You are here: Home > Medical Countermeasures Database > Atropine Sulfate
Atropine Sulfate - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Atropine Sulfate
2. Chemical Defense therapeutic area(s)
— including key possible usesAtropine Sulfate is used for treatment of nerve agent poisoning and organophosphate pesticide poisoning.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
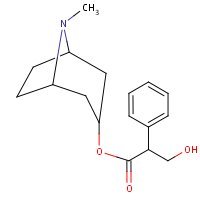
HSDB. Atropine
Mechanism of action
-
The most important therapeutic action of atropine is the inhibition of smooth muscle and glands innervated by postganglionic cholinergic nerves. It also has central nervous system activity, which may be stimulating or depressing depending upon the dose. Following the administration of usual clinical doses, atropine produces stimulation of the medulla and higher cerebral centers. This effect is manifested by mild central vagal excitation and moderate respiratory stimulation. Atropine sulfate also acts peripherally as a competitive antagonist of the muscarinic actions of acetylcholine. It does not prevent the release of acetylcholine but antagonizes the effect of acetylcholine on the effector cells. These actions include vasodilation, drying of the mouth, an increase in the pulse rate, inhibition of contractions of the gastrointestinal tract, ureter, and bladder, and reduction of salivary, bronchial, gastric and sweat gland secretions. Following clinical and larger doses, atropine sulfate causes dilation of the pupils and paralysis of accommodation and in narrow-angle glaucoma, can increase intraocular pressure.
Product Label:
ATROPINE SULFATE injection
[West-ward Pharmaceutical Corp.] Last revised: November
2011[DailyMed]
-
Antimuscarinics competitively inhibit the actions of acetylcholine or other cholinergic stimuli at autonomic effectors innervated by postganglionic cholinergic nerves, and to a lesser extent, on smooth muscles that lack cholinergic innervation. These drugs are referred to as antimuscarinics because at usual doses they principally antagonize cholinergic stimuli at muscarinic receptors and have little or no effect on cholinergic stimuli at nicotinic receptors. Antimuscarinics also have been referred to as anticholinergics (cholinergic blocking agents), but this term is appropriate only when it describes the antagonism of cholinergic stimuli at any cholinergic receptor, whether muscarinic or nicotinic. Since the functions antagonized by antimuscarinics principally are under the parasympathetic division of the nervous system, these drugs also have been referred to as parasympatholytics.
-
At autonomic ganglia, where cholinergic transmission involves nicotinic receptors, atropine or other tertiary amine antimuscarinics produce a partial cholinergic block only at relatively high doses. At the neuromuscular junction, where cholinergic receptors are principally or exclusively nicotinic, only extremely high doses of atropine or other tertiary amine antimuscarinics produce any degree of blockade. However, quaternary ammonium antimuscarinics generally possess varying degrees of nicotinic blocking activity and may interfere with ganglionic or neuromuscular transmission at doses that block muscarinic receptors. At high doses, quaternary ammonium antimuscarinics may produce substantial ganglionic blockade with resultant adverse effects (e.g., impotence, postural hypotension) and, in overdosage, they may cause a curariform neuromuscular block. There are considerable differences among antimuscarinics in the degree to which various pharmacologic effects are produced; this may result in part from existence of 4 or more subtypes of muscarinic receptors. The convention commonly used to classify muscarinic receptors designates pharmacologically defined muscarinic receptor subtypes as M1, M2, M3, and M4. These correspond to the genetically cloned receptor subtypes m1-m4. Although a fifth muscarinic gene product (designated m5) has been cloned, no functional correlate for this receptor has been unambiguously demonstrated, and its pharmacology appears to differ from that of other muscarinic receptor subtypes. Determining the precise location and role of the muscarinic receptor subtypes has been difficult, since multiple subtypes may be expressed in a tissue or cell, and much research remains to be done.
-
Receptors at various sites are not equally sensitive to inhibitory effects of antimuscarinics; therefore, the degree of inhibition at each site is dose-dependent. In general, the relative sensitivity of physiologic functions, proceeding from the most sensitive, is as follows: secretions of the salivary, bronchial, and sweat glands; pupillary dilation, ocular accommodation, and heart rate; contraction of the detrusor muscle of the bladder and smooth muscle of the GI tract; and gastric secretion and motility. An antimuscarinic, in a dose sufficient to depress gastric secretion, will usually also inhibit other, more sensitive functions to some degree. Therefore, if antimuscarinics are used to decrease gastric secretions, they are very likely to cause dryness of the mouth (xerostomia) and interfere with visual accommodation, and possibly cause difficulty in urinating.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
Summary of clinical and non-clinical studies
Nerve agents are phosphorous-containing organic chemicals that inhibit acetylcholinesterase (AChE) (Zilker, 2005). Inhibition of AChE leads to an accumulation of acetylcholine (ACh), which overstimulates glands and results in a range of symptoms including profuse sweating, rhinorrhea, salivation, and lacrimation, convulsions, coma, respiratory distress, and death (Shih and McDonough, 1999; Zilker, 2005). Nerve agents have been used in the Iran-Iraq War and in terrorist attacks in Japan and pose significant threats to both military and civilian populations. Atropine sulfate is an established treatment for muscarinic effects from organophosphate poisoning. Muscarinic effects include pinpoint pupils; blurred or dim vision; conjunctivitis; eye and head pain; hypersecretion by salivary, lacrimal, sweat, and bronchial glands; narrowing of the bronchi; nausea, vomiting, diarrhea, and crampy abdominal pains; urinary and fecal incontinence; and slow heart rate. Atropine sulfate is not an effective treatment for reversal of nicotinic effects which include skeletal muscle twitching, cramping, and weakness. Oximes, including pralidoxime (2-PAM), its chloride, and related oximes such as HI-6 are used as acetylcholinesterase (AChE) reactivators to treat severe OP poisoning with nicotinic and/or CNS manifestations. Seizures caused by CNS effects are treated with a benzodiazepine, such as diazepam. Because atropine sulfate and reactivators act in concert, they are often found together in treatments such as Mark-I kits developed by the military. Organophosphate derivatives treated with atropine sulfate include the military nerve agents soman (GD), sarin (GB), tabun (GA), and VX, a wide range of organophosphate pesticides, and carbamate insecticides that cause acetylcholinesterase inhibition. Atropine sulfate is ineffective against carbamate herbicides and other carbamate derivatives that function primarily through other mechanisms. Although organophosphate pesticides and nerve agents have common mechanisms, responses to exposure differ dramatically since exposure to nerve agents can result in death within a matter of minutes. Since the 1940s there have been numerous studies in human volunteers, in survivors from nerve agent attacks, and in nonclinical models to establish the consequences of exposure to nerve agents and to develop effective treatment regimens. The following provides a few examples demonstrating the efficacy and safety of atropine sulfate as a treatment against nerve agents. Guinea pigs treated with the nerve agent sarin (GB; 100 or 200 μg/kg) intravenously received 10 mg/kg of atropine sulfate alone or in combination with 30 μmol/kg of the oxime HLo-7 or the oxime HI-6 (Worek, Kirchner, and Szinicz, 1995). Respiratory depression and arrest, and subsequent circulatory failure, occurred within a few minutes for animals treated with sarin only. In contrast, respiratory and circulatory parameters improved for the treatment groups. Of the 3 treatment groups, the highest therapeutic efficacy and highest survival rate was observed for the atropine sulfate plus HLo-7 group. Atropine sulfate alone had some therapeutic effect in animals poisoned by 100 μg/kg of GB but was much less effective after 200 μg/kg of GB. In mice administered a 3 x median lethal dose (LD50; 3.45 μmol/kg) combination of GB/cyclosarin (GF), 24-hour median effective dose (ED50) values were 102.5, 18.22, and 1.96 μmol/kg for treatment groups that received atropine sulfate (17.4 mg/kg, IP) in combination with pralidoxime (2-PAM), obidoxime, or HI-6, respectively (Clement, 1994). Atropine sulfate plus HI 6-treated mice were ambulatory, eating, and unlike the other treatment groups, had no tremors. There was 100% mortality for all animals that received atropine sulfate only. Atropine sulfate alone and in combination with oximes administered intravenously was also used to treat organophosphate pesticide poisoning in 63 patients (Balali-Mood and Shariat, 1998). The patients were divided into three treatment groups: atropine alone, atropine plus obidoxime, and atropine plus 2-PAM. No statistically significant differences were observed in the major clinical findings and AChE activity on admission between the groups, but significant changes were observed during the treatment. Of the 3 groups, the severity of intoxication, and thus the number of respiratory complications, was greatest in the atropine plus 2-PAM group. However, no mortality occurred in the atropine plus 2-PAM group, whereas 12% and 50% of the patients died in the atropine only and the atropine plus obidoxime groups, respectively. Although not statistically significant (r=0.4747), AChE reactivation was only observed in the atropine plus 2-PAM group. In other clinical studies, atropine sulfate alone, or in combination with oximes, diazepam, intravenous fluids, and other systemic therapies, and atropine sulfate as a nasal drop formulation, were found to be effective in treating organophosphate toxicity (Yanagisawa, Morita, and Nakajima, 2006; Rajpal et al., 2009). Notably, even large doses of atropine delivered by accidental use of atropine autoinjectors by children, did not produce any life-threatening complications (Amitai et al., 1992). In summary, atropine sulfate in combination with oximes appears to be relatively safe and effective in treating nerve agent toxicity.
B. Link to clinical studies
Studies involving multiple populations
-
Two terrorist attacks with the nerve agent sarin affected citizens in Matsumoto and Tokyo, Japan in 1994 and 1995, killing 19 and injuring more than 6000. Sarin, a very potent organophosphate nerve agent, inhibits acetylcholinesterase (AchE) activity within the central, peripheral, and autonomic nervous systems. Acute and long-term Sarin effects upon humans were well documented in these two events. Sarin gas inhalation caused instantaneous death by respiratory arrest in 4 victims in Matsumoto. In Tokyo, two died in station yards and another ten victims died in hospitals within a few hours to 3 months after poisoning. Six victims with serum ChE below 20% of the lowest normal were resuscitated from cardiopulmonary arrest (CPA) or coma with generalized convulsion. Five recovered completely and one remained in vegetative state due to anoxic brain damage. EEG abnormalities persisted for up to 5 years. Miosis and copious secretions from the respiratory and GI tracts (muscarinic effects) were common in severely to slightly affected victims. Weakness and twitches of muscles (nicotinic effects) appeared in severely affected victims. Neuropathy and ataxia were observed in small number (less than 10%) of victims, which findings disappeared between 3 days and 3 months. Leukocytosis and high serum creatinine kinase (CK) levels were common. Hyperglycemia, ketonuria, low serum triglyceride, hypopotassemia were observed in severely affected victims, which abnormalities were attributed to damage of the adrenal medulla. Oximes, atropine sulfate, diazepam and ample intravenous infusion were effective treatments. Pralidoxime iodide IV reversed cholinesterase and symptoms quickly even if administered 6 h after exposure. Post Traumatic Stress Disorder (PTSD) was less than 8% after 5 years. However, psychological symptoms continue in victims of both incidents. In summary, both potent toxicity and quick recovery from critical ill conditions were prominent features. Conventional therapies proved effective in sarin incidents in Japan. (Class IV)
Yanagisawa N Morita H, Nakajima T. Sarin experiences in Japan: Acute toxicity and long-term effects. J Neurol Sci. 2006 Nov; 249(1):76-85. [PubMed Citation]
Adult
-
Atropine hypersensitivity is a rarely reported condition. However, in the military environment, such reactions are of significant concern given the threat of chemical warfare and the use of atropine as a nerve agent antidote. Upon deployment to regions where chemical attacks are a threat, each service member is issued three 2-mg intramuscular autoinjectors of atropine for self-treatment. In the case presented here, an active duty service member presented to his Aid Station to request red dog tags for a previously identified allergy to atropine. Sensitivity testing revealed a significant reaction to /less than/ 0.03 mg of intradermal atropine. This rarely reported reaction, in the military environment, poses a unique question regarding the suitability of deploying military members to areas where exposure to chemical warfare agents is possible. (Class III)
Hague JD, Derr JJ. Military Implications of Atropine Hypersensitivity Military Medicine 2004 May;169(5):389-91. [PubMed Citation]
-
Nerve agents (NA) (tabun, sarin, soman, VX) have been stocked around the world for some time and still present a major threat to civilian as well as to military populations. Since NA can be delivered through both an aerial spray system and a ballistic system, victims could suffer both NA intoxication and multiple trauma necessitating urgent surgical intervention followed by intensive care. These patients can be expected to be extremely precarious neurologically, respiratorily and hemodynamically. Moreover, their clinical signs can be misleading. Further exacerbating the problem is the fact that interactions of NA with the pharmacological agents used for resuscitation and:or during anesthesia can aggravate organ instability even more and possibly cause systemic collapse. There are no protocols for perioperative critical care and early assessment or for the administration of anaesthesia for surgical interventions in such combined multiple trauma and intoxicated casualties. The authors propose a scheme for the administration of critical care and anesthesia based on the scant anecdotal reports that have emerged after the occurrence of local accidents involving NA intoxication and on the neuropharmacological knowledge of the pesticide organophosphate poisoning database, these compounds being related chemical substances. (Class IV)
Weinbroum AA, Rudick V, Paret G, Kluger Y, Ben Abraham R. Anaesthesia and critical care considerations in nerve agent warfare trauma. Resuscitation 2000 Oct;47(2):113-23. [PubMed Citation]
-
Organophosphate (OP) compounds have been used as pesticides and in chemical warfare (nerve agents). Two nerve agents, tabun and sarin, were used by the Iraqi army against Iranian troops and innocent people. Hundreds of the exposed combatants died in the field. Atropine sulfate has been used successfully in large doses to counteract the muscarinic effects of OP poisoning. The effects of oximes in human OP poisoning have not been well studied. The authors aim was to study the effects of obidoxime and pralidoxime in OP pesticide poisoning. The patients were divided into three groups: atropine (A), obidoxime + atropine (OA) and pralidoxime + atropine (PA). Sixty-three patients (33 males, 30 females) with a mean age of 25 years were studied in different groups (43 A, 22 OA and 8 PA). There were no statistical significant differences in major clinical findings and acetylcholinesterase (AChE) activity on admission between the groups. Significant changes were observed during the treatment. Notwithstanding the severity of intoxication--particularly respiratory complications were more observed in the OA and PA groups--there were no fatalities in the PA group, whereas 4 (9%) and 6 (50%) patients in the A and OA groups died, respectively. AChE reactivation was only observed in the PA group, although it was not statistically significant (r = 0.4747). There was a good relationship between the AChE reactivation and outcome of the patients. High doses of obidoxime (8 mg/kg followed by 2 mg/kg/h) were found to be hepatotoxic and should be avoided. High doses of pralidoxime (30 mg/kg followed by 8 mg/kg/h) did not induce serious side effects and may be effective in some OP pesticides poisoning. (Class III)
Balali-Mood M and Shariat M. Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J Physiol Paris. 1998 92:375-78. [PubMed Citation]
-
The increased use of organophosphate (OP) insecticides and the ever increasing possibility of terror groups using nerve agents underscore the need to develop effective and safe antidotes against OP poisoning. While intramuscular administration of nerve gas antidotes like atropine sulfate has certain lacunae, intravenous route is neither practical nor feasible in the field conditions for mass casualties. The objective was to develop a novel atropine sulfate nasal drop formulation, evaluate and characterize it using scintigraphy and to carry out safety-efficacy study in human volunteers with a view to obtain early pharmacological effects in comparison to the existing options, particularly the conventional intramuscular route. Permeability studies were done using atropine sulphate solution containing variable amount of chitosan. A radiometric method was developed for scintigraphy studies while standard spectroscopy was used for the quantification of atropine sulfate in fluids. Concentration of atropine sulfate in nasal drops to produce therapeutic concentration in blood was calculated. Six volunteers (age range 18-53 years) were administered the formulation delivering 6 mg of atropine sulfate each. Bioavailability and atropinization were noted serially. Based on the results of in vitro, human scintigraphy and analytical data, 1% atropine sulfate-0.5% chitosan was chosen as the final nasal formulation. A human bioavailability curve was created which showed that the therapeutic concentration of the drug in blood was reached within 5 min with nasal drops suggesting that drug delivery through the nasal route is significantly better than the intramuscular route. Unpaired t-test between the means of baseline value of heart rate and that of each time interval showed that increase in heart rate of all the volunteers became significant at 15 min (P<0.01) and extremely significant at 30min (P<0.001). Correlation was evident from 5 min (c>0.7). Pupil diameter showed maximal increase at 30 min (P<0.01). This novel product, 1% atropine sulfate-0.5% chitosan nasal drops might be a safe and efficacious emergency treatment of organophosphorous poisoning with several advantages over the present management, including early atropinization and capability of mass treatment in least amount of time. (Class III)
Rajpal S, Mittal G, Sachdeva R, Chhillar M, Ali R, Agrawal SS, Kashyap R, Bhatnagar A. Development of atropine sulfate nasal drops and its pharmacokinetic and safety evaluation in healthy human volunteers. Environ Toxicol Pharmacol. 2009 27:206-11. [PubMed Citation]
Pediatric studies
-
OBJECTIVE: To evaluate the effects of high doses of atropine in children accidentally injected with automatic atropine injectors. These were distributed in Israel during the Persian Gulf Crisis as an antidote for chemical warfare agents. DESIGN AND SETTING: A national survey in pediatric emergency departments in Israel, involving 22 medical centers, with prospective data collection in 14 centers. PATIENTS: Children (n = 268) presenting to emergency departments following misuse of automatic atropine injectors.
-
MAIN OUTCOME MEASURES: Documentation of atropine dose and clinical manifestations; determination of a clinical severity score and its correlation with atropine dose; measurements of serum atropine levels in six patients. RESULTS: Over a period of 4 months, 268 cases were reported, of which 240 were clinically evaluated. The most common site of injection (75%) was the finger or palm. Doses were up to 17-fold higher than standard doses for age. In 116 children (48%), systemic effects of atropine were observed, and 20 (8%) had severe atropinization. Seizures and life-threatening arrhythmias were not reported, and there were no fatalities. The severity of atropinization was correlated with the dose following a classic nonlinear, dose-response relationship. Serum atropine levels (6.2 to 61.0 ng/mL) were much higher than those observed after administration of therapeutic doses. CONCLUSIONS: The high incidence of injection in the hand implies accidental use of automatic atropine injectors among children. The lack of mortality or life-threatening complications from injection of large doses of atropine attests to its relative safety in children. The low risk from atropine injections weighed against expected benefit as a lifesaving antidote justifies the distribution of personal atropine injectors to children at risk of organophosphorus nerve agent attack. (Class IV)
Amitai Y, Almog S, Singer R, Hammer R, Bentur Y, Danon YL. Atropine poisoning in children during the Persian Gulf crisis. A national survey in Israel. JAMA 1992 Aug;268(5):630-2. [PubMed Citation]
-
Following the FDATs approval of a pediatric dosage Atropen, the Pediatric Expert Advisory Panel was asked to review the existing guidelines and recommendations regarding the treatment of children exposed to nerve agents and the Mark-1 Kit (military Mark 1 kits contain autoinjectors: one autoinjector automatically delivers 2 mg atropine and the other automatically delivers 600 mg 2-PAM Cl); review the new literature on pediatric nerve agent exposure; and to develop recommendations and guidelines for this new device including modifications to the existing recommendations and guidelines if warranted. In May 2003, the first nationally accepted pediatric disaster and terrorism preparedness recommendations and treatment guidelines were issued by the Program for Pediatric Preparedness of the National Center for Disaster Preparedness (NCDP). These guidelines were based on a National Consensus Conference sponsored by the Program for Pediatric Preparedness and funded by the Agency for Healthcare Research and Quality and the EMS for Children Program of the Health Resources and Services Administration. At that time, the only available treatment for certain types of nerve gas exposure (predominantly those with anticholinesterase properties) was the Mark 1 kit. The recommendations were based on established usage of antidotes for cholinergic toxicity and were felt to be both safe and supported by the literature. It was stated that the Mark 1 Auto injector kits (although not approved for pediatric use) should be used as initial treatment for children with severe, life-threatening nerve agent toxicity for whom IV treatment is not possible or available, or for whom more precise IM (mg/kg) dosing would be logistically impossible. It was further felt that while not within the published dosage range for cholinergic toxicity, if a Mark 1 kit was the only source of atropine and pralidoxime available after a bona fide exposure it should be used to treat all children, even those younger than 3 years old. Furthermore, it was felt to be imperative to expedite approval of the pediatric auto injector kit (which contains both atropine and an oxime and is designed for children) that is currently produced and marketed abroad but not available in the United States. (Class IV)
Lynch M. Atropine Use in Children After Nerve Gas Exposure. J Pediatric Nursing 2005 20(6):477-84. [PubMed Citation]
Pregnancy, breast feeding studies
-
Atropine is found in human milk in trace amounts. Caution should be exercised when atropine is administered to a nursing woman (Class IV).
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
-
Atropine has been used as a premedication in obstetric anesthesia, as a test of fetoplacental insufficiency, & in the diagnosis of fetal asphyxia (atropine given to the mother produces changes in the fetal heart rate: first bradycardia, then tachycardia.) There is evidence of rapid placental transfer of atropine (Class IV).
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger, eds. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning, 2nd ed. Baltimore, MD: Williams and Wilkins, 1997, p. 843
-
Atropine crosses the placental barrier. Following IV administration of a single 12.5 mcg/kg dose of atropine sulfate in pregnant women, mean fetal blood (from the placental side of the cord) concentrations of atropine were 1.2 times those of the mother between 5-15 minutes after administration of the drug. In another study, fetal venous blood (from the cord) concentrations of atropine were 12 and 93% of simultaneous maternal venous concentrations 1 and 5 minutes after administration of the drug, respectively; fetal arterial blood (from the cord) concentrations were approximately 50% of simultaneous fetal venous blood concentrations (Class IV).
-
Although atropine, glycopyrrolate, hyoscyamine, and scopolamine cross the placenta, it is not known whether other antimuscarinics cross the placenta. Although atropine has been stated to distribute into milk in small quantities, there are minimal data to support this statement. It is unlikely that quaternary ammonium antimuscarinics distribute into milk; however, studies to determine this have apparently not been conducted (Class IV).
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
Clinical reviews
-
Successful management of incidents with chemical warfare agents strongly depends on the speed of medical help and the ability of helpers to react properly. Though the general principles of clinical toxicology, such as decontamination, stabilization, patient evaluation and symptomatic treatment are similar for many toxicants, chemical warfare agents deserve special attention because of their very high inhalative and cutaneous toxicity, rapid onset of the disease and multiple organ failures. This article describes the medical management of mass casualties with blister agents, nerve agents and blood agents from the viewpoint of a clinical toxicologist. Characteristic diagnostic signs, decontamination procedures and therapeutic schemes for these agents are described. Treatment options are discussed. The importance of planning (e.g. antidote availability) and preparedness is emphasized. (Class IV)
Zilker T Medical management of incidents with chemical warfare agents. Toxicology. 2005 Oct;214(3):221-31. [PubMed Citation]
-
Nerve agents (NA) are simple and cheap to produce but can produce casualties on a massive scale. They have already been employed by terrorist organizations and rogue states on civilians and armed forces alike. By inhibiting the enzyme acetylcholine esterase, NAs prevent the breakdown of the neurotransmitter acetylcholine. This results in over-stimulation of muscarinic and nicotinic receptors in the autonomic and central nervous systems and at the neuromuscular junction. Increased parasympathetic stimulation produces miosis, sialorrhea, bronchospasm and bronchorrhea. Effects at the neuromuscular junction cause weakness, fasciculations, and eventually paralysis. Central effects include altered behavior and mental status, loss of consciousness, seizures, or apnea. Most deaths are due to respiratory failure. Treatment with atropine competitively blocks the parasympathetic effects. Oximes like pralidoxime salvage acetylcholine esterase by "prying off" NA, provided the attachment has not "aged" to an irreversible bond. This reverses weakness. Benzodiazepines like diazepam are effective against NA induced seizures. Mortality has been surprisingly low. If victims can survive the first 15 to 20 min of a vapor attack, they will likely live. The low mortality rate to date underscores that attacks are survivable and research reveals even simple barriers such as clothing offer substantial protection. This article reviews the properties of NAs and how to recognize the clinical features of NA intoxication, employ the needed drugs properly, and screen out anxious patients who mistakenly believe they have been exposed (Class IV).
Cannard K. The acute treatment of nerve agent exposure J Neurol Sci 2006; 249:86-94. [PubMed Citation]
-
Chemical compounds capable of producing injury and death in humans are widely available. From the perspective of the clinician, there may be little difference between a terrorist event and an industrial accident. Both may involve large numbers of casualties, up to the thousands, depending on the method of agent dispersal. Both may involve dispersal by an explosion, thus producing conventionally injured patients as well as those with mixed chemical and conventional injuries. In both scenarios the strain placed on the hospital and the clinicians involved will be enormous. The skills available to the anesthesiologist in airway management and resuscitation will make anesthesia providers a critical resource in the health care system response to such an event. Prior knowledge and understanding of the agents involved, the pathophysiology of the injuries incurred, and the methods of treatment of these injuries will allow early recognition and treatment of the victims (Class IV).
Talmor D. Nonconventional Terror- The Anesthesiologist's Role in a Nerve Agent Event. Anesthesiology Clin 2007; 25:189-199. [PubMed Citation]
-
This review examines the potential use of nerve agents by a terrorist organization against a civilian population, which has become an increasingly apparent threat in the UK. Present guidelines for the use of atropine, particularly in children, following such an event are unclear. No precise agreement exists on the most appropriate dose of atropine, or the frequency with which it should be administered. This uncertainty leaves children vulnerable as potentially life-saving treatment may be crucially delayed. Guidelines must be standardized to allow rapid antidotal delivery and maximise the potential for survivors. This review examines the issues currently surrounding the use of atropine in children following a nerve agent attack and proposes strategies for treating exposed children (Class IV).
Sandilands EA, Good AM, Bateman DN. The use of atropine in a nerve agent response with specific reference to children: are current guidelines. Emerg Med J 2009; 26:690-694. [PubMed Citation]
-
Nerve agents (NAs) are the most lethal chemical weapons. The authors review the pathophysiology and management of NA poisoning of children. NAs cause cholinergic crisis. Children may manifest signs of cholinergic poisoning differently than adults. Children may be less likely to manifest miosis and glandular secretions. They may present with neurologic derangements alone. The goals of treatment should be to limit additional exposure, to provide respiratory support, and to prevent neurologic morbidity. Autoinjectors are optimal delivery vehicles for intramuscular antidotes and are likely to be used in civilian prehospital care. Antidotes include anticholinergics, oximes, and benzodiazepines. Several medications may be available within each class of antidotes. Clinicians will select an antidote based on the status of the individual victim, the accessibility of supportive care, and the availability of the drug. Atropine is well-tolerated and high doses may be required. The oxime pralidoxime chloride has a longer half-life in children. Currently, diazepam is the standard NA anticonvulsant. Midazolam may be the most effective intramuscular anticonvulsant after NA exposure, but, despite its efficacy, it is not an approved agent for seizures. Supportive care and long-term complications are summarized (Class IV).
Rotenberg JS, Jonathan Newmark J. Nerve Agent Attacks on Children: Diagnosis and Management. Pediatrics 2003; 112:648-58. [PubMed Citation]
-
Nerve agents are organophosphate compounds with anticholinesterase activity, which causes a build up of the neurotransmitter acetylcholine in both postsynaptic membrane and motor endplate receptors, leading to a cholinergic crisis. Nerve agents are the most potent weapons in the chemical arsenal and have been used in both military and civilian terrorist attacks in the past several decades (Class IV).
Schecter W. Cholinergic symptoms due to nerve agent attack: a strategy for management. Anesthesiol Clin N Am 2004; 22(3):579-90. [PubMed Citation]
-
Chemical agents have been used previously in wartime on numerous occasions, from World War I to the Gulf War. In 1994 and 1995, sarin nerve gas was used first in peacetime as a weapon of terrorism in Japan. The Tokyo subway sarin attack was the first large-scale disaster caused by nerve gas. A religious cult released sarin gas into subway commuter trains during morning rush hour. Twelve passengers died and about 5500 people were harmed. Sarin is a highly toxic nerve agent that can be fatal within minutes to hours. It causes the clinical syndrome of cholinergic hyperstimulation by inhibition of the crucial enzyme acetylcholinesterase. Therapy of nerve agent toxicity is divided into three categories, decontamination, respiratory support, and antidotes. All of these therapies may be given simultaneously. This article reviews toxicology and management of this acute chemical emergency. To help minimize the possible catastrophic impact on the public, we make several recommendations based on analysis of the Tokyo subway sarin attack and systematically review the current scientific literature (Class IV).
Tokuda Y, Kikuchi M, Takahashi O, Stein GH. Prehospital management of sarin nerve gas terrorism in urban settings: 10 years of progress after the Tokyo subway sarin attack. Resuscitation 2006; 68(2):193-202. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
The ability of the nerve agents tabun, sarin, soman, GF, VR, and VX to produce brain seizures and the effectiveness of the anticholinergics biperiden HCl or atropine SO4 as an anticonvulsant treatment were studied in a guinea-pig model. All animals were implanted a week prior to the experiment with cortical electrodes for electroencephalogram (EEG) recordings. On the day of exposure, the animals were pretreated with pyridostigmine (0.026 mg/kg, i.m.) 30 min prior to challenge with a 2xLD50 dose (s.c.) of a given agent. In separate experiments, animals were challenged with 5xLD50 (sc) of soman. One minute after agent challenge, the animals were treated intramuscularly (i.m.) with 2 mg/kg atropine SO4 admixed with 25 mg/kg 2-PAM Cl and then observed for the onset of seizure activity. Five minutes after the start of nerve agent- induced EEG seizures, animals were treated i.m. with different doses of biperiden HCl or atropine SO4 and observed for seizure termination. The anticonvulsant ED50 of biperiden HCl and atropine SO4 for termination of seizures induced by each nerve agent was calculated and compared. With equally toxic doses (2xLD50) of these agents, continuous EEG seizures (status epilepticus) developed in all animals challenged with soman, tabun, or VR, and in more than 90% of the animals challenged with GF or sarin. In contrast, only 50% of the animals developed seizures when challenged with VX. The times to onset of seizures for soman, tabun, GF, and sarin were very similar (5±8 min) while for VR, it was about 10 min. In the case of VX, not only was the time to seizure development longer (20.7 min), but the seizure activity in 19% of the animals terminated spontaneously within 5 min after onset and did not return. Under these conditions, the anticonvulsant ED50s of biperiden HCl for soman, GF, VR, tabun, sarin, and VX were 0.57, 0.51, 0.41, 0.2, 0.1, and 0.09 mg/kg, respectively, while those of atropine SO4 for soman, VR, tabun, GF, sarin, and VX were 12.2, 11.9, 10.4, 10.3, 5.1, and 4.1 mg/kg, respectively. In separate experiments, the anticonvulsant ED50 doses of biperiden for animals challenged with 2 or 5xLD50 of soman were 0.48 (95% confidence limits 0.25±0.73) or 0.57 (95% CI 0.38±0.84) mg/kg, respectively, while the anti convulsant ED50s for atropine (12.2 mg/kg, i.m.) were identical under these same two challenge conditions. The present study demonstrates that all nerve agents can produce status epilepticus and that the therapeutic effectiveness of atropine and biperiden roughly paralleled the seizurogenic potential of these agents.
Shih T-M, McDonough JH. Efficacy of biperiden and atropine as anticonvulsant treatment for organophosphorus nerve agent intoxication. Arch Toxicol 2000 May;74(3):165-72. [PubMed Citation]
-
Two guinea pig models were used to study the anticonvulsant potency of diazepam, midazolam, and scopolamine against seizures induced by the nerve agents tabun, sarin, soman, cyclosarin, O-ethyl S-(2-(diisopropylamino)ethyl)methylphosphonothioate (VX), and O-isobutyl S-(2-diethylamino)ethyl)- methyl phosphonothioate (VR). Animals instrumented for electroencephalogram recording were pretreated with pyridostigmine bromide (0.026 mg/kg i.m.) 30 min before challenge with 2 xLD50 (s.c.) of a nerve agent. In model A, atropine sulfate (2.0 mg/kg i.m.) and pyridine-2-aldoxime methylchloride (2-PAM; 25.0 mg/kg i.m.) were given 1 min after nerve agent challenge, and the tested anticonvulsant was given (i.m.) 5 min after seizure onset. In model B, a lower dose of atropine sulfate (0.1 mg/kg i.m.) was given along with 2-PAM 1 min after nerve agent challenge, and the anticonvulsant was given at seizure onset. With the lower dose of atropine, seizure occurrence increased to virtually 100% for all agents; the time to seizure onset decreased for sarin, cyclosarin, and VX; the signs of nerve agent intoxication were more severe; and coma resulted frequently with cyclosarin. The anticonvulsant ED50 doses for scopolamine or diazepam were, in general, not different between the two models, whereas the anticonvulsant ED50 values of midazolam increased 3- to 17-fold with the lower atropine dose. Seizure termination times were not systematically effected by the different doses of atropine. The order of anticonvulsant effectiveness within each model was scopolamine > or = midazolam > diazepam. The findings indicate that the dose of atropine given as antidotal therapy can significantly influence measures of nerve agent toxicity and responsiveness to anticonvulsant therapy.
Shih TM, Rowland TC, McDonough JH. Anticonvulsants for Nerve Agent-Induced Seizures: The Influence of the Therapeutic Dose of Atropine. JPET 2007 Jan;320(1):154-161. [PubMed Citation]
-
A total of eight anticholinergic drugs (aprophen, atropine, azaprophen, benactyzine, biperiden, procyclidine, scopolamine, trihexyphenidyl) were tested in parallel with diazepam for the ability to terminate seizure activity induced by the nerve agent soman. Guinea pigs, implanted with electrodes to record cortical electroencephalographic (EEG) activity, were pretreated with pyridostigmine Br (0.026 mg/kg, i.m.) and 30 min later challenged with 2xLD50 soman (56 microg/kg, s.c.) followed 1 min later by treatment with atropine SO4 (2 mg/kg, i.m.) and pralidoxime chloride (2-PAM Cl; 25 mg/kg, i.m.). All guinea pigs developed sustained seizure activity following this treatment. Dose-effect curves were determined for the ability of each drug to terminate seizure activity when anticonvulsant treatment was given either 5 or 40 min after seizure onset. Body weight gain and recovery of behavioral performance of a previously trained one-way avoidance task were measured after exposure. With the exception of atropine, all anticholinergic drugs were effective at lower doses than diazepam in terminating seizures when given 5 min after seizure onset; benactyzine, procyclidine and aprophen terminated seizures most rapidly while scopolamine, trihexyphenidyl, biperiden, and diazepam were significantly slower. When given 40 min after seizure onset, diazepam was the most potent compound tested, followed by scopolamine, benactyzine and biperiden; atropine was not effective when tested 40 min after seizure onset. For diazepam, the time to terminate the seizure was the same whether it was given at the 5- or 40-min delay. In contrast, most anticholinergics were significantly slower in terminating seizure activity when given at the 40-min delay relative to when they were given at the 5-min delay. Successful control of seizure activity, regardless of the drug, was predictive of survival of the lethal effects of nerve agent exposure, a more rapid behavioral recovery (body weight, avoidance performance) and greater protection from neuropathology. In contrast, failure of a drug treatment to terminate seizure activity was closely associated with an increased probability of acute (<24 h) and delayed (10-day survival) lethality, a slower behavioral recovery in survivors, and an increased incidence and degree of neuropathology.
McDonough JH Jr, Zoeffel LD, McMonagle J, Copeland TL, Smith CD, Shih TM. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman intoxicated guinea pigs. Epilepsy Research 2000 Jan;38(1):1-14. [PubMed Citation]
-
The ability of five organophosphorus nerve agents (tabun, sarin, soman, GF, and VX) to produce brain seizures and the effectiveness of atropine as an anticonvulsant treatment against these nerve agents were studied in two different animal models — the rat and guinea pig. All animals were implanted with cortical electrodes for EEG recordings. Five minutes after the start of nerve agent-induced EEG seizures, animals were treated intramuscularly (IM) with different doses of atropine sulfate and observed for seizure termination. The anticonvulsant ED50 of atropine sulfate for termination of seizures induced by each nerve agent was calculated and compared. In the rat model, selected oximes were administered either before, concurrent with, or following challenge with a 1.6xLD 50 dose of a given nerve agent to maximize seizure development with certain agent/oxime combinations. The choice and the timing of oxime administration significantly effected the incidence of seizure development by different nerve agents. When oxime administration did not effect seizure development (tabun, soman) the anticonvulsant ED 50 for atropine sulfate was the same, regardless of the nerve agent used to elicit the seizure. When oxime administration reduced the incidence of seizure occurrence (sarin, GF, VX), the anticonvulsant ED50 dose of atropine sulfate for a nerve agent was lower. In the guinea pig model, animals were pretreated with pyridostigmine prior to challenge with 2xLD 50 of a given agent, and treated 1 min later with atropine sulfate (2 mg/kg) and 2-PAM (25 mg/kg). Under these conditions, the incidence, latency of seizure development, and anticonvulsant ED50 s of atropine for soman-, tabun-, and GF-elicited seizures were virtually identical. With sarin, although the latency of seizure development was the same as with soman, tabun, and GF, seizures occurred with a lower incidence, and the anticonvulsant ED50 of atropine was lower. With VX, the latency of seizure development was notably longer, while the incidence of seizure development and anticonvulsant ED50 of atropine were significantly lower than with soman, tabun, or GF. In both models, a lower incidence of seizure development predicted a lower anticonvulsant dose of atropine. In the rat, the incidence of seizure development and the anticonvulsant effectiveness of atropine was highly dependent on the oxime used. In the guinea pig, higher doses of atropine sulfate were required to control soman-, tabun-, or GF-induced seizures, perhaps reflecting the lower cholinesterase reactivating ability of 2-PAM against these agents.
Shih TM and McDonough JH Jr. Organophosphorus Nerve Agents-Induced Seizures and Efficacy of Atropine Sulfate as Anticonvulsant Treatment. Pharmacol Biochem Behav 1999 Sep;64(1):147-153. [PubMed Citation]
-
This study evaluated the potency and rapidity of some anticholinergics (atropine, biperiden, and trihexyphenidyl) and benzodiazepines (diazepam and midazolam) as an anticonvulsant treatment against seizures induced by six nerve agents (tabun, sarin, soman, cyclosarin, VR, and VX) and summarized the relationship between anticonvulsant activity and nerve agent-induced lethality and neuropathology. Guinea pigs, previously implanted with cortical electrodes for EEG recording, were pretreated with pyridostigmine bromide (0.026 mg/kg im) 30 min prior to challenge with 2xLD50 dose (sc) of a given nerve agent; in a separate experiment, animals were challenged with 5x LD50 sc of soman. One minute after agent challenge the animals were treated im with 2 mg/kg atropine SO4 admixed with 25 mg/kg 2-PAM Cl. Five minutes after the start of EEG seizures, animals were treated im with different doses of anticholinergics or benzodiazepines and observed for seizure termination. The time to seizure onset, the time to seizure termination, and 24-h lethality were recorded. The anticonvulsant ED50 of each drug for termination of seizures induced by each agent was calculated and compared. Brain tissue from animals that survived 24 h was examined for pathology. All drugs were capable of terminating seizure activity, with midazolam and trihexyphenidyl being significantly more potent than the other drugs, and midazolam being more rapid in controlling seizure than atropine, trihexyphenidyl, or diazepam against each agent. Seizures induced by sarin or VX required lower doses of all the test anticonvulsants. The dose of a given drug that was an effective anticonvulsant against a 2xLD50 challenge of soman was equally effective against seizures induced by a 5xLD50 challenge. All nerve agents were capable of producing neuropathology. Seizure control was strongly associated with protection against acute lethality and brain pathology.
Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicology and Applied Pharmacology 2003 188: 69-80. [PubMed Citation]
-
The toxicity of a combination of isopropyl methylphosphonofluoridate (sarin; GB) and cyclohexyl methylphosphonofluoridate (GF) and the efficacy of various oxime reactivators in combination with atropine against the combined GB/GF challenge were evaluated in mice. The 24-h s.c. LD50 of the GB/GF combination was 1.15 mumol/kg (1.10-1.21; 95% confidence limits). Mice administered GB/GF displayed typical signs of nerve agent poisoning such as tremors and convulsions, with death most likely due to anoxia subsequent to respiratory arrest. The GB/GF LD50 value was comparable to the s.c. LD50 of 1.35 and 1.21 mumol/kg for GF and GB in mice, respectively. Combining the two nerve agents did not result in potentiation of the toxicity. In combination with atropine sulfate (17.4 mg/kg, i.p.), which alone did not reduce mortality, the oximes tested, 2-PAM, obidoxime and HI-6, were all effective when administered 5 min before 3 x LD50 dose of GB/GF with 24-h ED50 values of 102.5, 18.22 and 1.96 mumol/kg, respectively. Use of the GB/GF combination does not appear to confer any unique toxicity profile and appears to be easily treated with the standard therapy of a cholinolytic and oxime.
Clement, J.G. Toxicity of the combined nerve agents GB/GF in mice: efficacy of atropine and various oximes as antidotes. Arch Toxicol. 1994 68:64-66. [PubMed Citation]
-
During the past decade the oxime HI-6(1-[[[4-(aminocarbonyl)pyridinio]methoxy]methyl]-2- [(hydroxyimino)methyl] pyridinium dichloride) was shown to improve survival in nerve agent poisoning (in combination with atropine). Recent studies indicate, that HLo 7 (1-[[[4-(aminocarbonyl)pyridinio]methoxy]methyl]-2,4-bis [(hydroxyimino)methyl] pyridinium diiodide or dimethanesulfonate) is also an effective antidote in nerve agent poisoning but, with both oximes, data on restoration of respiration and circulation are scarce. The ability of HLo 7 or HI-6 with atropine to improve the respiratory and circulatory function in sarin-poisoned guinea-pigs was therefore investigated. Female Dunkin-Hartley guinea-pigs were anaesthetised with urethane (1.8 g/kg) and the arteria carotis, vena jugularis and trachea were cannulated. After baseline measurements the animals received 100 or 200 micrograms/kg sarin, and 2 min later the antidotes (all i.v.): 10 mg/kg atropine sulfate or a combination of atropine and HLo 7 or HI 6 (30 mumol/kg, each). Respiratory and circulatory parameters were recorded for the whole experimental period of 60 min or until the death of the animal. Brain and diaphragm acetylcholinesterase (AChE) activity was determined in each animal after the experiment. Poisoning by sarin resulted in a rapid respiratory arrest within 5 min. Atropine treatment was only partially effective in improving respiration after 100 micrograms/kg sarin but was ineffective after 200 micrograms/kg sarin. Therapy of sarin-poisoned animals with atropine plus oxime further improved respiration to various extents, restored circulation and increased survival time, HLo 7 being more effective than HI-6. Diaphragm and brain AChE were reactivated by HLo 7 and, to a minor extent, by HI-6. The results of this investigation suggest, that at equimolar doses (30 mumol/kg) the new bispyridinium dioxime HLo 7 has a higher therapeutic efficacy in sarin-poisoned guinea-pigs when compared to HI-6 (both in combination with atropine).
Worek, F., T. Kirchner, and L. Szinicz. Effect of atropine and bispyridinium oximes on respiratory and circulatory function in guinea-pigs poisoned by sarin. Toxicology. 1995;95:123-133. [PubMed Citation]
-
To develop therapeutics against lung injury and respiratory toxicity following nerve agent VX exposure, we evaluated the protective efficacy of a number of potential pulmonary therapeutics. Guinea pigs were exposed to 27.03 mg/m3 of VX or saline using a microinstillation inhalation exposure technique for 4 min and then the toxicity was assessed. Exposure to this dose of VX resulted in a 24-h survival rate of 52%. There was a significant increase in bronchoalveolar lavage (BAL) protein, total cell number, and cell death. Surprisingly, direct pulmonary treatment with surfactant, liquivent, N-acetylcysteine, dexamethasone, or anti-sense syk oligonucleotides 2 min post-exposure did not significantly increase the survival rate of VX-exposed guinea pigs. Further blocking the nostrils, airway, and bronchioles, VX-induced viscous mucous secretions were exacerbated by these aerosolized treatments. To overcome these events, the authors developed a strategy to protect the animals by treatment with atropine. Atropine inhibits muscarinic stimulation and markedly reduces the copious airway secretion following nerve agent exposure. Indeed, post exposure treatment with atropine methyl bromide, which does not cross the blood-brain barrier, resulted in 100% survival of VX-exposed animals. Bronchoalveolar lavage from VX-exposed and atropine-treated animals exhibited lower protein levels, cell number, and cell death compared to VX-exposed controls, indicating less lung injury. When pulmonary therapeutics were combined with atropine, significant protection to VX-exposure was observed. These results indicate that combinations of pulmonary therapeutics with atropine or drugs that inhibit mucous secretion are important for the treatment of respiratory toxicity and lung injury following VX exposure.
Nambiar MP, Gordon RK, Rezk PE, Katos AM, Wajda NA, Moran TS, Steele KE, Doctor BP, Sciuto AM. Medical countermeasure against respiratory toxicity and acute lung injury following inhalation exposure to chemical warfare nerve agent VX.Toxicology and Applied Pharmacology 2007 Mar;219(2-3):142-150 [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Atropine is rapidly and well absorbed after intramuscular administration. Atropine disappears rapidly from the blood and is distributed throughout the various body tissues and fluids. Much of the drug is destroyed by enzymatic hydrolysis, particularly in the liver; from 13 to 50% is excreted unchanged in the urine. Traces are found in various secretions, including milk.
-
The approximate Cmax of atropine following 1.67 mg atropine given intramuscularly to adults by the 2 mg AtroPen® delivery system was 9.6 ± 1.5 (mean ± SEM) ng/mL. The mean Tmax was 3 minutes. The T1/2 of intravenous atropine in adults 16-58 years is 3.0 ± 0.9 (mean ± SD) hours. The protein binding of atropine is 14 to 22% in plasma. There are gender differences in the pharmacokinetics of atropine. The AUC(0-inf) and Cmax were 15% higher in females than males. The half-life of atropine is slightly shorter (approximately 20 minutes) in females than males.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
-
Atropine is well absorbed from the GI tract. The drug appears to be absorbed principally from the upper small intestine. Atropine is also well absorbed following IM administration, oral inhalation, or endotracheal administration. Following oral administration of a single, radiolabeled, 2-mg dose of atropine in healthy, fasting adults, about 90% of the dose was absorbed. In this study, peak plasma concentrations were reached within 1 hour. Following IM administration, peak plasma concentrations are reached within 30 minutes. Following oral inhalation, atropine appears in serum within 15 minutes and peak concentrations are achieved within 1.5-4 hours.
-
Atropine-induced inhibition of salivation occurs within 30 minutes or 30 minutes to 1 hour and peaks within 1-1.6 or 2 hours after IM or oral administration, respectively; inhibition of salivation persists for up to 4 hours. Atropine-induced increase in heart rate occurs within 5-40 minutes or 30 minutes to 2 hours and peaks within 20 minutes to 1 hour or 1-2 hours after IM or oral administration, respectively. Following IV administration of the drug, peak increase in heart rate occurs within 2-4 minutes. Low doses of the drug cause a paradoxical decrease in heart rate. The ocular effects of atropine are delayed following systemic administration; in one study, near point of accommodation was increased within 2 or 4 hours after IM administration of a single 3-mg dose or oral administration of a single 4-mg dose, respectively. Bronchodilation (as determined by forced expiratory volume in 1 second [FEV1]) occurs within 15 minutes and is maximal within 15 minutes to 1.5 hours after oral inhalation of atropine. Based on peak inhibition of salivation in one study, 0.9-1.4 mg of orally administered drug was estimated to be approximately equivalent in effect to 0.6 mg administered IM.
-
Generally, those antimuscarinics having a quaternary ammonium group are incompletely absorbed from the GI tract since they are completely ionized. Generally, the tertiary amine antimuscarinics are readily absorbed from the GI tract. Tolterodine is well absorbed following oral administration, and absorption of the drug is rapid, with maximum serum concentrations of tolterodine occurring usually within 1-2 hours after administration of a dose. The presence of food in the GI tract may affect absorption of antimuscarinics. Scopolamine is well absorbed percutaneously following topical application. Following IM administration, atropine and glycopyrrolate are rapidly absorbed, reportedly reaching peak concentrations 15-50 minutes and peak antimuscarinic effects 30-45 minutes after administration, respectively. Following oral inhalation of usual doses, limited amounts of atropine or ipratropium reach systemic circulation. Systemic absorption of ipratropium following administration as a nasal spray also is limited but exceeds that resulting from oral inhalation of the drug as a nebulized solution or aerosol.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
-
In one in vitro study, atropine was about 18% bound to serum albumin.
-
Distribution of most antimuscarinics has not been determined. Atropine and glycopyrrolate are apparently rapidly distributed throughout the body since the drugs disappear rapidly from blood after IV administration. Atropine and hyoscyamine readily cross the blood-brain barrier; other tertiary amine antimuscarinics apparently penetrate the CNS since central effects have been observed.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
-
Atropine has a plasma half-life of about 2-3 hours. Following IM administration of atropine in one study, elimination of the drug (determined by urinary excretion of radiolabeled drug) appeared to be biphasic, with a half-life in the initial phase of about 2 hours and a half-life in the terminal phase of 12.5 hours or longer.
-
Atropine is metabolized in the liver to several metabolites including tropic acid, tropine (or a chromatographically similar compound), and, possibly, esters of tropic acid and glucuronide conjugates. Atropine is excreted mainly in urine. Approximately 77-94% of an IM dose of atropine is excreted in urine within 24 hours. About 30-50% of a dose is excreted in urine unchanged. In one study, about 50% of the dose was excreted in urine unchanged; about 33% as unknown metabolites, possibly esters of tropic acid; and less than 2% as tropic acid. In another study, tropine or a chromatographically similar compound was the major metabolite in urine. Small amounts of atropine may also be eliminated in expired air as carbon dioxide and in feces.
-
Antimuscarinics mainly are eliminated in the urine. The drugs generally are excreted in urine as unchanged drug and metabolites. Following oral administration, substantial amounts of antimuscarinics (especially quaternary ammonium compounds) may be eliminated in feces as unabsorbed drug.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
Children
-
The T1/2 of intravenous atropine in pediatric subjects under 2 years is 6.9 ± 3.3 (mean ± SD) hours; in children over 2 years, the T1/2 is 2.5 ± 1.2 (mean ± SD) hours.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
Pregnancy
-
Atropine readily crosses the placental barrier and enters the fetal circulation.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
-
Atropine crosses the placental barrier. Following IV administration of a single 12.5 mcg/kg dose of atropine sulfate in pregnant women, mean fetal blood (from the placental side of the cord) concentrations of atropine were 1.2 times those of the mother between 5-15 minutes after administration of the drug. In another study, fetal venous blood (from the cord) concentrations of atropine were 12 and 93% of simultaneous maternal venous concentrations 1 and 5 minutes after administration of the drug, respectively; fetal arterial blood (from the cord) concentrations were approximately 50% of simultaneous fetal venous blood concentrations. Although atropine has been stated to distribute into milk in small quantities, there are minimal data to support this statement.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
Geriatric
-
The T1/2 of intravenous atropine in adults 16-58 years is 3.0 ± 0.9 (mean ± SD) hours; in geriatric patients 65-75 years it is 10.0 ± 7.3 (mean ± SD) hours.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
Renal Impairment
-
Atropine apparently is not removed by hemodialysis.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
Animal
-
Atropine is well distributed throughout the body. The drug crosses the blood-brain barrier. Following IV administration of a single 0.1 mg/kg dose of radiolabeled atropine in dogs, peak CSF concentrations of the drug were 10.3 ng/mL, about 90% of the peak serum concentration.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
Oral dosage: the usual adult oral dosage of atropine sulfate is 0.4-0.6 mg (range: 0.1-1.2 mg) every 4-6 hours. As with other antimuscarinics, higher than recommended dosage sometimes has been required for therapeutic effect. Dosage should be titrated until therapeutic effect was achieved or adverse effects became intolerable (using the lowest possible effective dosage).
Parenteral dosage: the usual adult IM, IV, or subcutaneous dose of atropine sulfate is 0.4-0.6 mg (range: 0.3-1.2 mg).
Pesticide poisoning: for the treatment of muscarinic toxicity resulting from exposure to organophosphate anticholinesterase pesticides, the usual initial adult dose of atropine sulfate is 1-2 mg, preferably administered IV. Additional 2-mg doses may be administered IM or IV every 5-60 minutes until muscarinic signs and symptoms subside and repeated if they reappear. In severe cases, 2-6 mg may be given initially, preferably administered IV. Additional 2- to 6-mg doses may be administered IM or IV every 5-60 minutes until muscarinic signs and symptoms subside and repeated if they reappear. Subsequently, 0.5-1 mg may be administered orally at intervals of several hours as maintenance therapy until signs and symptoms completely subside. Up to 50 mg of the drug may be required during the first 24 hours; in some severe cases of intoxication, up to 2 g may be required over several days. In severe cases, atropine therapy should be gradually withdrawn to avoid abrupt recurrence of symptoms (e.g., pulmonary edema). Similar doses of atropine sulfate may be used in the treatment of muscarinic toxicity resulting from exposure to carbamate anticholinesterase pesticides. (Utilize Chemical Hazards Emergency Medical Management web site for comprehensive dosing recommendations - CHEMM Editor)
Pesticide poisoning: alternatively, the initial doses of atropine can be administered IM in an out-of-hospital setting. To facilitate out-of-hospital administration, atropine injection is commercially available in a prefilled auto-injector (e.g., AtroPen®); the auto-injector should be used by individuals who have received adequate training in the recognition and treatment of pesticide poisoning. AtroPen® is intended to be used for the initial treatment of muscarinic symptoms of pesticide poisoning (usually breathing difficulty secondary to increased secretions); definitive medical care should be sought immediately. For self-administration or administration by a caregiver in an out-of-hospital setting, the dose of atropine (AtroPen®) is based on severity of symptoms. Atropine should be administered as soon as symptoms of organophosphate or carbamate poisoning (e.g., tearing, excessive oral secretions, wheezing, muscle fasciculations) appear. For the treatment of adults with 2 or more mild symptoms of pesticide exposure (e.g., miosis or blurred vision, tearing, runny nose, hypersalivation or drooling, wheezing, muscle fasciculations, nausea/vomiting) when such exposure is known or suspected, one 2-mg IM dose of atropine sulfate should be administered. If the patient develops any severe symptoms (behavioral changes, severe breathing difficulty, severe respiratory secretions, severe muscle twitching, involuntary defecation or urination, seizures, unconsciousness), two additional 2-mg IM doses should be administered in rapid succession 10 minutes after the first dose. It is preferable that an individual other than the patient administer the second and third doses. For the treatment of adults who are either unconscious or present with any severe symptoms, three 2-mg doses should be administered IM in rapid succession. Additional treatment (i.e., supportive measures, additional doses of atropine, pralidoxime for organophosphate exposure, an anticonvulsant [e.g., diazepam] for seizures) generally is needed, and such treatment should be carried out under the supervision of trained medical personnel.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
Children (FDA)
Oral dosage: the usual oral dosage in children is 0.01 mg/kg or 0.3 mg/m2, but generally not exceeding 0.4 mg, every 4-6 hours.
Parenteral dosage
Parenteral dosage: the usual IM, IV, or subcutaneous dose in children is 0.01 mg/kg or 0.3 mg/m2, but generally not exceeding 0.4 mg. If necessary, these doses may be repeated every 4-6 hours.
Pesticide poisoning: in children, the usual IM or IV dose of atropine sulfate for the treatment of muscarinic toxicity resulting from exposure to organophosphate anticholinesterase pesticides is 0.05 mg/kg, repeated every 10-30 minutes until muscarinic signs and symptoms subside and repeated if they reappear. Similar doses of atropine sulfate may be used in the treatment of muscarinic toxicity resulting from exposure to carbamate anticholinesterase pesticides. (Utilize Chemical Hazards Emergency Medical Management web site for comprehensive dosing recommendations - CHEMM Editor)
Pesticide poisoning: alternatively, the initial doses of atropine can be administered IM in an out-of-hospital setting. To facilitate out-of-hospital administration for infants and children, atropine injection is commercially available in a prefilled auto-injector (e.g., AtroPen®); the auto-injector should be used by individuals who have received adequate training in the recognition and treatment of pesticide poisoning. The AtroPen® auto-injector containing atropine sulfate 0.25, 0.5, or 1 mg is intended for use in children weighing less than 7, 7-18, or 18-41 kg, respectively. AtroPen® is intended to be used for the initial treatment of muscarinic symptoms of pesticide poisoning (usually breathing difficulty secondary to increased secretions); definitive medical care should be sought immediately. When administered by a caregiver in an out-of-hospital setting, the dose of atropine (AtroPen®) is based on severity of symptoms and body weight. Mild symptoms of pesticide exposure include miosis or blurred vision, tearing, runny nose, hypersalivation or drooling, wheezing, muscle fasciculations, and nausea/vomiting. Treatment with atropine is indicated in infants and children with 2 or more mild symptoms of pesticide exposure when such exposure is known or suspected. Severe symptoms of pesticide exposure include behavioral changes, severe breathing difficulty, severe respiratory secretions, severe muscle twitching, involuntary defecation or urination, seizures, and unconsciousness. Treatment is indicated in infants and children who are unconscious or have any severe symptoms of pesticide exposure. Atropine should be administered as soon as symptoms of organophosphate or carbamate poisoning (e.g., tearing, excessive oral secretions, wheezing, muscle fasciculations) appear.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
Pregnancy (FDA)
Adequate animal reproduction studies have not been conducted with atropine. It is not known whether atropine can cause fetal harm when administered to a pregnant woman or if these agents can affect reproductive capacity. Atropine should be administered to a pregnant woman only if clearly needed.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
Atropine was not associated with an increased rate of malformation in a cohort study of pregnancies (401 first trimester, and 797 second and third trimester) , as noted in Bailey. Are there teratogenic risks associated with antidotes used in the acute management of poisoned pregnant women? Birth Defects Research (Part A) 67:133-140 (CHEMM Editor).
Nursing Mothers (FDA)
Atropine is found in human milk in trace amounts. Caution should be exercised when atropine is administered to a nursing woman.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Atropine sulfate has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Formulation
Atropine
Powder
Parenteral injection 0.25 mg/0.3 mL; 0.5 mg/0.7 mL; 1 mg/0.7 mL); 2 mg/0.7 mL Auto-Injector
Atropine Sulfate
Powder
Oral tablets 0.4 mg
Parenteral injection 0.05 mg/mL; 0.1 mg/mL; 0.4 mg/mL; 0.5 mg/mL; 1 mg/mL Atropine Sulfate Injection
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
Pralidoxime Chloride and Atropine
600 mg/2 mL Pralidoxime Chloride and 2.1 mg/0.7 mL Atropine; Auto-Injector (each drug in a separate chamber)
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
Shelf life
Stability
-
Atropine is one of the preferred antidotes for nerve gas exposure. Effective therapy requires large doses. Stockpiling of atropine in forms currently available commercially can be costly and can require a large storage area. Other problems in using the commercial product are the time required to break the large number of ampules needed for treatment, and the large volume of medication that must be administered. A preparation of concentrated atropine for injection from atropine powder is the ideal solution, but the stability of this preparation is unknown. A formulation for concentrated atropine sulfate solution (2 mg/mL) was developed using pharmaceutical-grade powder. The solution was prepared in isotonic saline solution and packaged in polypropylene syringes, which were sealed with syringe caps. Assessment of physical stability under several storage conditions was based on clarity, color, and pH monitoring. Chemical stability of the preparation was determined by using a stability-indicating high-performance liquid chromatographic method. All solutions remained clear and colorless, and the pH changed only slightly over the study period. The potency of the atropine sulfate solution remained above the United States Pharmacopeia limit of 93% for the duration of the study. The solutions were physically compatible and chemically stable when stored for 28 days at 35° C and exposed to light, 364 days at 23° C and exposed to light, or 364 days at 5° C and protected from light. Concentrated solutions of atropine sulfate can be compounded quickly and easily by using commercially available pharmaceutical-grade powder as the active ingredient. The extended shelf-life allows the preparation to be readily available in the field as required.
Donnelly RF, Corman, C. Physical compatibility and chemical stability of a concentrated solution of atropine sulfate (2 mg/mL) for use as an antidote in nerve agent casualties. Int J Pharm Compound. November 2008;12(6):550-2.
-
A massive nerve agent attack may rapidly deplete in-date supplies of atropine. The authors considered using atropine beyond its labeled shelf life. The objective was to determine the stability of premixed injectable atropine sulfate samples with different expiration dates. Methods: This was an in-vitro study using gas chromatography and mass spectrometry (GC/MS). Four atropine solutions (labeled concentration of 400 mg/mL) ranging from in date to 12 years beyond expiration (exp) and an additional sample of atropine sulfate (labeled concentration of 2,000 mg/mL) obtained from a World War II era autoinjector were assayed for atropine stability. Standards of atropine sulfate and tropine were prepared and quantified by GC/MS. Study samples were prepared by adding a buffer solution to free the base, extracting with an isopropanol/methylene chloride mixture and followed by evaporating the organic layer to dryness. Pentafluoropropionic anhydride and pentafluoropropanol were then added as derivatization reagents. Study samples were heated, the derivitization reagents were evaporated, and the remaining compound was reconstituted in ethyl acetate for injection into the GC/MS. All solutions were clear and colorless. Atropine concentrations were as follows: in date, 252 mg/mL; 2001 exp, 290 mg/mL; 1999 exp, 314 mg/mL; 1990 exp, 398 mg/mL; and WW II specimen, 1,475 mg/mL. Tropine was found in concentrations of /less than/10 mg/mL in all study samples. Significant amounts of atropine were found in all study samples. All samples remained clear and colorless, and no substantial amount of tropine was found in any study sample. Further testing is needed to determine clinical effect.
Schier JG, Ravikumar PR, Nelson LS, MD, Heller MB, Mary Ann Howland MA, Hoffman RS. Preparing for Chemical Terrorism: Stability of Injectable Atropine Sulfate. Acad Emerg Med. 2004 April; 11(4):329-34. [PubMed Citation]
-
A capillary gas-liquid chromatographic (GLC) and an ion-pair high performance liquid chromatographic (HPLC) method were developed for the assay of atropine sulfate and obidoxime chloride from a parenteral solution in commercial automatic injection devices. The injectors are aimed for the emergency treatment of poisoning by nerve agents. The two-step GLC method consists of extraction of atropine as a free base prior to GLC analysis using scopolamine as an internal standard. Obidoxime is determined directly in a diluted sample solution by reversed-phase HPLC using sodium 1-heptanesulfonate as a counter ion in the mobile phase. The relative standard deviation (R.S.D.) was I .81% for the GLC procedure with injectors containing only atropine and 2.37% for the GLC of atropine in atropine-obidoxime injectors. The R.S.D.s for the HPLC procedure of obidoxime in atropine obidoxime injectors was 0.82%. The corresponding R.S. D.s for the sampling of atropine-obidoxime injectors were 0.36% and 0.27%. The coefficient of determination (r') was 1.000 for both methods. The recoveries at the target concentration averaged 101.0% and 98.7% with a standard error of the mean of 0.30 for both methods. The retention times for atropine and obidoxime were 6.27 and 3.29 min, respectively.
Pohjola J, Harpf M. Determination of atropine and obidoxime in automatic injection devices used as antidotes against nerve agent intoxication. J Chromatogr A. 1994 Dec;686(2):350-354. [PubMed Citation]
Slep (DOD/FDA)
Storage
-
Atropine sulfate effloresces on exposure to air and is slowly affected by light. Atropine sulfate ophthalmic ointment should be stored in collapsible ophthalmic ointment tubes kept tightly closed and protected from heat at a temperature less than 40°C, preferably between 15-30°C; freezing should be avoided. Atropine sulfate ophthalmic solution should be stored in tight containers at a temperature of 8-27°C, or as specified by the manufacturer; freezing should be avoided. Atropine sulfate injections should be stored in single-dose or multiple-dose containers, preferably of USP Type I glass, at a temperature less than 40°C, preferably between 15-30°C; freezing of the injections should be avoided.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
-
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F). Keep from freezing. Protect from light.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
-
Store at 25°C (77°F); excursions permitted to 15 - 30°C (59 - 86°F) [see USP Controlled Room Temperature]
Keep from Freezing. Protect from Light.
Product Label:
ATNAA (atropine and pralidoxime chloride)
[Meridian Medical Technologies Inc.] Last revised May
2007[DailyMed]
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
THIS DRUG HAS NOT BEEN FOUND BY FDA TO BE SAFE AND EFFECTIVE, AND THIS LABELING HAS NOT BEEN APPROVED BY FDA.
Parasympatholytic agent (ophthalmic solution 1%): for use in producing cycloplegia and mydriasis. Useful for cycloplegic refraction or for pupil dilation in acute inflammatory conditions of the iris and uveal tract. For uveitis administer one or two drops topically to the eye(s) up to four times daily or as directed by a physician. The lacrimal sac should be compressed by digital pressure for two to three minutes after instillation. Heavily pigmented irides may require larger doses.
Product Label: ATROPINE CARE (atropine sulfate) solution/drops [Akorn, Inc.] Last revised: June 2012 [DailyMed]
-
THIS DRUG HAS NOT BEEN FOUND BY FDA TO BE SAFE AND EFFECTIVE, AND THIS LABELING HAS NOT BEEN APPROVED BY FDA.
For mydriasis and/or cycloplegia (ophthalmic ointment 1%): for cycloplegic refraction, for pupillary dilation desired in inflammatory conditions of the iris and uveal tract. A small amount in the conjunctival sac once or twice a day or as directed by a physician.
Product Label:
ATROPINE SULFATE ointment
[Bausch & Lomb Incorporated] Last revised: Aug 2012
[DailyMed]
-
ATROPINE SULFATE injection
Atropine is used as an antidote for pilocarpine, physostigmine, isoflurophate, choline esters, certain species of aminata and in poisoning by the organic phosphate cholinesterase inhibitors found in certain insecticides and by chemical warfare "nerve gases". Large doses relieve the muscarine-like symptoms and some of the central nervous system manifestations. The usual dose of atropine sulfate is 0.4 to 0.6 mg.
Adults suspected of contact with organic phosporous insecticides of the parathion type should be given atropine sulfate 0.8 mg intramuscularly. If an atropine effect is not apparent within 30 minutes or if definite symptoms of the poisoning occur (nausea, vomiting, diarrhea, pupillary constriction, pulmonary edema, fasciculations of eyelids and tongue, jerky ocular movements and excessive sweating, salivation and bronchial secretion), atropine sulfate 2 mg should be given intramuscularly at hourly intervals until signs of atropinization are observed. Up to 2 or 3 times of this dose (4 to 6 mg) may be required in severe cases.
Product Label:
ATROPINE SULFATE injection
[West-ward Pharmaceutical Corp.] Last revised: November
2011[DailyMed]
Children
-
ATROPINE SULFATE injection
Atropine is used as an antidote for pilocarpine, physostigmine,
isoflurophate, choline esters, certain species of aminata and in
poisoning by the organic phosphate cholinesterase inhibitors
found in certain insecticides and by chemical warfare "nerve
gases". Large doses relieve the muscarine-like symptoms and some
of the central nervous system manifestations. Suggested dosages
for pediatric patients are as follows:
7 - 16 lbs. - 0.1
mg 40
- 65 lbs. - 0.3 mg
17 - 24 lbs. - 0.15 mg
65 - 90 lbs. - 0.4 mg
24 - 40 lbs. - 0.2
mg
Over 90 lbs. - 0.4 to 0.6 mg
Product Label:
ATROPINE SULFATE injection
[West-ward Pharmaceutical Corp.] Last revised: November
2011[DailyMed]
8. Route of Administration/Monitoring
-
The Antidote Treatment - Nerve Agent, Auto-Injector (ATNAA) provides Atropine Injection and Pralidoxime Chloride Injection in separate chambers as sterile, pyrogen-free solutions for intramuscular injection. The ATNAA is a specially designed unit for automatic self-or buddy-administration by military personnel. When activated, the ATNAA sequentially administers atropine and pralidoxime chloride through a single needle. The recommended procedure is to inject the contents of the auto-injector into the muscles of an outer thigh or into the buttocks.
Product Label:
ATNAA (atropine and pralidoxime chloride)
[Meridian Medical Technologies Inc.] Last revised May
2007[DailyMed]
-
Treatment of organophosphorous nerve agent and insecticide poisoning should be instituted without waiting for the results of laboratory tests. Red blood cell and plasma cholinesterase, and urinary paranitrophenol measurements (in the case of parathion exposure) may be helpful in confirming the diagnosis and following the course of the illness. A reduction in red blood cell cholinesterase concentration to below 50% of normal has been seen only with organophosphorous ester poisoning.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
9. Adverse effects
-
Atropine sulfate may cause increased intraocular pressure. Topical application of atropine sulfate to the eye may cause adverse systemic effects. Systemic atropine toxicity may be manifested as flushing and dryness of the skin, blurred vision, rapid and irregular pulse, fever.
-
(Infants) abdominal distention in infants, mental aberration, and loss of neuromuscular coordination.
-
Severe systemic reactions to atropine are characterized by hypotension with progressive respiratory depression.
-
Ophthalmic atropine has been associated with cardiac arrhythmias (e.g., atrial fibrillation) in a few patients.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
-
Marked CNS disturbances, ranging from complete disorientation to an active delirium resembling that encountered in atropine overdosage may occur in these patients. Some patients may exhibit marked somnolence. Other manifestations may include dilated pupils, accelerated pulse rate, and dryness of mouth with a husky quality of the voice apparently caused by laryngeal paralysis.
-
Antimuscarinics generally should be used with caution in infants, since there have been a few isolated reports of respiratory distress, seizures, asphyxia, muscular hypotonia, and coma in children 6 weeks of age or younger who received dicyclomine orally. These symptoms reportedly occurred within minutes of ingestion, lasted 20-30 minutes, and eventually resolved with no long-term sequelae. The manufacturer states that because of the timing and nature of these reactions, they probably resulted from local irritation and/or aspiration rather than a direct pharmacologic effect; however, caution should be exercised in this age group.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
-
Individual tolerance varies greatly, but these systemic doses are likely to produce the following effects:
| Dose | Effect |
|---|---|
| 0.5 mg | Slight dryness of nose and mouth, bradycardia |
| 1 mg | Greater dryness of nose and mouth, with thirst; slowing, then acceleration of heart; slight mydriasis. |
| 2 mg | Very dry mouth, tachycardia, with palpitation, mydriasis, slight blurring of near vision. |
| 5 mg | Increase in the above symptoms plus disturbance of speech, difficulty in swallowing, headache, hot, dry skin, restlessness with asthenia |
| 10 mg and over | Above symptoms to extreme degree, plus ataxia, excitement, disorientation, hallucinations, delirium and coma. |
| 65 mg | May be fatal. |
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
-
A scarlatiniform rash may occur. Atropine may produce fever, particularly in children, through inhibition of heat loss by evaporation.
-
Although large doses of atropine may cause an alarming condition, recover is usual.
Product Label:
ATROPINE SULFATE injection
[West-ward Pharmaceutical Corp.] Last revised: November
2011[DailyMed]
-
Prolonged use may produce local irritation characterized by follicular conjunctivitis, vascular congestion, edema, exudate, and an eczematoid dermatitis. Severe reactions are manifested by hypotension with progressive respiratory depression. Coma and death have been reported in the very young.
Product Label: ATROPINE CARE (atropine sulfate) solution/drops [Akorn, Inc.] Last revised: June 2012 [DailyMed]
-
Atropine in toxic amounts leads to drowsiness, stupor, convulsions, & coma. A fatal dose may be as low as 1.6 mg & as high as 100 mg in children. Recovery in an adult has followed ingestion of 1 g. /It was/ observed that 175 ug/mg/kg (12.15 mg intramuscularly) produced initial peripheral autonomic effects such as tachycardia & dryness of mouth. Concomitant with these effects were central disturbances: somnolence, restlessness, ataxia, incoordination, hyperreflexia, hyperthermia & hypertension, disruption of awareness, & incoherent speech or inability to carry out instructions lasting 10-12 hr. Atropine sulfate 0.5 mg via nebulizer every 4 hr led to central anticholinergic intoxication; the patient recovered.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger, eds. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning, 2nd ed. Baltimore, MD: Williams and Wilkins, 1997, p. 843
-
/Symptoms of atropine toxicity/ include dryness of the mouth; flushed, hot, & dry skin; dilated & nonreactive pupils; tachycardia; hallucinations; & restlessness. Atropine toxicity is seen clinically (1) as a manifestation of an unusual sensitivity to a therapeutic dose; (2) after erroneous or excessive amounts have been admin by medical personnel, prescribed by pharmacy error, or voluntarily administered; (3) after covert use (a factitious atropine syndrome); (4) after excessive use of eyedrops by a confused older patient; & (5) occasionally after overexuberant use in the treatment of organophosphate or carbamate poisonings, especially when there is no significant depression of red cell cholinesterase levels. Atropine toxicity may follow oral ingestion; applications of eyedrops or eye ointment; subcutaneous, intramuscular, or iv injections; & inhalation. The clinical responses to both therapeutic doses & overdoses may be similar. Fatalities have followed doses far lower than those that have been tolerated by other individuals. Clinical symptoms correlate poorly with dosage; however, a general correlation of dosage with effects has been suggested.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger, eds. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning, 2nd ed. Baltimore, MD: Williams and Wilkins, 1997, p. 843
10. Contraindication(s)
-
In the face of life-threatening poisoning by organophosphorous nerve agents and insecticides, there are no absolute contraindications for the use of atropine.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
-
Conditions at which inhibition of postganglionic cholinergic nerves are undesirable, such as glaucoma and tachycardia. Also contraindicated in asthma, because the parenteral dose which might relive asthma would have an excessive drying effect upon mucous plugs in the bronchi. Prostatic hypertrophy, while not a contraindication, requires special attention to signs of urinary retention.
Product Label:
ATROPINE SULFATE injection
[West-ward Pharmaceutical Corp.] Last revised: November
2011[DailyMed]
-
Use of antimuscarinics in patients exposed to high environmental temperatures may result in heat prostration. In patients with fever, the risk of hyperthermia may be increased; therefore, antimuscarinics should be used with caution in patients who may be exposed to elevated environmental temperatures or in patients who are febrile. Patients should be advised of the risk of hyperthermia. Since antimuscarinics may produce drowsiness, dizziness, or blurred vision, patients should be warned not to engage in activities requiring mental alertness and/or visual acuity (e.g., operating a motor vehicle or other machinery, performing hazardous work) while taking these drugs.
-
Antimuscarinics should be used with caution in geriatric patients and children since they may be more susceptible to adverse effects of these drugs. Antimuscarinics should also be used with caution in patients with hyperthyroidism, hepatic or renal disease, or hypertension. Antimuscarinics block vagal inhibition of the SA nodal pacemaker and thus should be used with caution in patients with tachyarrhythmias, congestive heart failure, or coronary artery disease. Systemically administered antimuscarinics should be used cautiously in debilitated patients with chronic pulmonary disease, since a reduction in bronchial secretions may lead to inspissation and formation of bronchial plugs; however, antimuscarinics have been used effectively as bronchodilators when administered via oral inhalation. Antimuscarinics should be used with extreme caution in patients with autonomic neuropathy.
-
Antimuscarinics may produce a delay in gastric emptying with possible antral stasis in patients with gastric ulcer; therefore these drugs should be used cautiously in these patients. Antimuscarinics should be used with caution in patients with esophageal reflux or hiatal hernia associated with reflux esophagitis, since the drugs decrease gastric motility and relax the lower esophageal sphincter (decrease lower esophageal pressure); these effects promote gastric retention and aggravate reflux in these patients.
-
Antimuscarinics should be administered with extreme caution to patients with known or suspected GI infections (e.g., Clostridium difficile-associated diarrhea and colitis [also known as antibiotic-associated pseudomembranous colitis], shigellosis, dysentery), since these drugs may decrease GI motility and prolong symptomatology by causing retention of the causative organism or toxin(s). Because diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy, antimuscarinics should also be used with extreme caution in patients with diarrhea. In addition, antimuscarinics may further aggravate the diarrhea. Antimuscarinics should be used with extreme caution in patients with mild to moderate ulcerative colitis, since antimuscarinics may suppress intestinal motility and produce paralytic ileus with resultant precipitation or aggravation of toxic megacolon; the drugs are contraindicated in patients with severe ulcerative colitis or toxic megacolon complicating ulcerative colitis. Antimuscarinics also are contraindicated in patients with obstructive disease of the GI tract (e.g., pyloroduodenal stenosis, achalasia), cardiospasm, paralytic ileus, or intestinal atony (especially in geriatric or debilitated patients).
-
Antimuscarinics are contraindicated in patients with known hypersensitivity to the drugs. The drugs also are contraindicated in patients with angle-closure glaucoma; however, antimuscarinics can be administered safely to patients with open-angle glaucoma who are being treated with miotics. Antimuscarinics should be used with extreme caution in patients with partial obstructive uropathy and are contraindicated in patients with obstructive uropathy (e.g., bladder neck obstruction caused by prostatic hypertrophy). Antimuscarinics are also contraindicated in patients with myasthenia gravis unless the antimuscarinic is used to reduce adverse muscarinic effects of an anticholinesterase agent (e.g., neostigmine).
-
Antimuscarinics are contraindicated in patients with acute hemorrhage whose cardiovascular status is unstable.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesTitle: Study to know the efficacy of higher doses of pralidoxime in patients of organophosphorous poisoning.
Condition: Acute organophosphorus pesticide poisoning
Interventions: Drug: Pralidoxime (drug)
Title: Scopolamine treatment for patients with organophosphate poisoning
Condition: Neurotoxicity Syndromes
Intervention: Drug: Placebo
ClinicalTrials.gov. Atropine Sulfate
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesNo data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations-
Atropine plus pralidoxime with scopolamine add-on
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Critical nerve agent antidotes should be evaluated to determine if they can be safely and effectively administered to infants, children and adults via the intravenous and aerosol routes.
-
The accelerated development of promising antidotes for chemical nerve agents should be pursued.
-
Expanded labeling of currently licensed anticonvulsants for use against chemical nerve agents and their utilization in autoinjectors should be pursued.
-
Objective screening tests that can evaluate behavior and cognitive ability, and can differentiate chemically-induced neurological injury from pre-existing neurological illness need to be developed.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
Critical nerve agent antidotes should be evaluated to determine if they can be safely and effectively administered to infants, children and adults via the intravenous and aerosol routes.
-
The accelerated development of promising antidotes for chemical nerve agents should be pursued.
-
Expanded labeling of currently licensed anticonvulsants for use against chemical nerve agents and their utilization in autoinjectors should be pursued.
-
Objective screening tests that can evaluate behavior and cognitive ability, and can differentiate chemically-induced neurological injury from pre-existing neurological illness need to be developed.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
15. Study-related ethical concerns
— including review panel recommendationsNo data available at this time.
16. Global regulatory status
U.S.
Atropen auto-injector (Atropine
sulfate) injection
[Meridian Medical Technologies, Inc.]
The AtroPen® Auto-Injector is indicated for the treatment of poisoning by susceptible organophosphorous nerve agents having cholinesterase activity as well as organophosphorous or carbamate insecticides. The AtroPen®Auto-Injector should be used by persons who have had adequate training in the recognition and treatment of nerve agent or insecticide intoxication. Pralidoxime chloride may serve as an important adjunct to atropine therapy.
The AtroPen®is intended as an initial treatment of the muscarinic symptoms of insecticide or nerve agent poisonings (generally breathing difficulties due to increased secretions); definitive medical care should be sought immediately. The AtroPen® Auto-Injector should be administered as soon as symptoms of organophosphorous or carbamate poisoning appear (usually tearing, excessive oral secretions, wheezing, muscle fasciculations, etc.). In moderate to severe poisoning, the administration of more than one AtroPen® may be required until atropinization is achieved (flushing, mydriasis, tachycardia, dryness of the mouth and nose)... In severe poisonings, it may also be desirable to concurrently administer an anticonvulsant if seizure is suspected in the unconscious individual since the classic tonic-clonic jerking may not be apparent due to the effects of the poison. In poisonings due to organophosphorous nerve agents and insecticides it may also be helpful to concurrently administer a cholinesterase reactivator such as pralidoxime chloride.
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
Atnaa (Atropine and Pralidoxime
Chloride)
[MERIDIAN MEDICAL TECHNOLOGIES INC]
The ATNAA is indicated for the treatment of poisoning by susceptible organophosphorous nerve agents having anticholinesterase activity.
Product Label:
ATNAA (atropine and pralidoxime chloride)
[Meridian Medical Technologies Inc.] Last revised May
2007[DailyMed]
DuoDote is indicated for the treatment of poisoning by organophosphorous nerve agents as well as organophosphorous insecticides.
Product Label:
DUODOTE (atropine and pralidoxime chloride) kit
[Meridian Medical Technologies , Inc.] Last revised Sep
2009[DailyMed]
E.U.
Adults: 2 mg IM or IV every 5 to 60 min according to severity; continuation up to 24 h, 50 mg max
Children: 0.002-0.08 mg/kg IM or IV; continuation up to 24 h
Multidose preparations containing preservatives should be avoided in order to prevent the potential toxicity related to the preservatives.
Treatment may be needed for several days, especially for highly toxic organophosphate pesticides.
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
U.K.
Premedication; intra-operative bradycardia; with anticholinesterases for reversal of non-depolarising neuromuscular block; antidote to organophosphorous poisoning; symptomatic relief of gastro-intestinal disorders characterised by smooth muscle spasm; bradycardia; cardiopulmonary resuscitation; cycloplegia, anterior uveitis
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 703
17. Other potentially useful information
-
Patients exposed to vapor who have miosis and rhinorrhea will need no care unless (a) they have eye or head pain or nausea and vomiting; under these circumstances topical atropine or homatropine in the eye should relieve the symptoms and the patient can be discharged within an hour or so; or (b) the rhinorrhea is very severe; under these circumstances, atropine IM (2 mg in adults and 0.05 mg/kg in children) should relieve this and the patient can be discharged in an hour or so. Topical atropine and homatropine should not be used routinely for miosis because they cause visual impairment for about 24 hours.
ATSDR. Medical Management Guidelines for Nerve Agents: Tabun (GA); Sarin (GB); Soman (GD); and VX
CAS# Tabun (GA) 77-81-6, Sarin (GB) 107-44-8, Soman (GD) 96-64-0, VX 5078269-9
-
Octanol/Water Partition Coefficient: log Kow = 1.83
HSDB.
Atropine
18. Publications
Amitai Y, Almog S, Singer R, Hammer R, Bentur Y, Danon YL. Atropine poisoning in children during the Persian Gulf crisis. A national survey in Israel. JAMA 1992 Aug;268(5):630-2. [PubMed Citation]
ATSDR. Medical Management Guidelines for Nerve Agents: Tabun (GA); Sarin (GB); Soman (GD); and VX
CAS# Tabun (GA) 77-81-6, Sarin (GB) 107-44-8, Soman (GD) 96-64-0, VX 5078269-9
Balali-Mood M and Shariat M. Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J Physiol Paris. 1998 92:375-78. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Cannard K. The acute treatment of nerve agent exposure J Neurol Sci 2006; 249:86-94. [PubMed Citation]
Clement, J.G. Toxicity of the combined nerve agents GB/GF in mice: efficacy of atropine and various oximes as antidotes. Arch Toxicol.1994 68:64-66. [PubMed Citation]
ClinicalTrials.gov. Atropine Sulfate
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
Donnelly RF, Corman, C. Physical compatibility and chemical stability of a concentrated solution of atropine sulfate (2 mg/mL) for use as an antidote in nerve agent casualties. Int J Pharm Compound. November 2008;12(6):550-2.
Ellenhorn, M.J., S. Schonwald, G. Ordog, J. Wasserberger, eds. Ellenhorn's Medical Toxicology: Diagnosis and Treatment of Human Poisoning, 2nd ed. Baltimore, MD: Williams and Wilkins, 1997, p. 843
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
Hague JD, Derr JJ. Military Implications of Atropine Hypersensitivity Military Medicine 2004 May;169(5):389-91. [PubMed Citation]
HSDB. Atropine
Lynch M. Atropine Use in Children After Nerve Gas Exposure. J Pediatric Nursing 2005 20(6):477-84. [PubMed Citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 703
McDonough JH Jr, Zoeffel LD, McMonagle J, Copeland TL, Smith CD, Shih TM. Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman intoxicated guinea pigs. Epilepsy Research 2000 Jan;38(1):1-14. [PubMed Citation]
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 1277-89
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
Nambiar MP, Gordon RK, Rezk PE, Katos AM, Wajda NA, Moran TS, Steele KE, Doctor BP, Sciuto AM. Medical countermeasure against respiratory toxicity and acute lung injury following inhalation exposure to chemical warfare nerve agent VX.Toxicology and Applied Pharmacology 2007 Mar;219(2-3):142-150 [PubMed Citation]
Pohjola J, Harpf M. Determination of atropine and obidoxime in automatic injection devices used as antidotes against nerve agent intoxication. J Chromatogr A. 1994 Dec;686(2):350-354. [PubMed Citation]
Product Label:
ATNAA (atropine and pralidoxime chloride)
[Meridian Medical Technologies Inc.] Last revised May
2007[DailyMed]
Product Label:
ATROPEN auto-injector (atropine sulfate) injection
[Meridian Medical Technologies, Inc.] Last revised: July 2007
[DailyMed]
Product Label: ATROPINE CARE (atropine sulfate) solution/drops [Akorn, Inc.] Last revised: June 2012 [DailyMed]
Product Label:
ATROPINE SULFATE ointment
[Bausch & Lomb Incorporated] Last revised: Aug 2012
[DailyMed]
Product Label:
ATROPINE SULFATE injection
[West-ward Pharmaceutical Corp.] Last revised: November
2011[DailyMed]
Product Label:
DUODOTE (atropine and pralidoxime chloride) kit
[Meridian Medical Technologies , Inc.] Last revised Sep
2009[DailyMed]
Rajpal S, Mittal G, Sachdeva R, Chhillar M, Ali R, Agrawal SS, Kashyap R, Bhatnagar A. Development of atropine sulfate nasal drops and its pharmacokinetic and safety evaluation in healthy human volunteers. Environ Toxicol Pharmacol. 2009;27:206-11. [PubMed Citation]
Rotenberg JS, Jonathan Newmark J. Nerve Agent Attacks on Children: Diagnosis and Management. Pediatrics 2003; 112:648-58. [PubMed Citation]
Sandilands EA, Good AM, Bateman DN. The use of atropine in a nerve agent response with specific reference to children: are current guidelines. Emerg Med J 2009; 26:690-694. [PubMed Citation]
Schecter W. Cholinergic symptoms due to nerve agent attack: a strategy for management. Anesthesiol Clin N Am. 2004;22(3):579-90. [PubMed Citation]
Schier JG, Ravikumar PR, Nelson LS, MD, Heller MB, Mary Ann Howland MA, Hoffman RS. Preparing for Chemical Terrorism: Stability of Injectable Atropine Sulfate. Acad Emerg Med. 2004 April; 11(4):329-34. [PubMed Citation]
Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicology and Applied Pharmacology 2003 188: 69-80. [PubMed Citation]
Shih TM and McDonough JH Jr. Organophosphorus Nerve Agents-Induced Seizures and Efficacy of Atropine Sulfate as Anticonvulsant Treatment. Pharmacol Biochem Behav 1999 Sep;64(1):147-153. [PubMed Citation]
Shih T-M, McDonough JH. Efficacy of biperiden and atropine as anticonvulsant treatment for organophosphorus nerve agent intoxication. Arch Toxicol 2000 May;74(3):165-72. [PubMed Citation]
Shih TM, Rowland TC, McDonough JH. Anticonvulsants for Nerve Agent-Induced Seizures: The Influence of the Therapeutic Dose of Atropine. JPET 2007 Jan;320(1):154-161. [PubMed Citation]
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
Talmor D. Nonconventional Terror- The Anesthesiologist's Role in a Nerve Agent Event. Anesthesiology Clin 2007; 25:189-199. [PubMed Citation]
Tokuda Y, Kikuchi M, Takahashi O, Stein GH. Prehospital management of sarin nerve gas terrorism in urban settings: 10 years of progress after the Tokyo subway sarin attack. Resuscitation 2006; 68(2):193-202. [PubMed Citation]
Weinbroum AA, Rudick V, Paret G, Kluger Y, Ben Abraham R. Anaesthesia and critical care considerations in nerve agent warfare trauma. Resuscitation 2000 Oct;47(2):113-23. [PubMed Citation]
Worek, F., T. Kirchner, and L. Szinicz. Effect of atropine and bispyridinium oximes on respiratory and circulatory function in guinea-pigs poisoned by sarin. Toxicology. 1995; 95:123-133. [PubMed Citation]
Yanagisawa N Morita H, Nakajima T. Sarin experiences in Japan: Acute toxicity and long-term effects. J Neurol Sci. 2006 Nov;249(1):76-85. [PubMed Citation]
Zilker T Medical management of incidents with chemical warfare agents. Toxicology. 2005 Oct;214(3):221-31. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
