68(1):5-7.
Lavon O, Bentur Y. Does amyl nitrite have a role in the management of pre-hospital mass casualty cyanide poisoning? Clin Toxicol (Phila). 2010 Jul;48(6):477-84 (2010). [PubMed Citation]
Leavesley HB, Li L, Mukhopadhyay S, Borowitz JL, Isom GE. Nitrite-mediated antagonism of cyanide inhibition of cytochrome c oxidase in dopamine neurons. Toxicol Sci. 2010 Jun; 115(2):569-76. [PubMed Citation]
Ma J, Dasgupta PK, Zelder FH, Boss GR. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2012 Jul 29;736:78-84. [PubMed Citation]
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012
Moore SJ, Norris JC, Walsh DA, Hume AS. Antidotal use of methemoglobin forming cyanide antagonists in concurrent carbon monoxide/cyanide intoxication. J Pharmacol Exp Ther. 1987 Jul;242(1):70-3. [PubMed Citation]
Nakatani T, Kosugi Y, Mori A, Tajimi K, Kobayashi K; Changes in the parameters of oxygen metabolism in a clinical course recovering from potassium cyanide. Am J Emerg Med. 1993 May; 213-7. [PubMed Citation]
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1689-91
Product label: AMYL NITRITE inhalant [James Alexander Corporation] Last revised: July 2010 [DailyMed]
Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Review article: management of cyanide poisoning. Emerg Med Australas. 2012 Jun;24(3):225-38. [PubMed Citation]
Rella J, Marcus S, Wagner BJ. Rapid cyanide detection using the Cyantesmo kit. J Toxicol Clin Toxicol. 2004; 42(6):897-900. [PubMed Citation]
RePORTER. NIH. Countermeasures against chemical threats: countermeasures against cyanide.
RePORTER. NIH. Development of a field-deployable device to rapidly measure blood cyanide levels.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
US NLM. ChemIDplus lite. Amyl nitrite
Vick JA, Froehlich HL. Studies of cyanide poisoning. Arch Int Pharmacodyn Ther 1985 Feb;273(2):314-22. [PubMed Citation]
Vick JA, Froehlich H. Treatment of cyanide poisoning. Mil Med. 1991 Jul;156(7):330-9. [PubMed Citation]
Wurzburg H; Treatment of cyanide poisoning in an industrial setting. Vet Hum Toxicol. 1996 Feb 38(1):44-7. [PubMed Citation]
Yen D, Tsai J, Wang LM, Kao WF, Hu SC, Lee CH, Deng JF. The clinical experience of acute cyanide poisoning. Am J Emerg Med. 1995 Sep;13(5):524-8.
Youso SL, Rockwood GA, Lee JP, Logue BA Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2010 Sep 10;677(1):24-8. [PubMed Citation]
Youso SL, Rockwood GA, Logue BA. The analysis of protein-bound thiocyanate in plasma of smokers and non-smokers as a marker of cyanide exposure. J Anal Toxicol 2012 May;36(4):265-9. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
