You are here: Home > Medical Countermeasures Database > Diazepam
Diazepam - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Diazepam
2. Chemical Defense therapeutic area(s)
— including key possible usesThe anticonvulsant diazepam has been used therapeutically in the treatment of both convulsant (e.g., picrotoxin, hydrazine, strychnine, tetramethylenedisulfotetramine) and cholinergic (e.g., sarin (GB), soman (GD), cyclosarin (GF), tabun (GA), VX, and organophosphorus pesticides) agents to enhance the inhibitory effect of gamma-aminobutyric acid at GABA(a) receptors and limit excess central nervous system excitation and seizures.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
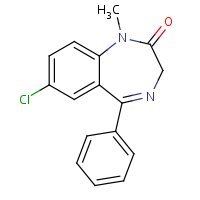
HSDB. Diazepam
Mechanism of action
-
In animals, diazepam appears to act on parts of the limbic system, the thalamus and hypothalamus, and induces calming effects. Diazepam, unlike chlorpromazine and reserpine, has no demonstrable peripheral autonomic blocking action, nor does it produce extrapyramidal side effects. However, animals treated with diazepam do have a transient ataxia at higher doses. Diazepam was found to have transient cardiovascular depressor effects in dogs, Long-term experiments in rats revealed no disturbances of endocrine function. Injections into animals have produced localized irritation of tissue surrounding injection sites and some thickening of veins after intravenous use. A study performed in 24 healthy male subjects comparing the I.M. Injection of 10 mg of diazepam in the mid-anterior/lateral thigh by the autoinjector versus 10 mg I.M. by a syringe (operated manually) indicates that the mean percent availability of the drug from the autoinjector is 100% of that obtained from the syringe.
Product label Diazepam Auto-injector [Meridian Medical Technologies, Inc.] Last revised: September 1997 [FDA]
-
Non-selective benzodiazepine (BZ) binding-site full agonists, exemplified by diazepam, act by enhancing the inhibitory effects of GABA at GABA(A) receptors containing either an alpha1, -2, -3 or -5 subunit. However, despite their proven clinical anxiolytic efficacy, such compounds possess a relatively narrow window between doses that produce anxiolysis and those that cause sedation, and are also associated with physical dependence and a potential for abuse. In the late 1980s and early 1990s a number of non-selective partial agonists, exemplified by bretazenil, pazinaclone and abecarnil, were described. Their reduced intrinsic efficacy relative to full agonists such as diazepam resulted in an improved preclinical pharmacological profile in that there was a large window between anxiolytic and sedative doses and their dependence and abuse liabilities were much lower. Unfortunately, these compounds failed, for a variety of reasons, to translate into clinical benefit, and as the public perception of BZs deteriorated interest in the area waned. However, the advent of molecular genetic and pharmacological approaches has begun to delineate which GABA(A) receptor subtypes are associated with the various pharmacological effects of the non-selective BZs. More specifically, the alpha2- and/or alpha3-containing GABA(A) receptors play a role in anxiety whereas the alpha1 subtype is involved in sedation, raising the possibility of a compound that selectively modulates alpha2- and/or alpha3-containing receptors but does not affect alpha1-containing receptors would be a non-sedating anxiolytic. In order to achieve selectivity for the alpha2/alpha3 subtypes relative to alpha1, two approaches may be used; selective affinity or selective efficacy. Selective affinity relies on a compound binding with higher affinity to the alpha2/alpha3 compared with alpha1 subtypes, but to date no such compounds have been described. On the other hand, subtype-selective efficacy relies on a compound binding to all subtypes but having different efficacies at various subtypes (relative selective efficacy, for example SL654198 or pagoclone) or having efficacy at some subtypes but none at others (absolute selective efficacy; for example, L-838417). The status of these and other BZ site compounds with claimed, but often not explicitly stated, GABA(A) subtype selectivity (such as ELB-139 and ocinaplon) will be reviewed in relation to their development as non-sedating anxiolytics for the treatment of generalised anxiety disorder.
Atack JR; The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs. 2005; 14 (5): 601-618. [PubMed Citation]
Summary of clinical and non-clinical studies
Organophosphorous (OP) insecticides and nerve agents are potent irreversible acetylcholinesterase (AChE) inhibitors, which subsequently cause hyperstimulation of the cholinergic receptors in the peripheral and central nervous systems (Joosen, van der Schans, Helden, 2010; Shih, Rowland, McDonough, 2007; Castro et al., 1992). Symptoms of OP intoxication in the central nervous system include seizures, status epilepticus (SE) and convulsions (Todorovic et al., 2012). Many Western countries pre-treat military populations with the reversible cholinesterase inhibitor pyridostigmine bromide to protect against OP intoxication, but its inability to cross the blood brain barrier leaves central AChE unprotected (Masson, 2011; Collombet, 2011). Standard treatment following OP intoxication encompasses an anticholinergic agent such as atropine, an AChE reactivator (oxime), and an anticonvulsant, such as diazepam. The inclusion of diazepam (Valium) in the treatment regimen for OP intoxication has been shown to be beneficial against convulsions and associated brain lesions (Harris et al., 1994). In rats, 10 mg/kg diazepam was effective in terminating SE induced by the OP agents paraoxon and diisopropyl fluorophosphates (DFP), although DFP induced SE became resistant to treatment with diazepam as SE progressed to 30 minutes (Todorovic et al., 2012). There was also a progressive loss in the anticonvulsant efficacy of diazepam if the treatment was delayed after seizure onset, as observed in guinea pigs exposed to soman (McDonough, McMonagle, Shih, 2010). Avizafone, a diazepam pro-drug used by the French military, and is able to terminate OP-induced epileptic seizures but only if it is injected in the first 20 minutes following nerve agent exposure (Collombet, 2011). Diazepam also decreased the incidence of convulsions and attenuated the cognitive impairment in rhesus monkeys exposed to the nerve agent soman (Castro et al., 1991). The diazepam-treated group was performing at 90% of their pre-soman exposure levels by Day 6 in contrast to the nondiazepam-treated group which did not perform at their pre-soman levels until Day 15. The therapeutic efficacy of atropine, obidoxime and diazepam was proven to be more effective when administered repetitively than when administered as a single shot treatment (Joosen, 2010). Diazepam is highly lipophilic, which allows it to enter the brain quickly but also quickly redistribute, allowing seizures to possibly recur if repetitive injections are not given (Rotenberg and Newmark, 2003). Diazepam, currently available in intra-muscular autoinjectors, has the potential for abuse, and in the absence of a nerve agent may affect mental alertness and physical dexterity. Several anticholinergic agents have been tested and compared to diazepam as a possible alternative in treating nerve agent seizures (McDonough et al., 2000; Harris et al., 1994; Shih et al., 2003). With the exception of atropine, eight anticholinergic drugs tested in parallel with diazepam for the ability to terminate soman-induced seizures in guinea pigs, were more effective at lower doses than diazepam at terminating seizures when given 5 minutes after seizure onset (McDonough et al., 2000). However, at 40 minutes after seizure onset, diazepam was the most potent compound tested, which may be more beneficial in a typical setting. The efficacy of diazepam is dependent on concurrent treatment with atropine unlike scopolamine (Harris et al., 1994) and a high dosage of atropine was needed to antagonize the added incapacitating effects of diazepam on motor performance in OP insecticide (dichlorvos) treated rats. Additionally, at 33.8 mg/kg atropine markedly increased the seizure threshold and prevented early respiratory distress induced by sarin in guinea pigs (Taysse et al., 2003). When 2 mg/kg of diazepam was administered concomitantly with pyrostigmine, pralidoxime, and 33.8 mg/kg of atropine, seizures were not observed but 62.5% of the animals displayed respiratory difficulties, which was not observed with avizafone. It appears that although replacements for the use of diazepam are being investigated, its efficacy as an anticonvulsant are limited by its time sensitivity and may be influenced by the dose of atropine which also has anticonvulsant properties (Shih, Rowland, McDonough, 2007).
B. Link to clinical studies
Pregnancy, breastfeeding studies
-
A 1989 report described dysmorphic features, growth restriction, and CNS defects in eight infants exposed either to diazepam, 30 mg/day or more, or oxazepam, 75 mg/day or more, throughout gestation. Three of the mothers denied use of drugs during pregnancy, but diazepam and its metabolite were demonstrated in their plasma in early pregnancy. The mothers did not use alcohol or street drugs, had regular prenatal care, and had no record of criminality or prostitution. The mean birth weight of the infants was 1.2 standard deviations below the Swedish average, only one having a weight above the mean, and one was small for gestational age. Six of the newborns had low Apgar scores primarily due to apnea, five needed resuscitation, all were hypotonic at birth, and all had neonatal drug withdrawal with episodes of opisthotonos and convulsions. Seven of the eight infants had feeding difficulties caused by a lack of rooting and sucking reflexes. Craniofacial defects observed in the infants (number of infants with defect shown in parentheses) were short nose with low nasal bridge (six), up tilted nose (six), slanted eyes (eight), epicanthic folds (eight), telecanthus (two), long eyelashes (three), highly arched palate (four), cleft hard palate and bifid uvula (two), low-set/abnormal ears (four), webbed neck (three), flat upper lip (five), full lips (four), hypoplastic mandible (five), and microcephaly (two). Other defects present were small, wide-spaced nipples (two), renal defect (one), inguinal hernia (two), and cryptorchidism (two). An infant with severe psychomotor retardation died of possible sudden infant death syndrome at 11 weeks of age. Microscopic examination of the brain demonstrated slight cortical dysplasia and an increased number of single-cell neuronal heterotopias in the white matter. Six other children had varying degrees of mental retardation, some had severely disturbed visual perception, all had gross motor disability, and hyperactivity and attention deficits were common. Extensive special examinations were conducted to identify other possible etiologies, but the only common factor in the eight cases was maternal consumption of benzodiazepines. Based on the apparent lack of other causes, the investigators concluded that the clinical characteristics observed in the infants probably represented a teratogenic syndrome due to benzodiazepines (Class IV).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.399-403
-
In a case of gross overdose, a mother who took 580 mg of diazepam as a single dose on about the 43rd day of gestation delivered an infant with cleft lip and palate, craniofacial asymmetry, ocular hypertelorism, and bilateral periauricular tags. The authors concluded that the drug ingestion was responsible for the defects. An association between diazepam and an increased risk of cleft lip or palate has been suggested by several studies. The findings indicated that 1st or 2nd trimester use of diazepam, and selected other drugs, is significantly greater among mothers of children born with oral clefts. However, a review of these studies, published in 1976, concluded that a causal relationship between diazepam and oral clefts had not yet been established, but even if it had, the actual risk was only 0.2% for cleft palate and only 0.4% for cleft lip with or without cleft palate (Class IV).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.399-403
-
Large retrospective studies showing no association between diazepam and cleft lip/palate have been published. The results of one of these studies has been criticized and defended. Although no association was found with cleft lip/palate, a statistically significant association was discovered between diazepam and inguinal hernia (Class IV).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.399-403
Clinical reviews
-
Sarin and VX were released on civilians in Japan on 11 occasions in the period 1994 to 1995. Clinicians must be prepared, therefore, to treat casualties from nerve agent exposure. This requires an understanding of the mechanisms of nerve agent toxicity and the factors that influence their clinical impact. Clinicians need to be able to make a rapid and accurate diagnosis and use atropine, an oxime and diazepam optimally (Class IV).
Vale A, Marrs TC, RiceP; Chemical terrorism and nerve agents. Medicine (UK) 2012; 40 (2): 77-79.
-
After more than 70 years of considerable efforts, research on medical defense against nerve agents has come to a standstill. Major progress in medical countermeasures was achieved between the 50's and 70's with the development of anticholinergic drugs and carbamate-based pretreatment, the introduction of pyridinium oximes as antidotes, and benzodiazepines in emergency treatments. These drugs ensure good protection of the peripheral nervous system and mitigate the acute effects of exposure to lethal doses of nerve agents. However, pyridostigmine and cholinesterase reactivators currently used in the armed forces do not protect/reactivate central acetylcholinesterases. Moreover, other drugs used are not sufficiently effective in protecting the central nervous system against seizures, irreversible brain damages and long-term sequelae of nerve agent poisoning. New developments of medical counter-measures focus on: (a) detoxification of organophosphorus molecules before they react with acetylcholinesterase and other physiological targets by administration of stoichiometric or catalytic scavengers; (b) protection and reactivation of central acetylcholinesterases, and (c) improvement of neuroprotection following delayed therapy. Future developments will aim at treatment of acute and long-term effects of low level exposure to nerve agents, research on alternative routes for optimizing drug delivery, and therapies. Though gene therapy for in situ generation of bioscavengers, and cell therapy based on neural progenitor engraftment for neuronal regeneration have been successfully explored, more studies are needed before practical medical applications can be made of these new approaches (Class IV).
Masson P; Evolution of and perspectives on therapeutic approaches to nerve agent poisoning. Toxicology Letters 2011; 206 (1): 5-13. [PubMed Citation]
-
The structure and biologic action of nerve agents is similar to organophosphates, commonly used as insecticides. Acetylcholine accumulation and binding to the cholinergic receptor site stimulates the affected organs producing a predictable set of clinical symptoms. Treatment of the affected patients will include decontamination, respiratory and hemodynamic support, as well as specific antidotes. The multiple casualties that may be expected present additional logistical and organizational problems. The specific skills of anesthesiologists will make them invaluable members of the care team in such a chemical mass casualty event (Class IV).
Talmor D; Nonconventional Terror- The Anesthesiologist's Role in a Nerve Agent Event. Anesthesiology Clin 2007; 25 (1): 189-199. [PubMed Citation]
-
Nerve agents (NA) are simple and cheap to produce but can produce casualties on a massive scale. They have already been employed by terrorist organizations and rogue states on civilians and armed forces alike. By inhibiting the enzyme acetylcholine esterase, NAs prevent the breakdown of the neurotransmitter acetylcholine. This results in over-stimulation of muscarinic and nicotinic receptors in the autonomic and central nervous systems and at the neuromuscular junction. Increased parasympathetic stimulation produces miosis, sialorrhea, bronchospasm and bronchorrhea. Effects at the neuromuscular junction cause weakness, fasciculations, and eventually paralysis. Central effects include altered behavior and mental status, loss of consciousness, seizures, or apnea. Most deaths are due to respiratory failure. Treatment with atropine competitively blocks the parasympathetic effects. Oximes like pralidoxime salvage acetylcholine esterase by "prying off" NA, provided the attachment has not "aged" to an irreversible bond. This reverses weakness. Benzodiazepines like diazepam are effective against NA induced seizures. Mortality has been surprisingly low. If victims can survive the first 15 to 20 min of a vapor attack, they will likely live. The low mortality rate to date underscores that attacks are survivable and research reveals even simple barriers such as clothing offer substantial protection. This article reviews the properties of NAs and how to recognize the clinical features of NA intoxication, employ the needed drugs properly, and screen out anxious patients who mistakenly believe they have been exposed (Class IV).
Cannard K; The acute treatment of nerve agent exposure. J Neurol Sci 2006; 249 (1): 86-94. [PubMed Citation]
-
Nerve agents (NAs) are the most lethal chemical weapons. We review the pathophysiology and management of NA poisoning of children. NAs cause cholinergic crisis. Children may manifest signs of cholinergic poisoning differently than adults. Children may be less likely to manifest miosis and glandular secretions. They may present with neurologic derangements alone. The goals of treatment should be to limit additional exposure, to provide respiratory support, and to prevent neurologic morbidity. Autoinjectors are optimal delivery vehicles for intramuscular antidotes and are likely to be used in civilian prehospital care. Antidotes include anticholinergics, oximes, and benzodiazepines. Several medications may be available within each class of antidotes. Clinicians will select an antidote based on the status of the individual victim, the accessibility of supportive care, and the availability of the drug. Atropine is well-tolerated and high doses may be required. The oxime pralidoxime chloride has a longer half-life in children. Currently, diazepam is the standard NA anticonvulsant. Midazolam may be the most effective intramuscular anticonvulsant after NA exposure, but, despite its efficacy, it is not an approved agent for seizures. Supportive care and long-term complications are summarized (Class IV).
Rotenberg JS, Newmark J; Nerve Agent Attacks on Children: Diagnosis and Management. Pediatrics 2003; 112 (3 Pt 1): 648-658. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
Near-lethal exposure to nerve agents produces prolonged epileptiform seizures requiring the administration of benzodiazepine anticonvulsant drugs, such as diazepam. Clinically, benzodiazepines are reported to lose anticonvulsant effectiveness the greater the delay between seizure onset and benzodiazepine treatment. This time-dependent diminished effectiveness of diazepam was tested in the present study. Seizures elicited by the nerve agent, soman, were produced in guinea pigs instrumented to record brain electrocorticographic (ECoG) activity. Different groups of animals were administered 10 mg/kg, intramuscularly, of diazepam at 5, 40, 60, 80, or 160 minutes after the onset of seizure activity. There was a progressive loss in the anticonvulsant efficacy of diazepam as the treatment was delayed after seizure onset, but no differences in the time for diazepam to stop seizures. The results show a diminished ability of diazepam to stop nerve-agent-induced seizures the longer treatment is delayed.
McDonough JH, McMonagle JD, Shih TM; Time-dependent reduction in the anticonvulsant effectiveness of diazepam against soman-induced seizures in guinea pigs. Drug Chem Toxicol 2010 Jul; 33 (3): 279-83. [PubMed Citation]
-
The nerve agent VX is most likely to enter the body via liquid contamination of the skin. After percutaneous exposure, the slow uptake into the blood, and its slow elimination result in toxic levels in plasma for a period of several hours. Consequently, this has implications for the development of toxic signs and for treatment onset. In the present study, clinical signs, toxicokinetics and effects on respiration, electroencephalogram and heart rate were investigated in hairless guinea pigs after percutaneous exposure to 500 ug/kg VX. We found that full inhibition of AChE and partial inhibition of BuChE in blood were accompanied by the onset of clinical signs, reflected by a decline in respiratory minute volume, bronchoconstriction and a decrease in heart rate. Furthermore, we investigated the therapeutic efficacy of a single dose of atropine, obidoxime and diazepam, administered at appearance of first clinical signs, versus that of repetitive dosing of these drugs on the reappearance of signs. A single shot treatment extended the period to detrimental physiological decline and death for several hours, whereas repetitive administration remained effective as long as treatment was continued. In conclusion, percutaneous VX poisoning showed to be effectively treatable when diagnosed on time and when continued over the entire period of time during which VX, in case of ineffective decontamination, penetrates the skin.
Joosen MJ, van der Schans MJ, van Helden HP; Percutaneous exposure to the nerve agent VX: Efficacy of combined atropine, obidoxime and diazepam treatment. Chemico-Biological Interactions 2010; 188 (1): 255-263. [PubMed Citation]
-
Two guinea pig models were used to study the anticonvulsant potency of diazepam, midazolam, and scopolamine against seizures induced by the nerve agents tabun, sarin, soman, cyclosarin, O-ethyl S-(2-(diisopropylamino)ethyl)methylphosphonothioate (VX), and O-isobutyl S-(2-diethylamino)ethyl)- methyl phosphonothioate (VR). Animals instrumented for electroencephalogram recording were pretreated with pyridostigmine bromide (0.026 mg/kg i.m.) 30 min before challenge with 2 x LD50 (s.c.) of a nerve agent. In model A, atropine sulfate (2.0mg/kg i.m.) and pyridine-2-aldoxime methylchloride (2-PAM; 25.0 mg/kg i.m.) were given 1 min after nerve agent challenge, and the tested anticonvulsant was given (i.m.) 5 min after seizure onset. In model B, a lower dose of atropine sulfate (0.1 mg/kg i.m.) was given along with 2-PAM 1 min after nerve agent challenge, and the anticonvulsant was given at seizure onset. With the lower dose of atropine, seizure occurrence increased to virtually 100% for all agents; the time to seizure onset decreased for sarin, cyclosarin, and VX; the signs of nerve agent intoxication were more severe; and coma resulted frequently with cyclosarin. The anticonvulsant ED50 doses for scopolamine or diazepam were, in general, not different between the two models, whereas the anticonvulsant ED50 values of midazolam increased 3- to 17-fold with the lower atropine dose. Seizure termination times were not systematically effected by the different doses of atropine. The order of anticonvulsant effectiveness within each model was scopolamine > or = midazolam > diazepam. The findings indicate that the dose of atropine given as antidotal therapy can significantly influence measures of nerve agent toxicity and responsiveness to anticonvulsant therapy.
Shih TM, Rowland TC, and McDonough JH. Anticonvulsants for Nerve Agent-Induced Seizures: The Influence of the Therapeutic Dose of Atropine. J PET 2007; 320 (1): 154-161. [PubMed Citation]
-
This investigation compared the efficacy of diazepam and the water-soluble prodiazepam-avizafone-in sarin poisoning therapy. Guinea pigs, pretreated with pyridostigmine 0.1 mg/kg, were intoxicated with 4LD(50) of sarin (s.c. route) and 1 min after intoxication treated by intramuscular injection of atropine (3 or 33.8 mg/kg), pralidoxime (32 mg/kg) and either diazepam (2 mg/kg) or avizafone (3.5 mg/kg). EEG and pneumo-physiological parameters were simultaneously recorded. When atropine was administered at a dose of 3 mg/kg, seizures were observed in 87.5% of the cases; if an anticonvulsant was added (diazepam (2 mg/kg) or avizafone (3.5 mg/kg)), seizure was prevented but respiratory disorders were observed. At 33.8 mg/kg, atropine markedly increased the seizure threshold and prevented early respiratory distress induced by sarin. When diazepam was administered together with atropine, seizures were not observed but 62.5% of the animals displayed respiratory difficulties. These symptoms were not observed when using avizafone. The pharmacokinetic data showed marked variation of the plasma levels of atropine and diazepam in different antidote combination groups, where groups receiving diazepam exhibited the lowest concentration of atropine in plasma. Taken together, the results indicate that avizafone is suitable in therapy against sarin when an anticonvulsant is judged necessary.
Taysse L, Calvet JH, Buee J, Christin D, Delamanche S, Breton P; Comparative efficacy of diazepam and avizafone against sarin-induced neuropathology and respiratory failure in guinea pigs: influence of atropine dose. Toxicol 2003 Jun; 188 (2-3): 197-209. [PubMed Citation]
-
This study evaluated the potency and rapidity of some anticholinergics (atropine, biperiden, and trihexyphenidyl) and benzodiazepines (diazepam and midazolam) as an anticonvulsant treatment against seizures induced by six nerve agents (tabun, sarin, soman, cyclosarin, VR, and VX) and summarized the relationship between anticonvulsant activity and nerve agent-induced lethality and neuropathology. Guinea pigs, previously implanted with cortical electrodes for EEG recording, were pretreated with pyridostigmine bromide (0.026 mg/kg im) 30 min prior to challenge with 2x LD50 dose (sc) of a given nerve agent; in a separate experiment, animals were challenged with 5x LD50 sc of soman. One minute after agent challenge the animals were treated im with 2 mg/kg atropine SO4 admixed with 25 mg/kg 2-PAM Cl. Five minutes after the start of EEG seizures, animals were treated im with different doses of anticholinergics or benzodiazepines and observed for seizure termination. The time to seizure onset, the time to seizure termination, and 24-h lethality were recorded. The anticonvulsant ED50 of each drug for termination of seizures induced by each agent was calculated and compared. Brain tissue from animals that survived 24 h was examined for pathology. All drugs were capable of terminating seizure activity, with midazolam and trihexyphenidyl being significantly more potent than the other drugs, and midazolam being more rapid in controlling seizure than atropine, trihexyphenidyl, or diazepam against each agent. Seizures induced by sarin or VX required lower doses of all the test anticonvulsants. The dose of a given drug that was an effective anticonvulsant against a 2x LD50 challenge of soman was equally effective against seizures induced by a 5x LD50 challenge. All nerve agents were capable of producing neuropathology. Seizure control was strongly associated with protection against acute lethality and brain pathology.
Shih TM, Duniho SM, McDonough JH; Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicology and Applied Pharmacology 2003; 188 (2): 69-80. [PubMed Citation]
-
These studies investigated the effectiveness of combination treatment with a benzodiazepine and an anticholinergic drug against soman-induced seizures. The anticholinergic drugs considered were biperiden, scopolamine, trihexaphenidyl, and procyclidine; the benzodiazepines were diazepam and midazolam. Male guinea pigs were implanted surgically with cortical screw electrodes. Electrocorticograms were displayed continually and recorded on a computerized electroencephalographic system. Pyridostigmine (0.026 mg x kg(-1), i.m.) was injected as a pretreatment to inhibit red blood cell acetylcholinesterase by 30-40%. Thirty minutes after pyridostigmine, 2 x LD50 (56 microg x kg(-1)) of soman was injected s.c., followed 1 min later by i.m. treatment with atropine (2 mg x kg(-1)) + 2-PAM (25 mg x kg(-1)). Electrographic seizures occurred in all animals. Anticonvulsant treatment combinations were administered i.m. at 5 or 40 min after seizure onset. Treatment consisted of diazepam or midazolam plus one of the above-mentioned anticholinergic drugs. All doses of the treatment compounds exhibited little or no antiseizure efficacy when given individually. The combination of a benzodiazepine and an anticholinergic drug was effective in terminating soman-induced seizure, whether given 5 or 40 min after seizure onset. The results suggest a strong synergistic effect of combining benzodiazepines with centrally active anticholinergic drugs and support the concept of using an adjunct to supplement diazepam for the treatment of nerve-agent-induced seizures.
Koplovitz I, Schulz S, Shutz M, Railer R, Macalalag R, Schons M, McDonough J; Combination anticonvulsant treatment of soman-induced seizures. J Appl Toxicol. 2001 Dec; 21 Suppl 1:S53-5. [PubMed Citation]
-
A total of eight anticholinergic drugs (aprophen, atropine, azaprophen, benactyzine, biperiden, procyclidine, scopolamine, trihexyphenidyl) were tested in parallel with diazepam for the ability to terminate seizure activity induced by the nerve agent soman. Guinea pigs, implanted with electrodes to record cortical electroencephalographic (EEG) activity, were pretreated with pyridostigmine Br (0.026 mg:kg, i.m.) and 30 min later challenged with 2xLD50 soman (56 microg:kg, s.c.) followed 1 min later by treatment with atropine SO4 (2 mg:kg, i.m.) and pralidoxime chloride (2-PAM Cl; 25 mg:kg, i.m.). All guinea pigs developed sustained seizure activity following this treatment. Dose-effect curves were determined for the ability of each drug to terminate seizure activity when anticonvulsant treatment was given either 5 or 40 min after seizure onset. Body weight gain and recovery of behavioral performance of a previously trained one-way avoidance task were measured after exposure. With the exception of atropine, all anticholinergic drugs were effective at lower doses than diazepam in terminating seizures when given 5 min after seizure onset; benactyzine, procyclidine and aprophen terminated seizures most rapidly while scopolamine, trihexyphenidyl, biperiden, and diazepam were significantly slower. When given 40 min after seizure onset, diazepam was the most potent compound tested, followed by scopolamine, benactyzine and biperiden; atropine was not effective when tested 40 min after seizure onset. For diazepam, the time to terminate the seizure was the same whether it was given at the 5- or 40-min delay. In contrast, most anticholinergics were significantly slower in terminating seizure activity when given at the 40-min delay relative to when they were given at the 5-min delay. Successful control of seizure activity, regardless of the drug, was predictive of survival of the lethal effects of nerve agent exposure, a more rapid behavioral recovery (body weight, avoidance performance) and greater protection from neuropathology. In contrast, failure of a drug treatment to terminate seizure activity was closely associated with an increased probability of acute (<24 h) and delayed (10-day survival) lethality, a slower behavioral recovery in survivors, and an increased incidence and degree of neuropathology.
McDonough JH Jr, Zoeffel LD, McMonagle J , Copeland TL, Smith CD, Shih TM; Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Research 2000; 38 (1): 1-14. [PubMed Citation]
-
This study evaluated the ability of six benzodiazepines to stop seizures produced by exposure to the nerve agent soman. Guinea pigs, previously prepared with electrodes to record electroencephalographic (EEG) activity, were pretreated with pyridostigmine (0.026 mg/kg, i.m.) 30 min before challenge with soman (56 microg/kg, s.c.) and then treated 1 min after soman exposure with atropine (2.0 mg/kg, i.m.) and pralidoxime chloride (2-PAM Cl; 25 mg/kg, i.m.). All animals developed seizures following this treatment. Benzodiazepines (avizafone, clonazepam, diazepam, loprazolam, lorazepam, and midazolam) were given i.m. 5 or 40 min after seizure onset. All benzodiazepines were effective in stopping soman-induced seizures, but there were marked differences between drugs in the rapidity of seizure control. The 50% effective dose (ED50) values and latencies for anticonvulsant effect for a given benzodiazepine were the same at the two times of treatment delay. Midazolam was the most potent and rapidly acting compound at both treatment times. Since rapid seizure control minimizes the chance of brain damage, use of midazolam as an anticonvulsant may lead to improved clinical outcome in the treatment of nerve agent seizures.
McDonough JH, McMonagle J, Copeland T, Zoeffel D, Shih TM; Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol, 1999 Nov; 73(8-9):473-476. [PubMed Citation]
-
This report describes studies of anticonvulsants for the organophosphorus (OP) nerve agent soman: a basic research effort to understand how different pharmacological classes of compounds influence the expression of seizure produced by soman in rats, and a drug screening effort to determine whether clinically useful antiepileptics can modulate soman-induced seizures in rats. Electroencephalographic (EEG) recordings were used in these studies. Basic studies were conducted in rats pretreated with HI-6 and challenged with 1.6 x LDs0 soman. Antimuscarinic compounds were extremely effective inblocking (pretreatment) or terminating soman seizures when given 5 min after seizure onset. However, significantly higher doses were required when treatment was delayed for more than 10 min, and some antimuscarinic compounds lost anticonvulsant efficacy when treatment was delayed for more than 40 min. Diazepam blocked seizure onset, yet seizures could recur after an initial period of anticonvulsant effect at doses </=2.5 mg/kg. Diazepam could terminate ongoing seizures when given 5 min after seizure onset, but doses up to 20 mg/kg were ineffective when treatment was delayed for 40 min. The GABA uptake inhibitor, tiagabine, was ineffective in blocking or terminating soman motor convulsions or seizures. The glutamate receptor antagonists, NBQX, GYKI 52466, and memantine, had weak or minimal antiseizure activity, even at doses that virtually eliminated signs of motor convulsions. The antinicotinic, mecamylamine, was ineffective in blocking or stopping seizure activity. Pretreatment with a narrow range of doses of a2-adrenergic agonist, clonidine, produced variable protection (40- 60%) against seizure onset; treatment after seizure onset with clonidine was not effective. Screening studies in rats, using HI-6 pretreatment, showed that benzodiazepines (diazepam, midazolam and lorazepam) were quite effective when given 5 min after seizure onset, but lost their efficacy when given 40 min after onset. The barbiturate, pentobarbital, was modestly effective in terminating seizures when given 5 or 40 min after seizure onset, while other clinically effective antiepileptic drugs, trimethadione and valproic acid, were only slightly effective when given 5 min after onset. In contrast, phenytoin, carbamazepine, ethosuximide, magnesium sulfate, lamotrigine, primidone, felbamate, acetazolamide, and ketamine were ineffective.
Shih T, McDonough JH Jr, Koplovitz I. Anticonvulsants for Soman-induced Seizure Activity. J Biomed Sci. 1999; 6 (2): 86-96. [PubMed Citation]
-
The effects of midazolam (MDZ), diazepam (DZ) and scopolamine (SCP) therapies on soman-induced electrocorticogram (ECoG) and biceps femoris electromyogram (EMG) activities and brain lesions were assessed in male rats. Animals received pyridostigmine (26 micrograms/kg, im) 30 min before soman (87.1 micrograms/kg, im) followed by therapy consisting of atropine (1.5 mg/kg) admixed with 2-PAM (25 mg/kg, im) 1 min later; MDZ (0.5 mg/kg), DZ (1.77 mg/kg) or SCP (0.43 mg/kg) was administered im at 1 min after the onset of convulsions (CVs). Typically, within 5 min after soman the ECoG profile changed to a full-blown, spike-and-dome epileptiform (SDE) pattern followed by CVs and increased amplitude of EMG activity. Treatment with SCP restored ECoG and EMG profiles by 30 min. At 2 hr after exposure only 1 animal demonstrated a slight abnormality in ECoG activity which was normal at 24 hr. Similarly, DZ and MDZ restored EcoG and EMG profiles by 30 min; however, in contrast to SCP, 83% of the animals demonstrated reappearance of SDE 2 hrs after soman. SCP therapy also enabled rats to move about in their cages by 30 min post treatment. In contrast, DZ- and MDZ-treated rats remained incapacitated as late as 2 hr post-exposure. Animals were euthanized at 24 hr, and the extent of soman-induced brain lesions was determined by light microscopic analysis. When present, brain lesions were minimal in SCP-treated rats. The mean brain lesion scores across all experimental conditions ranked as follows: soman control > MDZ > DZ > or = SCP = saline control. These observations suggest that SCP may be highly effective in severe soman intoxication.
Anderson DR, Harris LW, Chang FC, Baze WB, Capacio BR, Byers SL, Lennox WJ; Antagonism of soman-induced convulsions by midazolam, diazepam and scopolamine. Drug Chem Toxicol. 1997 Aug; 20 (3): 115-31. [PubMed Citation]
-
Diazepam (DZ) and scopolamine (SCP) are known to be beneficial when each is used in combination with atropine (AT) + oxime therapy against intoxication by soman, but the efficacy of each might be expected to vary with the dosage of AT. Thus, the therapeutic efficacy of SCP (5 doses; 0 - 0.86 mg/kg) versus DZ (5 doses; 0 - 5 mg/kg), when used in conjunction with AT (3 doses; 0.5 - 8 mg/kg) + 2-PAM (25 mglkg) therapy, was tested in groups of pyridostigmine pretreated guinea pigs exposed to 1.6, 2.0, 2.5 or 3.2 LD50s of soman. Response surface methodology was employed to describe the relationship between lethality and the AT/DZ or AT/SCP dosages. Results show that within the indicated dose ranges used, the efficacy of SCP is not dependent on the presence of AT, whereas AT is needed for DZ to maintain the lowest probability of death. These findings suggest that in guinea pigs SCP could supplement AT or replace DZ as therapy against nerve agent intoxication.
Harris LW, Gennings C, Carter WH, Anderson DR, Lennox WJ, Bowersox SL, Solana RP; Efficacy comparison of scopolamine (SCP) and diazepam (DZ) against soman-induced lethality in guinea pigs. Drug Chem Toxicol. 1994; 17 (1): 35-50. [PubMed Citation]
-
Six FDA approved, injectable compounds [benztropine (BZT); biperiden (BIP); dicyclomine (DCL); l-hyoscyamine (HYO); orphenadrine (ORP); scopolamine (SCP)] were each compared to diazepam (DZ, the standard) in male guinea pigs against ongoing soman-induced convulsive or sub-CV (CV/sub-CV) activity. Three trained graders concurrently assigned CV/sub-CV scores to each animal based on signs of intoxication at various times post-soman. Animals received (im) pyridostigmine (26 micrograms/kg) 30 min before soman (56 micrograms/kg; 2 x LD50), atropine (2 mg/kg) admixed with 2-PAM (25 mg/kg) at one min after soman, and the candidate drug preparation at 5.67 min post soman, a time when CV activity was assured. BIP and SCP were effective over dosage ranges between 10 and 0.3, and 1.0 and 0.13 mg/kg, respectively, while the other preparations were less effective at their respective maximum dosages. At the most effective dosages of SCP (1.0 mg/kg) and BIP (10 mg/kg), the CV/sub-CV scores were significantly lower (p < 0.05) than those of DZ. Only 33% survival was observed at each of two doses of ORP and one dose of HYO; therefore, no further testing was done with these compounds. Using freshly prepared solutions, DCL (up to 40 mg/kg) and BZT (up to 96 mg/kg) were tested with mixed results; DCL lowered lethality while BZT increased lethality. CV/sub-CV scores for the most effective dose of DCL and BZT were, however, lower than those of DZ. SCP is an antimuscarinic drug devoid of antinicotinic activity, while BIP possesses antimuscarinic, antinicotinic, antispasmodic and anti-N-methyl-D-aspartate activity. Recent evidence suggests that, in late stages of intoxication by nerve agents, noncholinergic, excitatory amino acid receptors may become involved and necessitate the use of a multi-action drug like BIP. The findings herein suggest that SCP and BIP are superior to DZ, but further studies are needed to determine which drug or drug class should be pursued in more advanced testing.
Anderson DR, Harris LW, Bowersox SL, Lennox WJ, Anders JC. Efficacy of injectable anticholinergic drugs against soman-induced convulsive/subconvulsive activity. Drug Chem Toxicol. 1994; 17 (2): 139-48. [PubMed Citation]
-
A review of the literature was conducted to provide an overview of organophosphorus (OP)-induced morphological changes in the non-human primate. Most studies have evaluated effects of the OP nerve agent soman (pinacolyl methylphosphonofluoridate), an irreversible inhibitor of acetylcholinesterase. Soman-induced acute and chronic morphological changes have been examined. The effects of nerve agent therapy (i.e. pyridostigmine, praloxidime chloride and atropine), with and without an anticonvulsant (i.e. diazepam, midazolam), on soman-induced lesions have also been studied. Acute changes in the central nervous system of rhesus and cynomolgus monkeys exposed to soman alone or soman and therapy, without an anticonvulsant, were characterized by neuronal degeneration and necrosis and neuropil edema. The lesions were usually present in the frontal cortex, entorhinal cortex, amygdaloid complex, caudate nucleus, thalamus and hippocampus. Morphologically, these lesions resemble lesions produced by hypoxic-ischemic injury or by seizures and are similar to soman-induced changes in other laboratory animals. Nerve agent therapy supplemented with an anticonvulsant reduced or prevented soman-induced acute neural lesions. Acute changes in non-neural tissues were limited to the heart (e.g. hemorrhage, myofiber necrosis, myocarditis) and skeletal muscle (e.g. myofiber necrosis). Heart lesions in the non-human primate are similar to OP-induced heart lesions in man. The pathogenesis of the acute lesions in both the central nervous system and heart is discussed. Consistent soman-induced chronic morphological changes have not been produced in the rhesus monkey or baboon.
Baze WB; Soman-induced morphological changes: an overview in the non-human primate. J Appl Toxicol. 1993 May-Jun; 13 (3): 173-7. [PubMed Citation]
-
The possibility that nerve agents will be used on the battlefield is real, the traditional therapy against nerve agent exposure consists of pyridostigmine pretreatment and atropine-pralidoxime chloride therapy administered after nerve agent exposure. This therapy regimen is extremely effective in preventing mortality in laboratory animals exposed to multilethal concentrations of nerve agent, yet these animals often display convulsions, brain damage, and behavioral incapacitation. We report here that the addition of diazepam to the traditional therapy for nerve agent (soman) exposure not only decreases the incidence of convulsions, but also attenuates the cognitive impairments of rhesus monkeys trained on a Serial Probe Recognition (SPR) task. Monkeys which received diazepam treatment required only 6 days before their performance on the SPR task returned to presoman exposure levels, compared to non-diazepam treated monkeys which required 15 days. Moreover, only 1 out of the 5 monkeys which received diazepam treatment suffered tonic-clonic convulsions; in contrast all 5 monkeys which did not receive diazepam treatment experienced severe convulsive episodes. These results suggest that diazepam would be an excellent adjunct to traditional nerve agent therapy to facilitate behavioral recovery from nerve agent intoxication that might be encountered by US military personnel on the battlefield or accidental organophosphate poisoning encountered in industrial or agricultural accidents.
Castro CA, Larsen T, Finger AV, Solana RP, McMaster SB; Behavioral efficacy of diazepam against nerve agent exposure in rhesus monkeys. Pharmacol Biochem Behav. 1992; 41 (1): 159-164. [PubMed Citation]
-
The aim of this study was to compare the anticonvulsive and protective effects of diazepam and midazolam in rats poisoned by chemical warfare agents. In rats treated with soman, sarin or VX, the anticonvulsive effects of midazolam and diazepam were of similar magnitude. Atropine and oxime HI-6 decreased the toxicity of soman, sarin and VX 1.65, 2.06 and 18.3 times, respectively. The introduction of diazepam and midazolam in the therapy of rats poisoned by VX and sarin led to further improvement of protective indices. Midazolam was even more effective than diazepam. A reliable protective effect was obtained with the lowest dose of both benzodiazepines used (0.5 mg/kg). The specific benzodiazepine antagonist flumazenil abolished, almost completely, the protective effect of both benzodiazepines. These data confirmed a significant role of the gabaergic system in poisoning with organophosphorus compounds, especially during the initial stage of intoxication.
Bokonjić D, Rosić N; Anticonvulsive and protective effects of diazepam and midazolam in rats poisoned by highly toxic organophosphorus compounds. Arh Hig Rada Toksikol. 1991 Dec; 42 (4): 359-65. [PubMed Citation]
-
Two benzodiazepine compounds, midazolam and diazepam, were administered as adjunctive treatment to soman-exposed rhesus monkeys to evaluate their effects on acute soman intoxication. Monkeys were pretreated orally with pyridostigmine, exposed to soman, and treated i.m. with atropine, pralidoxime chloride (2-PAM), and with midazolam, diazepam or sterile water (control). All monkeys that received the benzodiazepines recovered sooner and exhibited no convulsions. Neuronal degenerative and necrotic lesions were decreased or eliminated in the entorhinal cortex, caudate nucleus, and hippocampus of those animals that received benzodiazepine therapy. These findings support the continued evaluation of drugs with anticonvulsant activity as standard adjunct therapy for soman intoxication.
Hayward IJ, Wall HG, Jaax NK, Wade JV, Marlow DD, Nold JB; Decreased brain pathology in organophosphate-exposed rhesus monkeys following benzodiazepine therapy. J Neurol Sci. 1990 Aug; 98 (1): 99-106. [PubMed Citation]
-
The organophosphorus nerve agents soman and tabun were tested in the hen at doses 120-150 times higher than their acute LDs0, as it was assumed that these doses would produce delayed neuropathy. The animals were protected against the acute lethal effect of these agents by pretreatment with atropine, physostigmine, diazepam, and the oxime HI-6 or obidoxime. The surviving animals were followed for 30 days and the occurrence of delayed neuropathy was clinically diagnosed. Soman produced severe delayed neuropathy at a dose of 1.5 mg/kg, a dose which produced acute lethality in five animals out of six. Tabun elicited very mild neuropathic symptoms in one animal out of two at a dose of 6 mg/kg given on 2 consecutive days. Delayed neuropathy was not seen in the hens that survived the acute toxicity of a single dose of tabun, 12 mg/kg (three out of six) or 15 mg/kg (two out of six).
Willems JL, Nicaise M, De Bisschop HC; Delayed neuropathy by the organophosphorus nerve agents soman and tabun. Arch Toxicol. 1984; 55 (1): 76-77. [PubMed Citation]
Pregnant animal studies
-
Treatment of time-pregnant Long Evans rats with 1.25 mg/kg s.c. diazepam (2.5 mg/kg in Sprague Dawley rats) from gestational day 14 to 20 produced transient depression of an olfactory guided behavior (nest odor behavior) in suckling offspring. Enhanced drug sensitivity to diazepam was seen in adult male and female off-spring as indicated by increased temperature depression. In addition, increased sensitivity to an opiate (morphine) was noted for the female offspring in the tail flick test. Treatment of the pregnant dam with diazepam or clonazepam, a benzodiazepine with selective affinity for the central benzodiazepine receptor, resulted in a marked depression of cellular immune responses in the offspring of both sexes up to 2 months of age. Drug treatment during early fetal period (GD 12-16), at a time central benzodiazepine receptors are not present in all brain regions of the fetal brain, did not affect the quality of cellular immune responses, whereas treatment from GD 16 to 20 was effective. Prenatal diazepam effects are discussed in view of presence and functionality of both central and peripheral benzodiazepine binding sites in the fetus.
Schlumpf M, Ramseier H, Abriel H, Youmbi M, Baumann JB, Lictensteiger W; Diazepam effects on the fetus. Neurotoxicology 1989; 10 (3): 501-516. [PubMed Citation]
-
Organophosphates (OPs) inhibit the enzyme cholinesterase and cause accumulation of acetylcholine, and are known to cause seizures and status epilepticus (SE) in humans. The animal models of SE caused by organophosphate analogs of insecticides are not well characterized. SE caused by OPs paraoxon and diisopropyl fluorophosphate (DFP) in rats was characterized by electroencephalogram (EEG), behavioral observations and response to treatment with the benzodiazepine diazepam administered at various stages of SE. A method for SE induction using intrahippocampal infusion of paraoxon was also tested. Infusion of 200nmol paraoxon into the hippocampus caused electrographic seizures in 43/52 (82.7%) animals tested; and of these animals, 14/43 (30%) had self-sustaining seizures that lasted 4-18h after the end of paraoxon infusion. SE was also induced by peripheral subcutaneous injection of diisopropyl fluorophosphate (DFP, 1.25mg/kg) or paraoxon (1.00mg/kg) to rats pretreated with atropine (2mg/kg) and 2-pralidoxime (2-PAM, 50mg/kg) 30min prior to OP injection. SE occurred in 78% paraoxon-treated animals and in 79% of DFP-treated animals.Diazepam (10mg/kg) was administered 10min and 30min after the onset of continuous EEG seizures induced by paraoxon and it terminated SE in a majority of animals at both time points. DFP-induced SE was terminated in 60% animals when diazepam was administered 10min after the onset of continuous EEG seizure activity but diazepam did not terminate SE in any animal when it was administered 30min after the onset of continuous seizures. These studies demonstrate that both paraoxon and DFP can induce SE in rats but refractoriness to diazepam is a feature of DFP induced SE.
Todorovic MS, Cowan ML, Balint CA, Sun C, Kapur J; Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res 2012 Sep; 101 (3): 268-76. [PubMed Citation]
Non-clinical reviews
-
A review of the literature was conducted to provide an overview of organophosphorus (OP)-induced morphological changes in the non-human primate. Most studies have evaluated effects of the OP nerve agent soman (pinacolyl methylphosphonofluoridate), an irreversible inhibitor of acetylcholinesterase. Soman-induced acute and chronic morphological changes have been examined. The effects of nerve agent therapy (i.e. pyridostigmine, praloxidime chloride and atropine), with and without an anticonvulsant (i.e. diazepam, midazolam), on soman-induced lesions have also been studied. Acute changes in the central nervous system of rhesus and cynomolgus monkeys exposed to soman alone or soman and therapy, without an anticonvulsant, were characterized by neuronal degeneration and necrosis and neuropil edema. The lesions were usually present in the frontal cortex, entorhinal cortex, amygdaloid complex, caudate nucleus, thalamus and hippocampus. Morphologically, these lesions resemble lesions produced by hypoxic-ischemic injury or by seizures and are similar to soman-induced changes in other laboratory animals. Nerve agent therapy supplemented with an anticonvulsant reduced or prevented soman-induced acute neural lesions. Acute changes in non-neural tissues were limited to the heart (e.g. hemorrhage, myofiber necrosis, myocarditis) and skeletal muscle (e.g. myofiber necrosis). Heart lesions in the non-human primate are similar to OP-induced heart lesions in man. The pathogenesis of the acute lesions in both the central nervous system and heart is discussed. Consistent soman-induced chronic morphological changes have not been produced in the rhesus monkey or baboon.
Baze WB; Soman-induced morphological changes: an overview in the non-human primate. J Appl Toxicol. 1993 May-Jun; 13 (3): 173-7. [PubMed Citation]
-
This manuscript provides a survey of research findings catered to the development of effective countermeasures against nerve agent poisoning over the past decade. New neuropathophysiological distinctive features as regards organophosphate (OP) intoxication are presented. Such leading neuropathophysiological features include recent data on nerve agent-induced neuropathology, related peripheral or central nervous system inflammation and subsequent angiogenesis process. Hence, leading countermeasures against OP exposure are down-listed in terms of pre-treatment, protection or decontamination and emergency treatments. The final chapter focuses on the description of the self-repair attempt encountered in lesioned rodent brains, up to 3 months after soman poisoning. Indeed, an increased proliferation of neuronal progenitors was recently observed in injured brains of mice subjected to soman exposure. Subsequently, the latter experienced a neuronal regeneration in damaged brain regions such as the hippocampus and amygdala. The positive effect of a cytokine treatment on the neuronal regeneration and subsequent cognitive behavioral recovery are also discussed in this review. For the first time, brain cell therapy and neuronal regeneration are considered as a valuable contribution towards delayed treatment against OP intoxication. To date, efficient delayed treatment was lacking in the therapeutic resources administered to patients contaminated by nerve agents.
Collombet JM; Nerve agent intoxication: Recent neuropathophysiological findings and subsequent impact on medical management prospects. Toxicology and Applied Pharmacology 2011; 255 (3): 229-241. [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
A study performed in 24 healthy male subjects comparing the I.M. Injection of 10 mg of diazepam in the mid-anterior/lateral thigh by the autoinjector versus 10 mg I.M. by a syringe (operated manually) indicates that the mean percent availability of the drug from the autoinjector is 100% of that obtained from the syringe.
Product label U.S. Army's Diazepam Auto-injector [Meridian Medical Technologies, Inc.] Last revised: September 1997 [FDA]
-
The mean C, value from the autoinjector was 314 ng/mL (c.v. = l8.7%, range 185 to 439 ng/mL) and 48.6 ng/mL (c.v. = 19.8%, range 29.4 to 69.7 ng/mL) for diazepam and desmethyldiazepam, respectively, while the syringe gave corresponding values of 287 ng/mL (c.v. = 18.9%, range 174 to 37 ng/mL) and 47.2 ng/mL (c.v. = 19.4%, range 33.1 to 61.2 ng/mL) for diazepam and desmethyldiazepam, respectively. The corresponding mean Tmax values were 1.47 hours (c.v. = 69.9%, range 0.8 to 6 hours) and 61.0 hours (c.v. = 58.8%, range 24 to 144 hours) for diazepam and desmethyldiazepam for the autoinjector whereas the syringe gave values of 1.31 hours (c.v. = 32%, range 0.7 to 2.0 hours) and 54.5 hours (c.v. = 47.3%, range 12 to 96 hours) for diazepam and desmethyldiazepam, respectively.
Product label U.S. Army's Diazepam Auto-injector [Meridian Medical Technologies, Inc.] Last revised: September 1997 [FDA]
-
The pharmacokinetics of diazepam and its biologically active major metabolite desmethyldiazepam were investigated under clinically relevant situations. Both drugs were measured in the different specimens (blood, plasma, urine, bile) by a specific and sensitive gas-liquid chromatographic assay. The pharmacokinetic data were analyzed by the digital computer program SAAM-25 according to the two compartment open model. In healthy subjects the elimination half-life (T1/2(beta))is dependent on the age of the individuals, which is caused by changes in the apparent volume of distribution. The strong plasma protein binding of 95 to 98 percent determines the low hepatic clearance of diazepam. Only neglible amounts of a dose are excreted unchanged with the bile and into the urine. After multiple dosing with diazepam its T1/2(beta) is prolonged, which is caused by a lowered clearance. Thereby also desmethyldiazepam accumulates, since it is eliminated about three times slower than its parent compound. In patients with dysfunction of the liver (cirrhosis, hepatitis) diazepam and desmethyldiazepam exhibit a prolonged T1/2(beta) and a reduced clearance and the lowered plasma protein binding causes a larger volume of distribution. Animal and in vitro experiments demonstrate, besides species dependent differences, that the elimination of diazepam can be impaired by the accumulating desmethyldiazepam.
Klotz U. Important factors determining human distribution and elimination of diazepam. Fortschr Med. 1977; 95 (32): 1958-64. [PubMed Citation]
-
Benzodiazepines (BZDs) remain important agents in the management of epilepsy. They are drugs of first choice for status epilepticus and seizures associated with post-anoxic insult and are also frequently used in the treatment of febrile, acute repetitive and alcohol withdrawal seizures. Clinical advantages of these drugs include rapid onset of action, high efficacy rates and minimal toxicity. Benzodiazepines are used in a variety of clinical situations because they have a broad spectrum of clinical activity and can be administered via several routes. Potential shortcomings of BZDs include tolerance, withdrawal symptoms, adverse events, such as cognitive impairment and sedation, and drug interactions. Benzodiazepines differ in their pharmacologic effects and pharmacokinetic profiles, which dictate how the drugs are used. Among the approximately 35 BZDs available, a select few are used for the management of seizures and epilepsy: clobazam, clonazepam, clorazepate, diazepam, lorazepam and midazolam. Among these BZDs, clorazepate has a unique profile that includes a long half-life of its active metabolite and slow onset of tolerance. Additionally, the pharmacokinetic characteristics of clorazepate (particularly the sustained-release formulation) could theoretically help minimize adverse events. However, larger, controlled studies of clorazepate are needed to further examine its role in the treatment of patients with epilepsy.
Riss J, Cloyd J, Gates J, Collins S; Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008; 118 (2): 69-86. [PubMed Citation]
-
Acute repetitive seizures (ARS) are a debilitating part of episodic seizure activity that can sometimes progress to status epilepticus. Currently approved treatment that can be administered by non-medical personnel to patients with ARS is a diazepam rectal gel. While effective, rectal administration can be difficult, inconvenient and objectionable. A diazepam autoinjector has been developed to deliver diazepam via an intramuscular (IM) injection. This study evaluated the dose proportionality of the diazepam autoinjector and the consequent diazepam bioavailability relative to an equivalent dose of diazepam administered rectally as a commercial gel. This was a phase I, randomized, open-label, two-part, single-dose, crossover, single-centre pharmacokinetic study in 48 healthy young adult (aged 18-40 years) male and female subjects. Part I of the study (n=24) evaluated the dose proportionality of three strengths of the diazepam autoinjector (5, 10 and 15 mg) administered into the mid-outer thigh via a deep IM injection. Part II (n=24) assessed the relative bioavailability of the diazepam 10mg autoinjector versus the diazepam 10mg rectal gel. Parts I and II were run concurrently. Each subject completed screening up to 30 days prior to three (Part I) or two (Part II) dosing periods. Serial blood sampling for plasma diazepam and desmethyldiazepam (metabolite) concentrations, vital signs and adverse event (AE) assessments were performed at prespecified times. Treatments were separated by a 14-day washout period. In Part I, dose proportionality was demonstrated for the diazepam autoinjector at 5, 10 and 15 mg doses by increases in mean maximum plasma concentration (C(max)), mean area under the plasma concentration-time curve (AUC) from time zero to infinity (AUC(∞)), and mean AUC from time zero to time of last measurable concentration (AUC(last)). The median time to reach C(max) (t(max)) was consistent at 1 hour for each dose. In Part II of the study, IM administration via diazepam autoinjector (10 mg) resulted in plasma concentrations of both diazepam and desmethyldiazepam that were slightly higher and less variable than those observed following administration of diazepam rectal gel (10 mg). The geometric mean ratio (diazepam autoinjector/diazepam rectal gel) and 90% confidence intervals for diazepam C(max) and AUC(last) were 0.94 (0.84, 1.05) and 1.14 (1.08, 1.21), respectively, indicating that the overall bioavailability of the diazepam autoinjector was approximately 14% higher than that of diazepam rectal gel. Both treatments were generally well tolerated. Although the incidence of treatment-emergent AEs was higher with diazepam autoinjector compared with diazepam rectal gel (21.7% vs 13.6%), the difference can be attributed to injection site pain. Injection site pain also correlated with the diazepam autoinjector dose administered in Part I: 5 mg (4.3%), 10 mg (21.7%) and 15 mg (27.3%). However, no patients discontinued the trial due to injection site pain. No other AEs correlated with dose, and there was no evidence of respiratory depression with either administration. Results of the present study indicated that diazepam can be safely and reliably administered IM using a diazepam autoinjector.
Lamson MJ, Sitki-Green D, Wannarka GL, et al.; Pharmacokinetics of diazepam administered intramuscularly by autoinjector versus rectal gel in healthy subjects: a phase I, randomized, open-label, single-dose, crossover, single-centre study. Clin Drug Investig. 2011; 31 (8): 585-597. [PubMed Citation]
-
A diazepam 10-mg autoinjector was evaluated in bioequivalence and dose proportionality studies; both involved 24 young, healthy subjects and used randomized, open-label, 2-treatment, 2-period crossover designs with a 3-week washout period between treatments. The bioequivalence study compared a single diazepam 10-mg autoinjector with a conventional needle and syringe containing 10 mg of diazepam injectable. The dose proportionality study compared the pharmacokinetics of a single diazepam 10-mg autoinjector with that of 2 diazepam 10-mg autoinjectors given simultaneously (20 mg). Injections were intramuscular in the midanterolateral thigh in both studies. The studies showed that the diazepam autoinjector produced consistent plasma diazepam levels, with a rapid onset of absorption. The diazepam 10-mg autoinjector given intramuscularly was bioequivalent to a conventional syringe containing diazepam 10 mg. A single (10-mg) autoinjector and 2 (20-mg) diazepam autoinjectors administered simultaneously produced plasma diazepam concentrations that were essentially dose proportional.
Lehmann C, Wannarka GL; Bioavailability and dose proportionality of intramuscular diazepam administered by autoinjector. J Clin Pharmacol. 2008; 48 (4): 436-444. [PubMed Citation]
-
Twenty-two healthy volunteers aged 20-78 years received single 5-mg doses of diazepam by intravenous injection, by mouth in the fasting state, and by a deltoid intramuscular injection. The kinetic profile of diazepam by each route was determined from multiple plasma diazepam concentrations measured 7-14 days after each dose. After intravenous injection, diazepam volume of distribution (Vd) was larger in women than in men, but increased with age regardless of sex. Elimination half-life was longer in elderly than in young men (101 v 32 h, P < 0.025), partly due to the increased Vd as well as to a significant reduction in total metabolic clearance (0.24 v 0.46 ml/min/kg, P < 0.05). However, the prolonged half-life in elderly as opposed to young women (99 v 44 h; P < .01) was due mainly to increased Vd because clearance was not significantly changed (0.29 v 0.35 ml/min/kg). In all subjects, oral diazepam was rapidly absorbed; peak plasma levels were reached an average of 0.9 h after dosage. Absolute bioavailability averaged 94%, indicating essentially complete absorption. Neither age nor sex significantly influenced oral absorption. In all male subjects, and in 8 of 12 women, absorption of diazepam after deltoid intramuscular injection was rapid and essentially complete. However, in three young and one elderly women, absorption was slower and apparently incomplete. Axe as such did not significantly influence absorption of intramuscular diazepam.
Divoll M, Greenblatt DJ, Ochs HR, Shader RI; Absolute bioavailability of oral and intramuscular diazepam: effects of age and sex. Anesth Analg. 1983; 62 (1): 1-8. [PubMed Citation]
-
Diazepam is still one of the most used of the benzodiazepine group of drugs. Extensive studies over 10 years have defined a fairly complete profile of its kinetics. Minor aspects relating to patterns of its metabolism and excretion in certain age groups and in some disease states remain to be described quantitatively. However, there is more than sufficient kinetic information available for the requirements of good clinical practice. For optimum clinical benefit with minimum side-effects the following kinetic properties should be borne in mind: (a) there is a large interindividual variation (up to 30-fold) in dose/blood level ratios, especially when treatment is short-term; (b) the elimination half-life is prolonged in the elderly and the newborn and in some cases of liver disease; (c) there is accumulation of the active N-desmethylated metabolite during long-term treatment; and (d) administration of diazepam to pregnant women leads to rapid distribution from the maternal to fetal compartment: accumulation of both diazepam and desmethyldiazepam could cause prolonged sedation in the newborn. As there does not appear to be any clear relationship between the concentration of diazepam in the plasma and clinical effect, measurement of blood levels, other than for research purposes, is unnecessary. Based on kinetic data, a single administration of diazepam at night should be adequate for hypnotic and anxiolytic effects in most patients.
Mandelli M, Tognoni G, Garattini S; Clinical pharmacokinetics of diazepam. Clin Pharmacokinet. 1978; 3 (1): 72-91. [PubMed Citation]
-
The pharmacokinetics and plasma binding of diazepam were compared in man, dog, rabbit, guinea pig and rat. Diazepam (D) and its major metabolite, desmethyl-diazepam, were measured in blood and plasma by a specific and sensitive gas-liquid chromatography assay with an electron capture detector. After an intravenous bolus injection plasma levels of D declined biexponentially in all species examined and the data were analyzed according to the two-compartment open model. The binding of D and desmethyldiazepam has been determined at therapeutic concentrations by equilibrium dialysis in man (96.8 and 96.6%, respectively), dog (96.0 and 95.9%), rabbit (89.9 and 94.7%), guinea pig (91.3 and 78.6%) and rat (86.3 and 90.5%). In man, the elimination half-life, T1/2(beta), increased significantly (P less than .01) with decreasing total plasma clearance (Cl). Plasma binding affected Vd and Cl, but only Cl increased significantly (P less than .05), if more free D was available. This indicates that unbound drug is rate-determining for clearance by the liver, and that D fits into the restrictive elimination class in man. In the four animal species tested, Cl was a direct linear function of the body surface area. T1/2(beta) and the rates of drug clearance were characteristic figures for each species: from 1.1 hours and 81.6 ml/min/kg in the rat to 32.9 hours and 0.35 ml/min/kg in man, whereas T1/2(alpha), the half-life of distribution, varied only approximately 3-fold (0.3-1.0 hours) in the different species. A considerably higher extraction ratio than the unbound fraction of diazepam exists in these animal species, and blood clearance exceeds liver blood flow, giving reason to assume a much higher ability of the liver to metabolize D, and a species-dependent extrahepatic metabolism. The large variations described suggest that pharmacokinetic data or plasma binding results cannot simply be extrapolated to man.
Klotz U, Antonin KH, Bieck PR; Pharmacokinetics and plasma binding of diazepam in man, dog, rabbit, guinea pig and rat. J Pharmacol Exp Ther. 1976; 199 (1): 67-73. [PubMed Citation]
Pregnancy
-
Diazepam and its metabolite, desmethyldiazepam, freely cross the placenta and accumulate in the fetal circulation with newborn levels about 1 to 3 times greater than maternal serum levels. Transfer across the placenta has been demonstrated as early as 6 weeks' gestation. Of interest, fetal drug levels were independent of maternal serum concentrations and time from drug administration to sampling. These data suggest that diazepam accumulates in the fetal circulation and tissues during organogenesis. At term, equilibrium between the mother and fetus occurs in 5-10 minutes after IV administration. The maternal and fetal serum binding capacity for diazepam is reduced in pregnancy and is not correlated with albumin.
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.399-403
-
The transfer of drugs from the mother to the fetus is important from the view of possible harmful as well as therapeutic effects on both mother and fetus. In the first trimester of pregnancy this has been sparsely investigated. In 66 first-trimester pregnant women, who had applied for legal termination of the pregnancy, we have calculated different pharmacokinetic parameters of two benzodiazepine derivatives, diazepam and oxazepam, after administration of a single oral dose of 10 or 25 mg to the mother. The calculated pharmacokinetic parameters were within the normal range for healthy adults. The pharmacological active metabolite n-desmethyldiazepam was measured in concentrations near the detection limit. The penetration of diazepam and oxazepam from maternal serum to placental tissue in a 4 h period after drug administration was 31.5% and 49%, respectively, indicating a rapid transfer.
Jørgensen NP, Thurmann-Nielsen E, Walstad RA; Pharmacokinetics and distribution of diazepam and oxazepam in early pregnancy. Acta Obstet Gynecol Scand. 1988; 67 (6): 493-497. [PubMed Citation]
-
A mother who took 6-10 mg daily throughout pregnancy delivered a full-term, normally developed male infant. The infant was breastfed, and the mother continued to take her diazepam. Sedation was noted in the infant if nursing occurred <8 hours after taking a dose. Paired samples of maternal serum and breast milk were obtained on five occasions between 1 and 4 months after delivery. Milk concentrations of diazepam and desmethyldiazepam varied between 7.5 and 87 ng/mL and 1 9.2 and 77 ng/mL, respectively. The milk:serum ratios for diazepam varied between 0.14 and 0.21 in four samples but was 1.0 in one sample. The ratio for desmethyldiazepam varied from 0.10-0.18 in four samples and was 0.53 in the sample with the high diazepam ratio. A serum level was drawn from the infant on one occasion, revealing levels of diazepam and the metabolite of 0.7 and 46 ng/mL, respectively.
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.399-403
-
Previous investigations have revealed that prenatal exposure to diazepam (DZ) alters brain development and behavior in the offspring of rats and mice. In order to understand how DZ may affect the developing nervous system it is necessary to examine its metabolic fate in the neonate. It is therefore the aim of this study to investigate the disposition, metabolism, and persistence of DZ in the neonate. Dams were injected s.c. with 2.5 mg/kg of 14C DZ (10 muCi/day) on days 13-20 of gestation and their litters were fostered at birth. Dams killed within 24 hours postpartum and neonates killed at postnatal days 0, 10, and 20 were analyzed for 14C activity. Brain levels (pmoles DZ and metabolites/100 mg tissue SE) were 3.4 +/- 0.3 in the dam and in the neonates were 3.2 +/- 0.3 (day 0), 3.4 +/- 0.3 (day 10), and undetectable at day 20. Neonatal peripheral tissue 14C activity was undetectable by day 10. Brain regional analysis indicates 14C is highest in the colliculi at day 0, but not at day 10. Brain levels of DZ, oxazepam (OXA), N-desmethyldiazepam (NDZ), and the glucuronide (GLU) determined by high-performance liquid chromatography (HPLC), were GLU (49%), DZ (28%), and NDZ (24%) in the dam; GLU (52%), DZ (24%), and NDZ (25%) in the day 0 neonate; and GLU (32%), DZ (12%), NDZ (39%), and OXA (19%) at day 10. The distribution and metabolism of 14C DZ that persists in the neonate following prenatal exposure differs from that which occurs in the dam.
Simmons RD, Miller RK, Kellogg CK; Prenatal diazepam: distribution and metabolism in perinatal rats. Teratology 1983; 28 (2): 181-188. [PubMed Citation]
Hepatic Impairment
-
In six patients with cirrhosis and five patients with fibrosis of the liver elimination of diazepam (D) was compared after single and subchronic dosage. The pharmacokinetics of the major metabolite desmethyldiazepam (DD) was investigated in four healthy individuals and four patients with hepatic dysfunction and compared to its parent compound D. In the initial study, 11 patients with liver disease (cirrhosis and fibrosis) had a longer half-life (T 1/2(beta) of 99.2 +/- 23.2 hr after a single intravenous bolus of 0.1 mg/kg of D than to age-matched normal subjects (46.6 +/- 14.2). After subchronic treatment with 10 mg of D for 7 days T 1/2(beta) was prolonged only slightly (p = 0.043) in these patients (107.6 +/- 25.2 hr). Neither total plasma clearance (Cl) nor the apparent volume of distribution (VdSS or VdCl) showed significant changes. After intravenous injection of DD (0.1 mg/kg) plasma levels declined in the same biexponential manner as after D. The cross-over study in the four normal subjects demonstrated that DD was eliminated much more slowly than D. Whereas for D, T 1/2(beta) and Cl were 32.6 +/- 11.3 hr and 32.3 +/- 11.0 ml/min, respectively, the corresponding values for DD were 50.9 +/- 6.2 hr and 11.3 +/- 3.1 ml/min, respectively, the corresponding values for DD were 50.9 +/- 6.2 hr and 11.3 +/- 3.1 ml/min. The accumulation of DD after multiple dosage could be explained by the fact that it is formed faster from D than it is eliminated. In four patients with liver disease the elimination of D and the elimination of DD were altered. In these patients T 1/2(beta) for DD was prolonged (p = 0.015) to 108.2 +/- 40.3 hr. This prolongation was caused by a decrease in Cl of 4.6 +/- 1.1 ml/min, (p = 0.003) whereas Vd(Cl) did not change significantly. This indicates that at least two steps in diazepam metabolism are impaired in patients with liver disease.
Klotz U, Antonin KH, Brügel H, Bieck PR; Disposition of diazepam and its major metabolite desmethyldiazepam in patients with liver disease. Clin Pharmacol Ther. 1977; 21 (4): 430-6. [PubMed Citation]
-
This study investigates the separate effects of age and hepatocellular liver disease on the disposition and elimination of diazepam (Valium) in man. The drug was given either by rapid intravenous injection (0.1 mg/kg) or orally (10 mg) to 33 normal volunteers rnaging in age from 15 to 82 yr as well as to 9 individuals with alcoholic cirrhosis, 8 with acute viral hepatitis, and 4 with chronic active hepatitis. In the normal individuals, the terminal plasma half-life of diazepam, (t 1/2 (B)) exhibited a striking age-dependence; at 20 yr the t 1/2 (beta) was about 20 h, but it increased linearly with age to about 90 h at 80 yr. The plasma clearance of diazepam in the majority of the normal subjects was between 20 and 32 ml/min and showed no significant age-dependence. Cigarette smoking did not affect the half-life or the clearance. Additionally, neither the plasma binding (97.4 plus or minus 1.2%, mean plus or minus SD) nor the blood/plasma concentration ratio (0.58 plus or minus 0.16) of diazepam showed any age-related changes (P greater than 0.05). By contrast, analysis of the intravenous data according to a two-compartment open model indicated that both the initial distribution space (V1) and the volume of distribution at steady state [Vd(ss)] of diazepam increased linearly with age (P less than 0.005). The increase in Vd(ss) was secondary to the change in V1. It appears then that the prolongation of t 1/2 (beta) of diazepam with age is primarily dependent on an increase in the initial distribution volume of the drug. The plasma concentration/time course of the metabolite, desmethyldiazepam, was also affected by age. In older individuals, the initial presence and the peak values of desmethyldiazepam were observed later and the metabolite was present in lower concentrations. Despite the profound prolongation of t 1/2 (theta) with age, the constancy of diazepam clearance indicates that drug plasma concentrations will not accumulate any more in the old than the young, and chronic dosage more in the old than the young, and chronic dosage modifications based on pharmacokinetic considerations are unnecessary. Data obtained in patients with liver disease were compared with those found in age-matched control groups. Patients with cirrhosis showed a more than twofold prolongation in the half-life of diazepam (105.6 plus or minus 15.2 vs. 46.6 plus or minus 14.2 h, P less than 0.001).
Klotz U, Avant GR, Hoyumpa A, Schenker S, Wilkinson GR; The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest. 1975; 55 (2): 347-59. [PubMed Citation]
Animal
-
Five dogs received a single 1.0 mg/kg dose of diazepam (DZ) IV. Concentrations of DZ and its major metabolite desmethyldiazepam (DMDZ) were simultaneously measured in plasma and cisternal cerebrospinal fluid (CSF) for up to 8 h after the dose by electron-capture gas-liquid chromatography. DZ was rapidly eliminated from plasma (half-life 0.3--1.3 h); DZ disappearance was mirrored by formation of DMDZ, which in turn was eliminated slowly, Both DZ and DMDZ rapidly penetrated CSF and concentrations in CSF declined parallel with those in plasma. Despite rapid uptake, the extent of CSF transfer of DZ and DMDZ was limited by plasma protein binding. Mean CSF:plasma concentrtion ratios for DZ (range 0.023--0.137) and DMDZ (range 0.047--0.119) were highly correlated with the unbound fraction in plasma (r = 0.95 and 0.80, respectively). Thus DZ and DMDZ concentrations in CSF, presumed to reflect concentrations at the site of action, are determined by unbound plasma concentrations. The intensity of pharmacologic action is more likely to correlate with unbound than with total plasma concentrations.
Greenblatt DJ, Ochs HR, Lloyd BL; Entry of diazepam and its major matabolite into cerebrospinal fluid. Psychopharmacology (Berl) 1980; 70 (1): 89-93. [PubMed Citation]
Adults (FDA)
-
For the symptomatic treatment of anxiety, as an adjunct to other anticonvulsants in the prophylaxis of epileptic seizures, and as an adjunct in the relief of acute, painful musculoskeletal conditions and spasticity: the usual adult oral dosage of diazepam as conventional tablets or oral solution is 2-10 mg 2-4 times daily.
-
For the management of agitation associated with acute alcohol withdrawal: the usual adult oral dosage of diazepam as conventional tablets or oral solution is 10 mg 3 or 4 times daily during the first 24 hours, followed by 5 mg 3 or 4 times daily as needed.
-
Moderate or severe anxiety: the adult IV dose of diazepam for moderate or severe, acute anxiety is 2-5 mg or 5-10 mg, respectively. In acute conditions, the manufacturers state that diazepam may be administered hourly if necessary, although an interval of 3-4 hours is usually satisfactory. Some clinicians recommend that the adult dosage not exceed 30 mg within an 8-hour period.
-
For the management of agitation associated with acute alcohol withdrawal in adults: 10 mg may be administered IV initially; however, some clinicians recommend an initial dose of up to 20 mg. The manufacturers state that, if necessary, additional doses of 5-10 mg may be administered every hour, although an interval of 3-4 hours is usually satisfactory. For acute alcohol withdrawal, some clinicians recommend 10 mg of diazepam IV initially, followed by 10 mg at 20- to 30-minute intervals until the patient is calm.
-
To provide light anesthesia and anterograde amnesia in adults prior to electrical cardioversion: 5-15 mg of diazepam is given IV within 5-10 minutes prior to the procedure.
-
To reduce anxiety prior to endoscopy: diazepam is administered slowly IV immediately before the procedure; dosage is titrated to obtain the desired sedative response, such as slurring of speech. Generally, IV administration of up to 10 mg is adequate, but up to 20 mg may be required especially if opiates are not given concomitantly. If IV administration is not feasible, 5-10 mg of diazepam may be given IM approximately 30 minutes prior to endoscopy in adults.
-
For preoperative sedation in adults: 10 mg of diazepam may be administered parenterally 1-2 hours prior to surgery. Some clinicians have recommended a dose of up to 20 mg preoperatively.
-
For painful musculoskeletal conditions and spasticity in adults: 5-10 mg may be administered IV initially and 3-4 hours later if necessary. For tetanus in adults, larger doses may be required and up to 20 mg has been given every 2-8 hours.
-
Anticonvulsant: the usual initial IV anticonvulsant dose of diazepam in adults is 5-10 mg. Since diazepam may have a short duration of action after IV administration, it may be necessary to readminister the drug. The initial dose may be repeated at 10- to 15-minute intervals until a maximum total of 30 mg has been given. After seizures are terminated, appropriate maintenance anticonvulsant therapy should be instituted. If necessary, the initial dose of diazepam may be repeated in 2-4 hours. Diazepam may be given IM if seizures make IV administration impossible.
-
For sedation in intubated and mechanically ventilated adults during treatment in critical care settings: intermittent diazepam injections of 0.03-0.1 mg/kg may be given every 0.5-6 hours; however, more frequent administration may be required for the management of acutely agitated patients.
-
Status epilepticus: when parenteral solutions of diazepam are administered rectally for the management of status epilepticus, the usual dosage in adults is 0.5 mg/kg (not to exceed 20 mg).
-
For treatment of nerve agent or organophosphate induced seizures see CHEMM Acute Patient Care Guidelines for dosing recommendations - CHEMM Editor
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2622-2625
Children (FDA)
-
Dosage adjustment: children 6 months of age or older may receive an initial oral dosage of 1-2.5 mg 3 or 4 times daily as conventional tablets or oral solution. Alternatively, some clinicians recommend 0.12-0.8 mg/kg or 3.5-24 mg/m2 orally in 3 or 4 divided doses daily as conventional tablets. Dosage is adjusted gradually according to response and tolerance.
-
As an adjunct in the management of epilepsy in children: 6-15 mg daily (and occasionally up to 30 mg daily) in divided doses as conventional tablets or oral solution has been used by some clinicians.
-
Diazepam IV administration: in children, IV diazepam should be given slowly over a 3-minute period. The manufacturers recommend that the initial dose not exceed 0.25 mg/kg; the dose may be repeated at 15- to 30-minute intervals to a maximum total of 0.75 mg/kg.
-
For the management of seizures (e.g., status epilepticus) in children 30 days to 5 years of age: the usual initial IV dose of diazepam is 0.1-0.5 mg; this dose may be repeated every 2-5 minutes until a maximum total of 5-10 mg has been given. In children 5 years of age or older, the initial IV dose for the management of seizures is 1 mg; this dose may be repeated every 2-5 minutes until a maximum total of 10 mg has been given.
-
For tetanus in children: the manufacturers recommend 1-2 mg of diazepam for infants older than 30 days to 5 years of age and 5-10 mg for children older than 5 years of age administered slowly IV. This dose may be repeated every 3-4 hours as needed.
-
Although the safety and efficacy of parenteral diazepam in infants 30 days of age or younger have not been established, neonates with agitation due to opiate withdrawal have received 0.5-2 mg IM every 8 hours followed by gradual reduction in dosage.
-
Sedation in intubated and mechanically ventilated children older than 12 years of age during treatment in critical care settings: intermittent diazepam injections of 0.03-0.1 mg/kg may be given every 0.5-6 hours; however, more frequent administration may be required for the management of acutely agitated patients.
-
Status epilepticus: when parenteral solutions of diazepam are administered rectally for the management of status epilepticus, the usual dosage in children is 0.5 mg/kg (not to exceed 20 mg).
-
Acute repetitive seizures: when diazepam is administered rectally as the commercially available gel for the management of acute repetitive seizures, the dose should be individualized for maximum benefit. Children 2-5 years of age should receive 0.5 mg/kg and those 6-11 years of age should receive 0.3 mg/kg; adults and children 12 years of age and older should receive 0.2 mg/kg. These age-adjusted doses were based on the observation that diazepam clearance in children declines with age until about 12 years of age, at which time adult values are reached. The actual dose to be administered is determined by rounding up to the nearest commercially available dose (i.e., the next multiple of 2.5 mg). Using this method of rounded dosing, patients will receive 90-180% of the dose calculated on a weight and age basis. The safety of this dosing method has been established in clinical studies in adults and children 2 years of age and older.
-
For treatment of nerve agent or organophosphate induced seizures see CHEMM Acute Patient Care Guidelines for dosing recommendations - CHEMM Editor
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2622-2625
Geriatric (FDA)
-
The initial oral dosage of diazepam as conventional tablets or oral solution for geriatric or debilitated patients should be 2-2.5 mg once or twice daily. Dosage is adjusted gradually according to response and tolerance.
-
In geriatric or debilitated patients or in patients receiving other sedative drugs, single parenteral doses of 2-5 mg of diazepam should be used, and dosage should be slowly increased if needed.
-
For geriatric or debilitated patients, the dose of the rectal gel should be adjusted downward to reduce the likelihood of ataxia and oversedation. The 2.5-mg applicator also may be used to provide a partial replacement dose (supplemental dose) for patients who partially expel the recommended dose within 5 minutes after administration.
-
For treatment of nerve agent or organophosphate induced seizures see CHEMM Acute Patient Care Guidelines for dosing recommendations - CHEMM Editor
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2622-2625
Hepatic Impairment (FDA)
-
In mild and moderate cirrhosis, average half-life is increased. The average increase has been variously reported from 2-fold to 5-fold, with individual half-lives over 500 hours reported. There is also an increase in volume of distribution, and average clearance decreases by almost half. Mean half-life is also prolonged with hepatic fibrosis to 90 hours (range 66 - 104 hours), with chronic active hepatitis to 60 hours (range 26 - 76 hours), and with acute viral hepatitis to 74 hours (range 49 - 129). In chronic active hepatitis, clearance is decreased by almost half.
Product label Valium (Diazepam) tablet [Roche Products Inc] Last revised: April 2010 [DailyMed]
-
Five medically stable male patients with cirrhosis and four healthy age- and sex-matched controls received single 5-mg oral doses of diazepam (DZ) daily for 22 consecutive days. Plasma concentrations of DZ and its major metabolite desmethyldiazepam (DMDZ) were measured daily during the period of dosing and in the 7-day washout period that followed. Clinical self-ratings of sedation, fatigue, mood state, and sleep patterns were obtained daily during the period of dosage with the use of visual analogue scales. Steady-state plasma DZ concentrations were higher (165 and 98 ng/ml), and DMDZ concentrations tended to be higher (399 and 206 ng/ml), in cirrhotics than in controls. Increases in self-rated daytime sedation also were greater in cirrhotics than in controls and correlated strongly with total DZ and DMDZ plasma concentration during the first 2 wk of diazepam dosing. Thus, reduced clearance of DZ in cirrhotics leads to increased cumulation during long-term dosing. This in turn is associated with increased clinical sedation. Sedative effects are partly offset as treatment proceeds because of adaptation or tolerance. Based on kinetic findings, diazepam can be given safely to cirrhotic patients provided daily dosage is reduced by approximately 50%.
Ochs HR, Greenblatt DJ, Eckardt B, et al; Repeated diazepam dosing in cirrhotic patients: cumulation and sedation. Clin Pharmacol Ther 1983; 33 (4): 471-476.
Pregnancy (FDA)
FDA Pregnancy Risk Category:
-
D; POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective.
Product label Valium (Diazepam) tablet [Roche Products Inc] Last revised: April 2010 [DailyMed]
Nursing Mothers (FDA)
Lactation Risk Category:
-
L3 Moderately Safe; There are no controlled studies in breastfeeding women, however, the risk of untoward effects to a breastfed infant is possible; or, controlled studies show only a minimal non-threatening adverse effects. Drugs should be given only if the potential benefit justifies the potential risk to the infant.
-
L4 Possibly Hazardous (if used chronically); There is possible evidence of risk to breastfed infant or to breastmilk production, but the benefits from the use in breastfeeding mothers may be acceptable despite the risk to the infant (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective.)
Hale TW, Medications and Mother's Milk. Amarillo, TX: Hale Publishing L.P., 2006 p.250-252
Emergency Use Authorization (FDA/CDC)
-
No Emergency Use Authorization for Diazepam has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (Public Law 108-276).
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
5. Current available formulations/shelf life
Formulations
-
Oral Solution 5 mg/5 mL
-
Oral Solution, concentrate 5 mg/mL
-
Oral Tablets 2 mg, 5 mg, 10 mg
-
Parenteral Injection 5 mg/mL
-
Rectal Gel 5 mg/mL (2.5, 10, and 20 mg) (in prefilled applicators with pediatric universal or adult applicator tips)
FDA; Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Patent and Generic Drug Product Data Last Updated: August 09, 2012
-
CANA (Convulsive Antidote, Nerve Agent) is a sterile solution packaged within a device that delivers its entire 2 mL contents automatically upon activation. Each mL contains 5 mg diazepam.
Product label U.S. Army's Diazepam Auto-injector [Meridian Medical Technologies, Inc.] Last revised: September 1997 [FDA]
Shelf life
Shelf life
-
5 years (oral tablets)
-
3 years (parenteral formulation)
International Programme on Chemical Safety: Poisons Information Monograph for Diazepam (181) April (1998)
Storage
-
Diazepam tablets and extended-release capsules should be stored in tight, light-resistant containers at 15-30°C.
-
Diazepam injection should be protected from light and stored at 15-30°C; freezing should be avoided.
-
Diazepam oral solution and oral concentrate solution should be stored at 15-30°C.
-
Diazepam rectal gel should be stored at a controlled room temperature of 25° but may be exposed to temperatures ranging from 15-30°C.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2622-2625
6. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Insomnia: diazepam is also used in the treatment of insomnia. Failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric or medical illness. Worsening of insomnia or the emergence of new abnormalities of thinking or behavior may be the consequence of an unrecognized psychiatric or physical disorder.
-
Anxiety, (adjunct): Parenteral diazepam is indicated as adjuncts prior to endoscopic procedures if apprehension, anxiety, or acute stress reactions are present and to diminish patient's recall of the procedure. Safety and efficacy have not been established for the use of diazepam prior to bronchoscopy or laryngoscopy.
-
Panic disorders (treatment): Diazepam is used in the treatment of panic disorders.
-
Headache, tension (treatment): Diazepam is used in the treatment of tension headache.
-
Tremors (treatment): Oral diazepam is also used in the treatment of familial, senile, or essential action tremors.
Thomson/Micromedex 2007; Drug Information for the Health Care Professional: Volume 1, 27th Edition. Greenwood Village, CO. 2007 p.512-535
-
No therapy has been proved to be effective for patients with severe chloroquine poisoning, which is usually fatal. In a retrospective study of 51 cases, we found that ingestion of more than 5 g of chloroquine was an accurate predictor of a fatal outcome, and therefore chose this dose as the criterion for severe chloroquine poisoning. We selected as a control group 11 consecutive patients who had ingested more than 5 g of chloroquine between July 1983 and December 1985. We then undertook a prospective study to determine whether a better outcome could be obtained with immediate mechanical ventilation and the administration of diazepam and epinephrine. Eleven consecutive patients who ingested more than 5 g of chloroquine in 1986 received this combination therapy. Ten of these patients survived, whereas only one control had survived (P = 0.0003). There was no significant difference between the combination-therapy and control groups in age (29 +/- 3 vs. 27 +/- 2 years), amount of chloroquine ingested (7.5 +/- 0.5 vs. 8.5 +/- 0.8 g), systolic arterial pressure (74 +/- 2 vs. 74 +/- 3 mm Hg), or QRS duration (0.14 +/- 0.01 vs. 0.14 +/- 0.01 second). In our combination-therapy group, blood chloroquine levels ranged from 40 to 80 mumol per liter, whereas a literature search showed that no patient in whom blood levels were more than 25 mumol per liter had survived. These preliminary data suggest that combining early mechanical ventilation with the administration of diazepam and epinephrine may be effective in the treatment of severe chloroquine poisoning.
Riou B, Barriot P, Rimailho A, Baud FJ; Treatment of severe chloroquine poisoning. N Engl J Med. 1988 Jan 7; 318 (1): 1-6. [PubMed Citation]
Children
-
Preoperative sedation: although the manufacturers have not established pediatric dosage recommendations for preoperative sedation, some clinicians have recommended IM administration of 0.4 mg/kg of diazepam in children older than 2 years of age 1-2 hours prior to surgery.
-
Acute anxiety reactions in children, some clinicians recommend 0.04-0.2 mg/kg of diazepam IV; this dose may be repeated in 3-4 hours, but dosage should not exceed 0.6 mg/kg in an 8-hour period.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2622-2625
-
OBJECTIVE: To report a case of status epilepticus in a patient with anticonvulsant hypersensitivity syndrome (AHS) that was controlled successfully using continuous intravenous infusion diazepam. AHS and alternatives for treatment of status epilepticus are also reviewed. DESIGN: Single case report. SETTING: 217-bed children's university hospital. PATIENT: Four-year-old, 20-kg girl, diagnosed with idiopathic tonic-clonic epilepsy, who developed AHS to phenobarbital and phenytoin and status epilepticus unresponsive to lorazepam. RESULTS: Diazepam intravenous infusion at dosages titrated to 8 mg/h was used successfully to control seizures for eight days until signs and symptoms of AHS had resolved and maintenance therapy with valproic acid was started. CONCLUSIONS: Continuous intravenous infusion diazepam is a reasonable therapeutic choice for the management of status epilepticus in a patient with AHS when traditional therapy such as phenytoin and phenobarbital cannot be used.
Bertz RJ, Howrie DL; Diazepam by continuous intravenous infusion for status epilepticus inanticonvulsant hypersensitivity syndrome. Ann Pharmacother. 1993 Mar; 27 (3): 298-301. [PubMed Citation]
7. Route of Administration/Monitoring
-
Oral as a tablet or solution, IM or IV injection and a rectal gel.
FDA; Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Patent and Generic Drug Product Data Last Updated: August 09, 2012
-
Dosage of diazepam must be individualized, and the smallest effective dosage should be used (especially in geriatric or debilitated patients or in those with liver disease or low serum albumin) to avoid oversedation. The doses recommended by the manufacturers for IM and IV administration are identical. Because of the unpredictable response of children to CNS drugs, diazepam therapy should be initiated with the lowest dosage and increased as required. Since diazepam and its metabolites have long elimination half-lives, time to reach steady-state plasma concentrations should be considered when dosage adjustments are made.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2622-2625
-
Routine observation of vital signs, especially Glasgow Coma Scale (GCS) and airway patency, is indicated. Measurement of partial pressure of carbon dioxide via expired air or arterial blood gases is the best way to assess respiratory compromise from sedation.
Dart RC (ed): Medical Toxicology, 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2004 p.811-823
8. Adverse effects
-
Side effects most commonly reported were drowsiness, fatigue and ataxia; venous thrombosis and phlebitis at the site of injection. Other adverse reactions less frequently reported include:
-
CNS: confusion, depression, dysarthria, headache, hypoactivity, slurred speech, syncope, tremor, vertigo.
-
G.I.: constipation, nausea.
-
G.U.: incontinence, changes in libido, urinary retention.
-
Cardiovascular: bradycardia, cardiovascular collapse, hypotension.
-
EENT: blurred vision, diplopia, nystagmus.
-
Skin: urticaria, skin rash.
-
Other: hiccups, changes in salivation, neutropenia, jaundice.
-
Paradoxical reactions such as acute hyper-excited states, anxiety, hallucinations, increased muscle spasticity, insomnia, rage, sleep disturbances and stimulation have been reported; should these occur, use of the drug should be discontinued.
-
Minor changes in EEG patterns, usually low-voltage fast activity, have been observed in patients during and after diazepam therapy and are of no known significance.
-
In peroral endoscopic procedures, coughing, depressed respiration, dyspnea, hyperventilation, laryngospasm and pain in throat or chest have been reported.
-
Because of isolated reports of neutropenia and jaundice, periodic blood counts and liver function tests are advisable during long-term therapy.
Product label U.S. Army's Diazepam Auto-injector [Meridian Medical Technologies, Inc.] Last revised: September 1997 [FDA]
9. Contraindication(s)
-
Diazepam is contraindicated in patients with a known hypersensitivity to diazepam.
-
Due to lack of sufficient clinical experience, diazepam is contraindicated in pediatric patients under 6 months of age.
-
Diazepam is contraindicated in patients with myasthenia gravis, severe respiratory insufficiency, severe hepatic insufficiency, and sleep apnea syndrome.
-
Diazepam may be used in patients with open-angle glaucoma who are receiving appropriate therapy, but is contraindicated in acute narrow-angle glaucoma.
Product label Valium (Diazepam) tablet [Roche Products Inc] Last revised: April 2010 [DailyMed]
-
Intravenous administration of diazepam with the auto-injector is contraindicated.
Product label U.S. Army's Diazepam Auto-injector [Meridian Medical Technologies, Inc.] Last revised: September 1997 [FDA]
10. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues-
Title: Exploring the Effects of Diazepam and Lorazepam.
Condition: Healthy Subjects
Interventions: Drug: Diazepam; Drug: Lorazepam; Drug: placebo
ClinicalTrials.gov; Exploring the Effects of Diazepam and Lorazepam. (NIH)
-
Title: Study the Efficacy and Safety of Diazepam Auto-injector for Controled Episodes of Acute Repetitive Seizures.
Condition: Epilepsy
Intervention: Drug: Diazepam
ClinicalTrials.gov; Study the Efficacy and Safety of Diazepam Auto-injector for Controled Episodes of Acute Repetitive Seizures. (NIH)
-
Title: Intranasal Midazolam Versus Rectal Diazepam for Treatment of Seizures.
Condition: Seizures
Interventions: Drug: Midazolam; Drug: Diazepam
ClinicalTrials.gov; Intranasal Midazolam Versus Rectal Diazepam for Treatment of Seizures. (NIH)
-
Title: Efficacy and Safety Study Comparing Lorazepam and Diazepam for Children in the Emergency Department With Seizures (Status 2).
Condition: Status Epilepticus
Intervention: Drug: Lorazepam or Diazepam
ClinicalTrials.gov; Efficacy and Safety Study Comparing Lorazepam and Diazepam for Children in the Emergency Department With Seizures (Status 2). (NIH)
-
Title: Phase III Randomized Study of Diazepam Vs Lorazepam Vs Placebo for Prehospital Treatment of Status Epilepticus.
Condition: Status Epilepticus
Intervention: Drug: Diazepam; Drug: Lorazepam
ClinicalTrials.gov; Phase III Randomized Study of Diazepam Vs Lorazepam Vs Placebo for Prehospital Treatment of Status Epilepticus. (NIH)
11. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesNo data available at this time.
12. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations-
SHORT-TERM (0-3 YEARS)
Research that uses the latest technologies to better understand the mechanisms of injury for a variety of classes of chemical threat agents should be supported.
An assessment of the current military medical chemical defense products for use in the civilian population should be undertaken.
Countermeasures for chemical threat agents that protect all populations, e.g., the very young, the elderly, immunocompromised and pregnant individuals, should be evaluated and developed.
The expansion of label indications for the use of relevant licensed products following exposure to chemical threat agents should be pursued.
-
MID-TERM (3-5YEARS)
Research to determine the health effects of "low-level" exposure to chemical threat agents, with emphasis on the organ, tissue, genetic, and molecular levels, should be supported.
Appropriate animal models useful in the study of the effects of chemical threat agents, including non-human primates, should be developed.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
13. Needed studies for non Chemical Defense clinical indications
— including review panel recommendationsNo data available at this time.
14. Study-related ethical concerns
— including review panel recommendationsEthical concerns include:
-
Informed consent for data collection in disaster/mass casualty situation
-
Central institutional review boards (IRBs)
-
How to conduct multicenter studies
Poison centers
-
Pediatric Emergency Care Applied Research Network
Hospital consortia.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
15. Global regulatory status
U.S.
-
Diazepam Tablets
Diazepam is indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
In acute alcohol withdrawal, diazepam may be useful in the symptomatic relief of acute agitation, tremor, impending or acute delirium tremens and hallucinosis.
Diazepam is a useful adjunct for the relief of skeletal muscle spasm due to reflex spasm to local pathology (such as inflammation of the muscles or joints, or secondary to trauma); spasticity caused by upper motor neuron disorders (such as cerebral palsy and paraplegia); athetosis; and stiff-man syndrome.
Oral diazepam may be used adjunctively in convulsive disorders, although it has not proved useful as the sole therapy.
Product label Valium (Diazepam) tablet [Roche Products Inc] Last revised: April 2010 [DailyMed]
-
Diazepam Injection
As an adjunct prior to endoscopic procedures if apprehension, anxiety or acute stress reactions are present, and to diminish the patient's recall of the procedures.
Diazepam is a useful premedication (the IM route is preferred) for relief of anxiety and tension in patients who are to undergo surgical procedures. Intravenously, it is used prior to cardioversion for the relief of anxiety and tension and to diminish the patient's recall of the procedure.
Product label Diazepam (Diazepam) Injection [Baxter Healthcare Corporation] Last revised: February 2006 [DailyMed]
-
Diazepam Gel
Diazepam rectal gel is a gel formulation of diazepam intended for rectal administration in the management of selected, refractory, patients with epilepsy, on stable regimens of AEDs, who require intermittent use of diazepam to control bouts of increased seizure activity.
Product label Diastat (Diazepam) Gel [Valeant Pharmaceuticals International] Last revised: October 2007 [DailyMed]
U.K.
-
Short-term use in anxiety or insomnia; adjunct in acute alcohol withdrawal; status epilepticus; febrile convulsions; muscle spasm; peri-operative use.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.191
16. Other potentially useful information
-
Diazepam injection is classified by the Drug Enforcement Administration as a schedule IV controlled substance.
Product label Valium (Diazepam) tablet [Roche Products Inc] Last revised: April 2010 [DailyMed]
-
The anticonvulsant diazepam has been used therapeutically in the treatment of both convulsant (e.g., picrotoxin, hydrazine, strychnine, tetramethylenedisulfotetramine) and cholinergic (e.g., sarin (GB), soman (GD), cyclosarin (GF), tabun (GA), VX, and organophosphorus pesticides) agents to enhance the inhibitory effect of gamma-aminobutyric acid at GABA(a) receptors and limit excess central nervous system excitation and seizures.
Department of Homeland Security (DHS). Chemical Security Analysis Center (CSAC). 2011. Chemical Segregation by Toxidrome for the Chemical Terrorism Risk Assessment. PowerPoint available in Report on the Toxic Chemical Syndrome Definition and Nomenclature Workshop (May, 2012)
-
Octanol/Water Partition Coefficient: log Kow = 2.82
HSDB. Diazepam
17. Publications
Atack JR. The benzodiazepine binding site of GABA(A) receptors as a target for the development of novel anxiolytics. Expert Opin Investig Drugs. 2005; 14 (5): 601-618. [PubMed Citation]
Anderson DR, Harris LW, Bowersox SL, Lennox WJ, Anders JC. Efficacy of injectable anticholinergic drugs against soman-induced convulsive/subconvulsive activity. Drug Chem Toxicol 1994; 17 (2): 139-48. [PubMed Citation]
Anderson DR, Harris LW, Chang FC, Baze WB, Capacio BR, Byers SL, Lennox WJ; Antagonism of soman-induced convulsions by midazolam, diazepam and scopolamine. Drug Chem Toxicol. 1997 Aug; 20 (3): 115-31. [PubMed Citation]
Bertz RJ, Howrie DL; Diazepam by continuous intravenous infusion for status epilepticus inanticonvulsant hypersensitivity syndrome. Ann Pharmacother. 1993 Mar; 27 (3): 298-301. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Bokonjić D, Rosić N; Anticonvulsive and protective effects of diazepam and midazolam in rats poisoned by highly toxic organophosphorus compounds.
Arh Hig Rada Toksikol. 1991 Dec; 42 (4): 359-65. [PubMed Citation]
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.399-403
Cannard K; The acute treatment of nerve agent exposure. J Neurol Sci 2006; 249 (1): 86-94. [PubMed Citation]
Castro CA, Larsen T, Finger AV, Solana RP, McMaster SB; Behavioral efficacy of diazepam against nerve agent exposure in rhesus monkeys. Pharmacol Biochem Behav. 1992; 41 (1): 159-164. [PubMed Citation]
Collombet JM; Nerve agent intoxication: Recent neuropathophysiological findings and subsequent impact on medical management prospects. Toxicology and Applied Pharmacology 2011; 255 (3): 229-241. [PubMed Citation]
Dart RC (ed): Medical Toxicology, 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2004 p.811-823
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
Divoll M, Greenblatt DJ, Ochs HR, Shader RI; Absolute bioavailability of oral and intramuscular diazepam: effects of age and sex. Anesth Analg. 1983; 62 (1): 1-8. [PubMed Citation]
FDA; Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Patent and Generic Drug Product Data Last Updated: August 09, 2012
Greenblatt DJ, Ochs HR, Lloyd BL; Entry of diazepam and its major matabolite into cerebrospinal fluid. Psychopharmacology (Berl) 1980; 70 (1): 89-93. [PubMed Citation]
Hale TW, Medications and Mother's Milk. Amarillo, TX: Hale Publishing L.P., 2006 p.250-252
Harris LW, Gennings C, Carter WH, Anderson DR, Lennox WJ, Bowersox SL, Solana RP; Efficacy comparison of scopolamine (SCP) and diazepam (DZ) against soman-induced lethality in guinea pigs. Drug Chem Toxicol. 1994; 17 (1): 35-50. [PubMed Citation]
Hayward IJ, Wall HG, Jaax NK, Wade JV, Marlow DD, Nold JB; Decreased brain pathology in organophosphate-exposed rhesus monkeys following benzodiazepine therapy. J Neurol Sci. 1990 Aug; 98 (1): 99-106. [PubMed Citation]
HSDB. Diazepam
International Programme on Chemical Safety: Poisons Information Monograph for Diazepam (181) April (1998)
Joosen MJ, van der Schans MJ, van Helden HP; Percutaneous exposure to the nerve agent VX: Efficacy of combined atropine, obidoxime and diazepam treatment. Chemico-Biological Interactions 2010; 188 (1): 255-263. [PubMed Citation]
Jørgensen NP, Thurmann-Nielsen E, Walstad RA; Pharmacokinetics and distribution of diazepam and oxazepam in early pregnancy. Acta Obstet Gynecol Scand. 1988; 67 (6): 493-497. [PubMed Citation]
Klotz U, Avant GR, Hoyumpa A, Schenker S, Wilkinson GR; The effects of age and liver disease on the disposition and elimination of diazepam in adult man. J Clin Invest. 1975; 55 (2): 347-59. [PubMed Citation]
Klotz U, Antonin KH, Bieck PR; Pharmacokinetics and plasma binding of diazepam in man, dog, rabbit, guinea pig and rat. J Pharmacol Exp Ther. 1976; 199 (1): 67-73. [PubMed Citation]
Klotz U, Antonin KH, Brügel H, Bieck PR; Disposition of diazepam and its major metabolite desmethyldiazepam in patients with liver disease. Clin Pharmacol Ther. 1977; 21 (4): 430-6. [PubMed Citation]
Klotz U. Important factors determining human distribution and elimination of diazepam. Fortschr Med. 1977; 95 (32): 1958-64. [PubMed Citation]
Koplovitz I, Schulz S, Shutz M, Railer R, Macalalag R, Schons M, McDonough J; Combination anticonvulsant treatment of soman-induced seizures. J Appl Toxicol. 2001 Dec; 21 Suppl 1:S53-5. [PubMed Citation]
Lamson MJ, Sitki-Green D, Wannarka GL, et al.; Pharmacokinetics of diazepam administered intramuscularly by autoinjector versus rectal gel in healthy subjects: a phase I, randomized, open-label, single-dose, crossover, single-centre study. Clin Drug Investig. 2011; 31 (8): 585-597. [PubMed Citation]
Lehmann C, Wannarka GL; Bioavailability and dose proportionality of intramuscular diazepam administered by autoinjector. J Clin Pharmacol. 2008; 48 (4): 436-444. [PubMed Citation]
Mandelli M, Tognoni G, Garattini S; Clinical pharmacokinetics of diazepam. Clin Pharmacokinet. 1978; 3 (1): 72-91. [PubMed Citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.191
Masson P; Evolution of and perspectives on therapeutic approaches to nerve agent poisoning. Toxicology Letters 2011; 206 (1): 5-13. [PubMed Citation]
McDonough JH Jr, McMonagle J, Copeland T, Zoeffel D, Shih TM; Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol 1999; 73 (8-9): 473-478. [PubMed Citation]
McDonough JH, McMonagle JD, Shih TM; Time-dependent reduction in the anticonvulsant effectiveness of diazepam against soman-induced seizures in guinea pigs. Drug Chem Toxicol 2010 Jul; 33 (3): 279-83. [PubMed Citation]
McDonough JH Jr, Zoeffel LD, McMonagle J , Copeland TL, Smith CD, Shih TM; Anticonvulsant treatment of nerve agent seizures: anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Research 2000; 38 (1): 1-14. [PubMed Citation]
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2622-2625
Ochs HR, Greenblatt DJ, Eckardt B, et al; Repeated diazepam dosing in cirrhotic patients: cumulation and sedation. Clin Pharmacol Ther 1983; 33 (4): 471-476.
Product label Diastat (Diazepam) Gel [Valeant Pharmaceuticals International] Last revised: October 2007 [DailyMed]
Product label Diazepam (Diazepam) Injection [Baxter Healthcare Corporation] Last revised: February 2006 [DailyMed]
Product label U.S. Army's Diazepam Auto-injector [Meridian Medical Technologies, Inc.] Last revised: September 1997 [FDA]
Product label Valium (Diazepam) tablet [Roche Products Inc] Last revised: April 2010 [DailyMed]
Riou B, Barriot P, Rimailho A, Baud FJ; Treatment of severe chloroquine poisoning. N Engl J Med. 1988 Jan 7; 318 (1): 1-6. [PubMed Citation]
Riss J, Cloyd J, Gates J, Collins S; Benzodiazepines in epilepsy: pharmacology and pharmacokinetics. Acta Neurol Scand. 2008; 118 (2): 69-86. [PubMed Citation]
Rotenberg JS, Newmark J; Nerve Agent Attacks on Children: Diagnosis and Management. Pediatrics 2003; 112 (3 Pt 1): 648-658. [PubMed Citation]
Schlumpf M, Ramseier H, Abriel H, Youmbi M, Baumann JB, Lictensteiger W; Diazepam effects on the fetus. Neurotoxicology 1989; 10 (3): 501-516. [PubMed Citation]
Shih T, McDonough JH Jr, Koplovitz I. Anticonvulsants for Soman-induced Seizure Activity. J Biomed Sci. 1999; 6 (2): 86-96. [PubMed Citation]
Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003 Apr 15;188(2):69-80. [PubMed Citation]
Shih TM, Rowland TC, and McDonough JH. Anticonvulsants for Nerve Agent-Induced Seizures: The Influence of the Therapeutic Dose of Atropine. J PET 2007; 320 (1): 154-161. [PubMed Citation]
Simmons RD, Miller RK, Kellogg CK; Prenatal diazepam: distribution and metabolism in perinatal rats. Teratology 1983; 28 (2): 181-188. [PubMed Citation]
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
Talmor D; Nonconventional Terror- The Anesthesiologist's Role in a Nerve Agent Event. Anesthesiology Clin 2007; 25 (1): 189-199. [PubMed Citation]
Taysse L, Calvet JH, Buee J, Christin D, Delamanche S, Breton P; Comparative efficacy of diazepam and avizafone against sarin-induced neuropathology and respiratory failure in guinea pigs: influence of atropine dose. Toxicol 2003 Jun; 188 (2-3): 197-209. [PubMed Citation]
Thomson/Micromedex 2007; Drug Information for the Health Care Professional: Volume 1, 27th Edition. Greenwood Village, CO. 2007 p.512-535
Todorovic MS, Cowan ML, Balint CA, Sun C, Kapur J; Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res 2012 Sep; 101 (3): 268-76. [PubMed Citation]
Tsung-Ming Shih, Tami C. Rowland, and John H. McDonough. Anticonvulsants for Nerve Agent-Induced Seizures: The Influence of the Therapeutic Dose of Atropine. J PET 2007; 320 (1): 154-161. [PubMed Citation]
Vale A, Marrs TC, Rice P; Chemical terrorism and nerve agents. Medicine (UK) 2012; 40 (2): 77-79.
Department of Homeland Security (DHS). Chemical Security Analysis Center (CSAC). 2011. Chemical Segregation by Toxidrome for the Chemical Terrorism Risk Assessment. PowerPoint available in Report on the Toxic Chemical Syndrome Definition and Nomenclature Workshop (May, 2012)
Willems JL, Nicaise M, De Bisschop HC; Delayed neuropathy by the organophosphorus nerve agents soman and tabun. Arch Toxicol. 1984; 55 (1): 76-77. [PubMed Citation]
18. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
