You are here: Home > Medical Countermeasures Database > HI-6
HI-6 - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
HI-6
2. Chemical Defense therapeutic area(s)
— including key possible usesAntidote for organophosphorous nerve agent poisoning including chlorosarin, cyclosarin (GF), R-33 (VR), R-VX, sarin (GB), tabun (GA), VX, chlorosoman, soman (GD), and organophosphorous pesticides.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
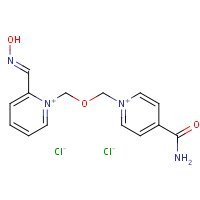
US NLM. ChemIDplus Lite. HI-6
Mechanism of action
-
Current treatment of organophosphorus poisoning, resulting in overstimulation and desensitization of muscarinic and nicotinic receptors by acetylcholine (ACh), consists of the administration of atropine and oxime reactivators. However, no versatile oxime reactivator has been developed yet and some mortality still remains after application of standard atropine treatment, probably due to its lack of antinicotinic action. In our study, we focused on the interesting non-acetylcholinesterase property of oximes, i.e. antinicotinic effect of reactivators. Two standard reactivators (HI-6, obidoxime) and two new compounds (K027 and K203) were chosen for in vitro (patch clamp) and in vivo (nerve-evoked muscle contraction) testings. Both examinations showed antinicotinic effects of the reactivators. In vitro inhibition of acetylcholine-evoked currents by obidoxime, HI-6 and K203 was equivalent while K027 was less potent. Similar order of potency was observed by the in vivo examinations. We thus confirm previous in vitro results, which describe antinicotinic effects of oxime reactivators, and furthermore, we show in vivo antagonism of oxime reactivators exerted by the inhibition of ACh effect on the nicotinic receptor in the neuromuscular junction. Taking together, the effects of tested oxime reactivators indicate an antagonism on both embryonic and adult form of the muscle nicotinic receptors.
Soukup O, Krůšek J, Kaniaková M, Kumar UK, Oz M, Jun D, Fusek J, Kuča K, Tobin G. Oxime reactivators and their in vivo and in vitro effects on nicotinic receptors. Physiol Res. 2011;60(4):679-86. [PubMed Citation]
-
Organophosphorus poisoning manifests as a cholinergic syndrome due to an inhibition of acetylcholinesterase. It is treated symptomatically by anticholinergics and oxime reactivators are used as causal antidotes. Reactivators possess a complex mechanism of action and interact at various levels of the cholinergic transmission. The aim of this study was to investigate the effect of standard oxime reactivators (HI-6, obidoxime, trimedoxime, methoxime and pralidoxime) on the hemicholinium-3 sensitive carriers, which are involved in the high-affinity choline uptake (HACU) transport, a key regulatory step in the synthesis of acetylcholine. The activity of the carriers was estimated in vitro on hippocampal synaptosomes using the substrate (3H)-choline and the competitive inhibitor (3H)-hemicholinium-3. Furthermore, the effect of the reactivators on the fluidity of hippocampal membranes was assessed. All tested compounds, except methoxime, showed an acute inhibitory effect on the carriers, however, only at μM concentrations. Trimedoxime showed the highest potency to inhibit HACU among all tested compounds (I(max) 62%, IC(50)=3μM). All compounds, except HI-6, influenced also a membrane fluidity in the region of the hydrophilic heads of phospholipid bilayer, nevertheless, only methoxime was able to penetrate more deeply into the hydrocarbon core. We suggest that the direct interaction of oxime reactivators with the carrier protein (HI-6 and trimedoxime) and/or the changes in carrier conformation mediated by alterations in membrane fluidity (trimedoxime, obidoxime and pralidoxime) could occur here. The influence of reactivators on the carriers could be unfavorable in the case of their prolonged administration in vivo. From this point of view, the application of methoxime appears to be the best.
Soukup O, Kristofikova Z, Jun D, Tambor V, Ripova D, Kuca K. The interaction of standard oxime reactivators with hemicholinium-3 sensitive choline carriers. Toxicol Lett. 2012 Aug 3;212(3):315-9. [PubMed Citation]
-
The in vivo sensitivity of the molecular forms of the enzyme acetylcholinesterase to inhibition by either soman or sarin, reactivation by HI-6 and the time course of recovery following inhibition by soman were investigated in mice. Administration of HI-6 (50 mg/kg, i.p.) immediately after soman (100 micrograms/kg, s.c.) or sarin (150 micrograms/kg, s.c.) resulted in an apparent selective reactivation of the 10S and 16S molecular forms of acetylcholinesterase and no reactivation of the 4S form of diaphragm acetylcholinesterase. The apparent selectivity of the reactivation of the molecular forms of the acetylcholinesterase was probably due to the fact that the 10S and 16S forms of acetylcholinesterase are located primarily extracellularly and the 4S form intracellularly. The HI-6 was restricted primarily to the extracellular compartment due to its quaternary, hydrophilic nature. If the administration of HI-6 was delayed until 60 min following soman (100 micrograms/kg, s.c.) injection, no reactivation of any of the molecular forms of acetylcholinesterase could be found in the diaphragm. The soman-inhibited acetylcholinesterase had probably aged and, thus, was not susceptible to reactivation by HI-6. The time course of recovery of the molecular forms in the diaphragm occurred rather quickly with the smaller 4S and 10S forms recovering to control levels faster than the larger 16S form. It took between 8 and 16 days for the 16S form to recover to normal. In the brain, hypothalamic acetylcholinesterase molecular forms such as the 4S recovered faster than the 10S form which had not recovered to control 16 days after soman administration; the 16S form of acetylcholinesterase was not detected in the brain.
Clement JG, Rosario S, Bessette E, Erhardt N. Soman and sarin inhibition of molecular forms of acetylcholinesterase in mice. Time course of recovery and reactivation by the oxime HI-6. Biochem Pharmacol. 1991 Jul 5;42(2):329-35.
-
The antidotal treatment of organophosphorus poisoning is still a problematic issue since no versatile antidote has been developed yet. In our study, we focused on an interesting property, which does not relate to the reactivation of inhibited acetylcholinesterase (AChE) of some oximes, but refers to their anti-muscarinic effects which may contribute considerably to their treatment efficacy. One standard reactivator (HI-6) and two new compounds (K027 and K203) have been investigated for their antimuscarinic properties. Anti-muscarinic effects were studies by means of an in vitro stimulated atrium preparation (functional test), the [(3)H]-QNB binding assay and G-protein coupled receptor assay (GPCR, beta-Arrestin Assay). Based on the functional data HI-6 demonstrates the highest anti-muscarinic effect. However, only when comparing [(3)H]-QNB binding results and GPCR data, K203 shows a very promising compound with regard to anti-muscarinic potency. The therapeutic impact of these findings has been discussed.
Soukup O, Kumar UK, Proska J, Bratova L, Adem A, Jun D, Fusek J, Kuca K, Tobin G. Environ Toxicol Pharmacol. The effect of oxime reactivators on muscarinic receptors: functional and binding examinations. 2011 May;31(3):364-70. [PubMed Citation]
-
The toxicity of organophosphorus (OP) nerve agents is manifested through irreversible inhibition of acetylcholinesterase (AChE) at the cholinergic synapses, which stops nerve signal transmission, resulting in a cholinergic crisis and eventually death of the poisoned person. Oxime compounds used in nerve agent antidote regimen reactivate nerve agent-inhibited AChE and halt the development of this cholinergic crisis. Due to diversity in structures of OP nerve agents, none of the currently available oximes is able to reactivate AChE inhibited by different nerve agents. To understand the mechanism for the differential activities of oximes toward AChE inhibited by diverse nerve agents in order to aid the design of new broad-spectrum AChE reactivators, we undertook site-directed mutagenesis and molecular modeling studies. Recombinant wild-type and mutant bovine (Bo) AChEs were inhibited by two bulky side-chain nerve agents, GF and VR, and used for conducting reactivation kinetics with five oximes. A homology model for wild-type Bo AChE was built using the recently published crystal structure of human AChE and used to generate models of 2-PAM and HI-6 bound to the active-sites of GF- and VR-inhibited Bo AChEs before nucleophilic attack. Results revealed that the peripheral anionic site (PAS) of AChE as a whole plays a critical role in the reactivation of nerve agent-inhibited AChE by all 4 bis-pyridinium oximes examined, but not by the mono-pyridinium oxime 2-PAM. Of all the residues at the PAS, Y124 appears to be critical for the enhanced reactivation potency of H oximes.
Luo C, Chambers C, Pattabiraman N, Tong M, Tipparaju P, Saxena A. Y124 at the peripheral anionic site is important for the reactivation of nerve agent-inhibited acetylcholinesterase by H oximes. Biochem Pharmacol. 2010 Nov 1;80(9):1427-36. [PubMed Citation]
-
Oxime-induced reactivation of organophosphorus (OP) nerve agent-inhibited acetylcholinesterase (AChE) is a very important step for the treatment of nerve agent toxicity. Therefore, extensive efforts are being made to develop more efficient and broad-spectrum oximes to replace the currently used oximes 2-PAM or obidoxime. In the 1970s and 1980s, several H oximes (such as HI-6 and HLo-7) were found to be very potent reactivators of non-aged soman-inhibited AChE. Later these oximes were shown to rapidly reactivate GF- and VR-inhibited AChE as well. However, the mechanism for the high potency of these H oximes is still unknown. In this study, the relationship between the reactivation rate constant of nerve agent-inhibited rhesus monkey AChE, human AChE and guinea pig AChE and the size of the alkoxyl (OR) group of nerve agents was analyzed. Results demonstrate that for nerve agent-inhibited rhesus monkey and human AChEs, reactivation by H oximes accelerated as the size of the OR group was increased. But with guinea pig AChE, reactivation by H oximes declined as the size of the OR group was increased. Reactivation kinetic study using GF- and VR-inhibited wild-type and mutant bovine AChEs has shown that mutations of Y124Q and W286A particularly reduced reactivation by these H oximes. Since these 2 amino acid residues are highly conserved in all AChEs sequenced to date, it is unlikely that the remarkable reduction observed in H oxime reactivation with guinea pig AChE is caused by a change in these two amino acid residues.
Luo C, Chambers C, Yang Y, Saxena A. Mechanism for potent reactivation ability of H oximes analyzed by reactivation kinetic studies with cholinesterases from different species. Chem Biol Interact. 2010 Sep 6;187(1-3):185-90. [PubMed Citation]
-
There is an ongoing debate whether oximes can effectively counteract the effects of organophosphorus compounds (OP) on brain acetylcholinesterase (AChE) activity and whether there are differences in the kinetic properties of brain and erythrocyte AChE. In order to investigate the kinetics of AChE from different tissues and species the well established dynamically working in vitro model with real-time determination of membrane-bound AChE activity was adapted for use with brain AChE. The enzyme reactor, that was loaded with brain, erythrocyte or muscle AChE, was continuously perfused with substrate and chromogen while AChE activity was on-line analyzed in a flow-through detector. It was possible to determine the Michaelis-Menten constants of human erythrocyte, muscle and brain AChE which were almost identical. In addition, the inhibition kinetics of sarin and paraoxon as well as the reactivation kinetics of obidoxime and HI 6 were determined with human, swine and guinea pig brain and erythrocyte AChE. It was found that the inhibition and reactivation kinetics of brain and erythrocyte AChE were highly comparable in all tested species. These data support the view that AChE from different tissue has similar kinetic properties and that brain AChE is comparably susceptible toward reactivation by oximes.
Herkert NM, Freude G, Kunz U, Thiermann H, Worek F. Comparative kinetics of organophosphates and oximes with erythrocyte, muscle and brain acetylcholinesterase. Toxicol Lett. 2012 Mar 7;209(2):173-8. [PubMed Citation]
-
The reactivation of nerve agent-inhibited acetylcholinesterase (AChE) by oxime is the most important step in the treatment of nerve agent poisoning. Since the evaluation of nerve agent antidotes cannot be conducted in humans, results from animal experiments are extrapolated to humans. Guinea pig is one of the animal models that is frequently used for conducting nerve agent antidote evaluations. Several investigations have demonstrated that the efficacy of an oxime primarily depends on its ability to reactivate nerve agent-inhibited AChE. If the in vitro oxime reactivation of nerve agent-inhibited animal AChE is similar to that of human AChE, it is likely that the results of an in vivo animal study will reliably extrapolate to humans. Therefore, the goal of this study was to compare the reactivation of guinea pig and human AChEs inhibited by six different G and V type nerve agents. Reactivation kinetic studies with five mono- and bis-pyridinium oximes showed that oxime reactivation of nerve agent-inhibited human AChE in most cases was faster than guinea pig AChE. The most significant enhancement was observed in the reactivation of human AChE inhibited by nerve agents containing bulky side chains GF, GD, and VR, by H-series oximes HLo-7, HI-6, and ICD-585. In these cases, species-related differences observed between the two AChEs, based on the second-order reactivation rate constants, were 90- to over 400-fold. On the other hand, less than 3-fold differences were observed in the rates of aging of nerve agent-inhibited guinea pig and human AChEs. These results suggest that the remarkable species-related differences observed in the reactivation of nerve agent-inhibited guinea pig and human AChEs were not due to differences in the rates of aging. These results also suggest that guinea pig may not be an appropriate animal model for the in vivo evaluation of oxime therapy.
Luo C, Tong M, Chilukuri N, Brecht K, Maxwell DM, Saxena A. An in vitro comparative study on the reactivation of nerve agent-inhibited guinea pig and human acetylcholinesterases by oximes. Biochemistry. 2007 Oct 23;46(42):11771-9 [PubMed Citation]
-
Non-human primates are valuable animal models that are used for the evaluation of nerve agent toxicity as well as antidotes and results from animal experiments are extrapolated to humans. It has been demonstrated that the efficacy of an oxime primarily depends on its ability to reactivate nerve agent-inhibited acetylcholinesterase (AChE). If the in vitro oxime reactivation of nerve agent-inhibited animal AChE is similar to that of human AChE, it is likely that the results of an in vivo animal study will reliably extrapolate to humans. Therefore, the goal of this study was to compare the aging and reactivation of human and different monkey (Rhesus, Cynomolgus, and African Green) AChEs inhibited by GF, GD, and VR. The oximes examined include the traditional oxime 2-PAM, two H-oximes HI-6 and HLo-7, and the new candidate oxime MMB4. Results indicate that oxime reactivation of all three monkey AChEs was very similar to human AChE. The maximum difference in the second-order reactivation rate constant between human and three monkey AChEs or between AChEs from different monkey species was 5-fold. Aging rate constants of GF-, GD-, and VR-inhibited monkey AChEs were very similar to human AChE except for GF-inhibited monkey AChEs, which aged 2-3 times faster than the human enzyme. The results of this study suggest that all three monkey species are suitable animal models for nerve agent antidote evaluation since monkey AChEs possess similar biochemical/pharmacological properties to human AChE.
Luo C, Tong M, Maxwell DM, Saxena A. Comparison of oxime reactivation and aging of nerve agent-inhibited monkey and human acetylcholinesterases. Chem Biol Interact. 2008 Sep 25;175(1-3):261-6. [PubMed Citation]
-
The purpose of this work was to evaluate the possible non-reactivating effects of toxogonin (1,1'[oxybis(methylene)]bis[4-[hydroxyimino) methyl]pyridinium]-dichloride), HI-6 (1-[[[(4-aminocarbonyl)pyridinio] methoxy]methyl]-2-[(hydroxyimino)methyl]pyridinium-dichloride) and HLö-7 (pyridinium, 1-[[[4-(aminocarbonyl)pyridino]methoxy] methyl]-2,4-bis-[(hydroxyimino)methyl]diiodide) on the release of acetylcholine from cholinergic nerves. The oximes have been tested in our rat bronchial smooth muscle model, with respect to the effects of oximes on the K+ (51 mM)-evoked release of [3H]acetylcholine in the presence and absence of soman (1.0 microM). Toxogonin (100 microM) had no effect on the K(+)-evoked release of [3H]acetylcholine in the presence or absence of soman (1.0 microM). Similar results were found for HI-6 (100 microM). In contrast, HLö-7 (100 microM) enhanced the K(+)-evoked release of [3H]acetylcholine in the absence of soman. In the presence of soman HLö-7 did not alter the release of [3H]acetylcholine induced by K+ stimulation. The potentiating effect of HLö-7 on the release of [3H]acetylcholine could be blocked by the L-, N- and P-Ca2+ channel blockers verapamil (0.1 and 1.0 microM), omega-conotoxin GVIA (1.0 microM) and omega-agatoxin IV-A (0.2 microM), respectively. Muscarinic receptor antagonists (atropine (10 microM), pirenzepine (M1) (1.0 microM) and methoctramine (M2) (1.0 microM) had no effects on the HLö-7 (100 microM)-enhanced release of [3H]acetylcholine. Protein kinase inhibitors (H-7 (20 microM), calphostin C (1.0 microM) and KN-62 (10 microM) inhibited the HLö-7 (100 microM)-enhanced K(+)-evoked release of [3H]acetylcholine. The results showed that only HLö-7 had a direct enhancing effect on the release of acetylcholine through activation or opening of Ca2+ channels and a subsequent protein phosphorylation in the nerve terminal.
Aas P. In vitro effects of toxogonin, HI-6 and HLö-7 on the release of [3H]acetylcholine from peripheral cholinergic nerves in rat airway smooth muscle. Eur J Pharmacol. 1996 Apr 22;301(1-3):59-66. [PubMed Citation]
Summary of clinical and non-clinical studies
Organophosphorous (OP) nerve agents (including soman, sarin, tabun, GF, VX, and Russian VX) and pesticides inhibit acetylcholinesterase (AChE), subsequently causing a toxic accumulation of acetylcholine (ACh) and over-stimulation of cholinergic receptors in the peripheral and central nervous systems. The current antidotal treatment includes a muscarinic ACh receptor antagonist to block the over-stimulation of cholinergic receptors by ACh, an anticonvulsant to protect against seizures, and an oxime to reactivate OP-inhibited AChE. The bis pyridinium oxime HI-6 (asoxime; 1-[[[4-(aminocarbonyl pyridinio] methoxy] methyl]-2-[(hydroxyimino)methyl]-pyridinium dichloride) is currently available for use in defined military settings in Canada, Sweden and the Czech Republic, and is under development in a number of other countries (Lundy et al., 2011). HI-6 is a broad spectrum reactivator of AChE that is inhibited by nerve agents; it has a reactivation potency of 40-79% for Russian VX, sarin and cyclosarin (Kuca et al., 2007). HI-6 has consistently been the most effective reactivator of soman-inhibited AChE when tested in a variety of animal models (blood and tissue), and humans (blood) compared to other oximes, including the currently developed pralidoxime (2-PAM) and obidoxime (Koplovitz and Stewart, 1994; Shih, 1993; Worek et al., 1998; Puu et al., 1986). HI-6 was also more effective at increasing survival following 4 LD50 soman challenge in guinea pigs than oximes HLo-7 and pyrimidoxime (Lundy et al., 1992). HI-6 is also highly effective against GF (Lundy et al., 1992; Luo et al., 2008) but lacks efficacy against tabun (Koplovitz and Stewart, 1994; Kuca et al., 2007). Atropine dose-dependently increased the effectiveness of HI-6 against soman and tabun; adjunctive treatment with the anticonvulsant diazepam further enhanced the efficacy of HI-6 and atropine against soman (Koplovitz et al., 1995). The toxicities of HI-6 are low with an LD50 of 400 mg/kg and approximately 615 mg/kg in rhesus monkeys and dogs, respectively. Because of the efficacy and broad spectrum of nerve agent reactivation, efforts to increase the usage and licensing of HI-6 are underway (Lundy, 2011). An HI-6 salt derivative (HI-6 dimethanesulphonate) with increased water solubility, for potential use in an autoinjector, is currently under development and has been shown to have the same reactivation potency in vivo as the currently used HI-6 chloride (Kassa et al., 2007).
B. Link to clinical studies
Adult
-
Three oximes currently being evaluated for adoption as replacement nerve agent therapy by various countries were compared for therapeutic efficacy against the toxic organophosphate inhibitors soman and tabun under a standard set of conditions. These oximes together with PAM-CI and toxogonin, were also compared for efficacy against GF, an agent weaponized by Iraq The order of effectiveness against soman was HI-6 > HLo-7 > pyrimidoxime HLo-7 was very effective against tabun poisoning while HI-6 and pyrimidoxime were of moderate value Against GF, HI-6 and HLo-7 were extremely effective, toxogonin was moderately effective, and PAM-CI and pyrimidoxime were the least effective HI-6 provided a high level of protection against all of the agents tested as did HLo-7 to a slightly lesser degree. The other oximes suffered from their lack of effects against one or more of the organophosphates. (Class IV)
Lundy PM, Hansen AS, Hand BT, Boulet CA. Comparison of several oximes against poisoning by soman, tabun and GF. Toxicology. 1992;72(1):99-105. [PubMed Citation]
-
The efficacy of the oxime HI-6 was studied as a treatment for organophosphorus poisoning. HI-6 was given four times daily as a single intramuscular injection of 500 mg accompanied by atropine and diazepam therapy. Oxime treatment was started on admission and continued for a minimum of 48 h and a maximum of 7 d. HI-6 rapidly reactivated human blood acetylcholinesterase inhibited by dimethoxy organophosphorus compounds, while the dimethoxy-inhibited enzyme was mainly resistant to the treatment by HI-6. Although both HI-6 and pralidoxime chloride reactivated the red blood cell cholinesterase in quinalphos-poisoned subjects, the return of enzyme activities was more rapid following the use of HI-6. The general improvement of poisoned patients, which was sometimes more rapid than the rise of acetylcholinesterase activity, pointed to direct pharmacological effects of HI-6. No undesirable side-effects were noted in patients when HI-6 plasma concentrations were maintained at levels far above the therapeutic concentration for up to 7 d. (Class IV)
Kusić R, Jovanović D, Randjelović S, Joksović D, Todorovic V, Bosković B, Jokanović M, Vojvodić V. HI-6 in man: efficacy of the oxime in poisoning by organophosphorus insecticides. Hum Exp Toxicol. 1991 Mar;10(2):113-8. [PubMed Citation]
-
A 20-year-old male who attempted suicide by injecting subcutaneously 10 ml of Sistemin 40 (40% dimethoate) was admitted 16 h later. General weakness, muscular fibrillations and a marked inhibition of red blood cell and serum cholinesterases were the prominent signs of intoxication. The antidotal treatment of intermittent boluses of atropine, oxime HI-6 and diazepam was combined with symptomatic therapy. Cholinesterase activity decreased within the next 3 d. In contrast to the marked general improvement of the patient, the return of cholinesterase activities was very slow. The patient was discharged 24 d after the poisoning with no notable consequences which could be ascribed to the intoxication. (Class IV)
Jovanović D, Randjelović S, Joksović D. A case of unusual suicidal poisoning by the organophosphorus insecticide dimethoate. Hum Exp Toxicol. 1990 Jan;9(1):49-51. [PubMed Citation]
Clinical reviews
-
The oximes pralidoxime (2-PAM), its dimethanesulphonate salt derivative P2S, and obidoxime (toxogonin) are currently licensed and fielded for the treatment of chemical warfare (CW) organophosphorous (OP) nerve agent poisoning. While they are effective against several of the identified threat CWOP agents, they have little efficacy against others such as soman (GD) and cyclosarin (CF). In addition, they are also significantly less effective than other investigational oximes against the nerve agent known as Russian VX (RVX). Among the oximes currently being investigated, two in particular, HI-6 (asoxime) and MMB-4 (ICD-039, methoxime) have been proposed as replacement therapies for the currently licensed oximes. HI-6 has been safely used in individuals to treat OP insecticide poisoning, as well as in human volunteers, although its efficacy against OP nerve agent poisoning in humans cannot be demonstrated due to ethical considerations. It is currently available for use in defined military settings in Canada, Sweden and the Czech Republic, and is also under development in a number of other countries. The oxime MMB-4 has not yet been studied clinically, but is fielded by the Czech Republic, and is being developed by the United States armed services as a replacement for the currently fielded 2-PAM. This review compares the effectiveness of HI-6 and MMB-4 against nerve agent threats where comparisons can be made. HI-6 has been demonstrated to be generally a superior reactivator of nerve agent inhibited enzyme, particularly with human and non-human primate derived enzyme, and has also shown better protective effects against the lethality of most OP agents in a variety of species. Both compounds appear to be clearly superior to the available oximes, obidoxime and 2-PAM. (Class IV)
Lundy PM, Hamilton MG, Sawyer TW, Mikler J. Comparative protective effects of HI-6 and MMB-4 against organophosphorous nerve agent poisoning. Toxicology. 2011 Jul 29;285(3):90-6. [PubMed Citation]
-
The traditional therapeutic treatment of organophosphate cholinesterase inhibitor (nerve agents) poisoning consists of co-treatment with an antimuscarinic (atropine) and a reactivator of inhibited acetylcholinesterase (AChE), which contains a nucleophilic oxime function. Two oximes are presently widely available for clinical use, pralidoxime and obidoxime (toxogonin), but both offer little protection against important nerve agent threats. This has highlighted the real need for the development and availability of more effective oximes for human use, a search that has been going on for up to 30 years. However, despite the demonstration of more effective and safe oximes in animal experiments, no additional oximes have been licensed for human use. HI-6, (1-[[[4(aminocarbonyl)-pyridinio]methoxy]methyl]-2(hydroxyimino)pyridinium dichloride; CAS 34433-31-3) has been studied intensively and has been proved effective in a variety of species including non-human primates and appears from clinical experience to be safe in humans. These studies have led to the fielding of HI-6 for use against nerve agents by the militaries of the Czech republic, Sweden, Canada and under certain circumstances the Organisation for the Prohibition of Chemical Weapons. Nevertheless HI-6 has not been granted a license for clinical use, must be used only under restricted guidelines and is not available for civilian use as far as is known. This article will highlight those factors relating to HI-6 that pertain to the licensing of new compounds of this type, including the mechanism of action, the clinical and pre-clinical demonstration of safety and its efficacy against a variety of nerve agents particularly in non-human primates, since no relevant human population exists. This article also contains important data on the use of HI-6 in baboons, which has not been available previously. The article also discusses the possibility of successful therapy with HI-6 against poisoning in humans relative to doses used in non-human primates and relative to its ability to reactivate inhibited human AChE (Class IV).
Lundy PM, Raveh L, Amitai G. Development of the bisquaternary oxime HI-6 toward clinical use in the treatment of organophosphate nerve agent poisoning. Toxicol Rev. 2006;25(4):231-43. [PubMed Citation]
-
Prophylactic approaches against intoxication with organophosphates (OP)/nerve agents can be based on following principles: keeping acetylcholinesterase (AChE), the key enzyme for toxic action of OP/nerve agents, intact (protection of cholinesterases) is a basic requirement for effective prophylaxis. It can be reached using simple chemicals such as reversible inhibitors (preferably carbamates), which are able to inhibit AChE reversibly. AChE inhibited by carbamates is resistant to OP/nerve agent inhibition. After spontaneous recovery of the activity, normal AChE serves as a source of the active enzyme. Detoxification is realised by administration of the enzymes splitting the OP or exploitating specific enzymes (cholinesterases). OP/nerve agent is bound to the exogenously administered proteins (enzymes) and, thus, the agent level in the organism is decreased ("scavenger" effect). The antidotes currently used for the treatment of OP poisoning (also simple chemicals) can be tested as prophylactics. This principle can be considered as a treatment "in advance". The problem with their use is the timing, duration and achievement of sufficient levels of these antidotes after the administration. At present, PYRIDOSTIGMINE seems to be common prophylactic antidote; prophylactics PANPAL (tablets with pyridostigmine, trihexyphenidyle and benactyzine), TRANSANT (transdermal patch containing HI-6) are other means introduced into different armies as prophylactics. Future development will be focused on scavengers (cholinesterases and other enzymes) acting before the binding of nerve agent to the target sites, and on other drugs reversible cholinesterase inhibitors (e.g. huperzine A, physostigmine, acridine derivatives etc.) including non-traditional routes of administration (Class IV).
Bajgar J, Fusek J, Kassa J, Kuca K, Jun D. Chemical aspects of pharmacological prophylaxis against nerve agent poisoning. Curr Med Chem. 2009;16(23):2977-86. [PubMed Citation]
-
The increasing threat of nerve agent use for terrorist purposes against civilian and military population calls for effective therapeutic preparedness. At present, administration of atropine and an oxime are recommended, although effectiveness of this treatment is not proved in clinical trials. Here, monitoring of intoxications with organophosphorus (OP) pesticides may be of help, as their actions are closely related to those of nerve agents and intoxication and therapy follow the same principles. To this end, the clinical course of poisoning and the effectiveness of antidotal therapy were investigated in patients requiring artificial ventilation being treated with atropine and obidoxime. However, poisoning with OP pesticides shows extremely heterogeneous pictures of cholinergic crisis frequently associated with clinical complications. To achieve valuable information for the therapy of nerve agent poisoning, cases resembling situations in nerve agent poisoning had to be extracted: (a) intoxication with OPs forming reactivatable OP-AChE-complexes with short persistence of the OP in the body resembling inhalational sarin intoxication; (b) intoxication with OPs resulting rapidly in an aged OP-AChE-complex resembling inhalational soman intoxication; (c) intoxications with OPs forming a reactivatable AChE-OP complex with prolonged persistence of the OP in the body resembling percutaneous VX intoxication. From these cases it was concluded that sufficient reactivation of nerve agent inhibited non-aged AChE should be possible, if the poison load was not too high and the effective oximes were administered early and with an appropriate duration. When RBC-AChE activity was higher than some 30%, neuromuscular transmission was relatively normal. Relatively low atropine doses (several milligrams) should be sufficient to cope with muscarinic symptoms during oxime therapy (Class IV).
Thiermann H, Szinicz L, Eyer P, Felgenhauer N, Zilker T, Worek F. Lessons to be learnt from organophosphorus pesticide poisoning for the treatment of nerve agent poisoning. Toxicology. 2007 Apr 20;233(1-3):145-54. [PubMed Citation]
-
The use of organophosphorus pesticides results in toxicity risk to non-target organisms. Organophosphorus compounds share a common mode of action, exerting their toxic effects primarily via acetylcholinesterase (AChE) inhibition. Consequently, acetylcholine accumulates in the synaptic clefts of muscles and nerves, leading to overstimulation of cholinergic receptors. Acute cholinergic crisis immediately follows exposure to organophosphate and includes signs and symptoms resulting from hyperstimulation of central and peripheral muscarinic and nicotinic receptors. The current view of the treatment of organophosphate poisoning includes three strategies, i.e. the use of an anticholinergic drug (e.g., atropine), cholinesterase-reactivating agents (e.g., oximes) and anticonvulsant drugs (e.g., benzodiazepines). Oximes, as a part of antidotal therapy, ensure the recovery of phosphylated enzymes via a process denoted as reactivation of inhibited AChE. However, both experimental results and clinical findings have demonstrated that different oximes are not equally effective against poisonings caused by structurally different organophosphorus compounds. Therefore, antidotal characteristics of conventionally used oximes can be evaluated regarding how close the certain substance is to the theoretical concept of the universal oxime. Pralidoxime (PAM-2), trimedoxime (TMB-4), obidoxime (LüH-6), HI-6 and HLö-7 have all been demonstrated to be very effective in experimental poisonings with sarin and VX. TMB-4 and LüH-6 may reactivate tabun-inhibited AChE, whereas HI-6 possesses the ability to reactivate the soman-inhibited enzyme. An oxime HLö-7 seems to be an efficient reactivator of AChE inhibited by any of the four organophosphorus warfare agents. According to the available literature, the oximes LüH-6 and TMB-4, although relatively toxic, are the most potent to induce reactivation of AChE inhibited by the majority of organophosphorus pesticides. Since there are no reports of controlled clinical trials on the use of TMB-4 in human organophosphate pesticide poisoning, LüH-6 may be a better option (Class IV).
Antonijevic B, Stojiljkovic MP. Unequal efficacy of pyridinium oximes in acute organophosphate poisoning. Clin Med Res. 2007 Mar;5(1):71-82. [PubMed Citation]
-
Organophosphate (OP) poisoning poses great danger to both military and civilian populations. OP induced brain injury is characterized by rapid loss of consciousness, seizures, central respiratory inhibition as well as long-term behavioral changes in sub-lethal injuries. The pharmacological treatment of OP poisoning is based on anticholinergic and anticonvulsant drugs as well as oximes, which reactivate the non-aged inhibited enzyme. The commonly used oximes are quaternary compounds with questionable capacity to penetrate through the blood-brain barrier. This implies that the main beneficial effect of oximes may result from reactivation of AChE activity in respiratory muscles rather than in the brain. Importantly, data accumulated over the last few decades suggests a potential beneficial role for oximes in the brain, despite their polarity. Albeit the concentration of oximes in the central nervous system is significantly lower than in the plasma, they do gain access into the brain and are able to reactivate inhibited local AChE. Oximes may also attenuate OP-induced brain insult via different mechanisms other than AChE reactivation. In this review, we focus on the ability of oximes to act in the brain and protect the central nervous system from OP-induced injury, either by direct reactivation of AChE or by other pharmacological mechanisms. While this is a poorly investigated field we believe that the data supports the potential role of oximes in mitigating OP-induced neuronal injury, thus making them valuable in the treatment of severe casualties (Class IV).
Shrot S, Markel G, Dushnitsky T, Krivoy A. The possible use of oximes as antidotal therapy in organophosphate-induced brain damage. Neurotoxicology. 2009 Mar;30(2):167-73. [PubMed Citation]
-
The cholinesterase-inhibiting organophosphorus compounds referred to as nerve agents (soman, sarin, tabun, GF agent, and VX) are particularly toxic and are considered to be among the most dangerous chemical warfare agents. Included in antidotal medical countermeasures are oximes to reactivate the inhibited cholinesterase. Much experimental work has been done to better understand the properties of the oxime antidotal candidates including the currently available pralidoxime and obidoxime, the H oximes HI-6 and Hlö-7, and methoxime. There is no single, broad-spectrum oxime suitablefor the antidotal treatment of poisoning with all organophosphorus agents. If more than one oxime is available, the choice depends primarily on the identity of the responsible organophosphorus compound. The H oximes appear to be very promising antidotes against nerve agents because they are able to protect experimental animals from toxic effects and improve survival of animals poisoned with supralethal doses. They appear more effective against nerve agent poisoning than the currently used oximes pralidoxime and obidoxime, especially in the case of soman poisoning. On the other hand, pralidoxime and especially obidoxime seem sufficiently effective to treat poisonings with organophosphorus insecticides that have relatively less toxicity than nerve agents (Class IV).
Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol. 2002;40(6):803-16. [PubMed Citation]
-
There are important differences between on-target military attacks against relatively well protected Armed Forces and nerve agent attacks initiated by terrorists against a civilian population. In contrast to military personnel, civilians are unlikely to be pre-treated with pyridostigmine and protected by personal protective equipment. Furthermore, the time after exposure when specific therapy can first be administered to civilians is likely to be delayed. Even conservative estimates suggest a delay between exposure and the first administration of atropine/oxime of at least 30 minutes. The organophosphorus nerve agents are related chemically to organophosphorus insecticides and have a similar mechanism of toxicity, but a much higher mammalian acute toxicity, particularly via the dermal route. Nerve agents phosphonylate a serine hydroxyl group in the active site of the enzyme, acetylcholinesterase (AChE), which results in accumulation of acetylcholine and, in turn, causes enhancement and prolongation of cholinergic effects and depolarisation blockade. The rate of spontaneous reactivation of AChE is variable, which partly accounts for differences in acute toxicity between the nerve agents. With soman in particular, an additional reaction occurs known as 'aging'. This consists of monodealkylation of the dialkylphosphonyl enzyme, which is then resistant to spontaneous hydrolysis and reactivation by oximes. Monodealkylation occurs to some extent with all dialkylphosphonylated AChE complexes; however, in general, is only of clinical importance in relation to the treatment of soman poisoning, where it is a very serious problem. With soman, aging occurs so fast that no clinically relevant spontaneous reactivation of AChE occurs before aging has taken place. Hence, recovery of function depends on resynthesis of AChE. As a result, it is important that an oxime is administered as soon after soman exposure as possible so that some reactivation of AChE occurs before all the enzyme becomes aged. Even though aging occurs more slowly and reactivation occurs relatively rapidly in the case of nerve agents other than soman, early oxime administration is still clinically important in patients poisoned with these agents. Experimental studies on the treatment of nerve agent poisoning have to be interpreted with caution. Some studies have used prophylactic protocols, whereas the drugs concerned (atropine, oxime, diazepam) would only be given to a civilian population after exposure. The experimental use of pyridostigmine before nerve agent exposure, although rational, is not of relevance in the civilian context. With the possible exception of the treatment of cyclosarin (GF) and soman poisoning, when HI-6 might be preferred, a review of available experimental evidence suggests that there are no clinically important differences between pralidoxime, obidoxime and HI-6 in the treatment of nerve agent poisoning, if studies employing pre-treatment with pyridostigmine are excluded (Class IV).
Marrs TC, Rice P, Vale JA. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol Rev. 2006;25(4): 297-323. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
This was a study of the efficacy of antidotes comprising of HI-6 (1-[[[4-(aminocarbonyl)-pyridinio]-methoxy]-methyl]-2-[(hydroxyimino) methyl] pyridinium dichloride), atropine and midazolam on soman induced neurodegeneration and the expression of c-Fos, Calpain, and Bax levels in discrete rat brain areas. Therapeutic regime consisting of HI-6 (50 mg/kg, i.m), atropine (10 mg/kg, i.m) and midazolam (5 mg/kg, i.m) protected animals against soman (2 x LD50, s.c) lethality completely at 2 h and 80% at 24 h. HI-6 treatment reactivated soman inhibited plasma and RBC cholinesterase up to 40%. Fluoro-Jade B (FJ-B) staining of neurodegenerative neurons showed that soman induced significant necrotic neuronal cell death, which was reduced by this antidotal treatment. Soman increased the expression of neuronal proteins including c-Fos, Bax and Calpain levels in the hippocampus, cerebral cortex and cerebellum regions of the brain. This therapeutic regime also reduced the soman induced Bax, Calpain expression levels to near control levels in the different brain regions studied, except a mild induction of c-Fos expression in the hippocampus. CONCLUSION: Rats that received antidotal treatment after soman exposure were protected from mortality and showed reduction in the soman induced expression of c-Fos, Bax and Calpain and necrosis. Results highlight the need for timely administration of better antidotes than standard therapy in order to prevent the molecular and biochemical changes and subsequent long term neurological effects induced by nerve agents.
RamaRao G, Afley P, Acharya J, Bhattacharya BK. Efficacy of antidotes (midazolam, atropine and HI-6) on nerve agent induced molecular and neuropathological changes. BMC Neurosci. 2014 Apr 4;15:47. [PubMed Citation].
-
Sulfur mustard (SM) is a blister agent with cytotoxic mechanism of action. There is no suitable treatment based on administration of an antidote. In this study, Wistar rats were exposed to SM in doses of 0-40 mg/kg body weight and treated with the compound HI-6. The treatment provided no significant effect on ferric reducing antioxidant power of blood and plasma. However, HI-6 caused an increase in the level of thiobarbituric acid reactive substances. This stressogenic response was presumably the cause of the significant elevation of the blood level of both glutathione reductase and reduced glutathione. HI-6 appears to be suitable for enhancing prophylactically oxidative stress protection from small oxidative insult.
Pohanka M, Sobotka J, Svobodova H, Stetina R. Sulfur mustard induced oxidative stress and its alteration using asoxime (HI-6). Interdiscip Toxicol. 2013 Dec;6(4):198-202.
-
This study compared the efficacy of HI6 and 2-PAM against nerve agent (soman, tabun, sarin, and VX) -induced lethality in the atropinesterase-free rabbits pretreated with vehicle (controls) or pyridostigmine. Treatment was administered at signs or 2 min after agent challenge and consisted of oxime (100 mumol/kg) + atropine (13 mg/kg) (alone or together with diazepam). Twenty-four-h LD, values were calculated for soman- and tabun-intoxicated animals, whereas 24-h survival was noted in animals given 10 L&s of sarin or VX. In pyridostigmine and control rabbits intoxicated with soman and treated with oxime + atropine (alone or together with diazepam), HI6 was 3-5 times more effective than 2-PAM. In contrast, HI6 was less effective than 2-PAM against tabun poisoning. In pyridostigmine-pretreated animals exposed to tabun, efficacy was increased more than 3-fold when compare to tabun-challenged animals treated with atropine + HI6 alone. Both oximes were highly effective against satin and VX. These findings suggest that HI6 could replace 2-PAM as therapy for nerve agent poisoning, because it is superior to Z-PAM against soman, and when used in pyridostigmine-pretreated animals, it affords excellent protection against all four nerve agents when used in combination with atropine (alone or together with diazepam) therapy.
Koplivitz I, Stewart JR. A comparison of the efficacy of HI6 and 2-PAM against soman, tabun, sarin, and VX in the rabbit. Toxicol Lett. 1994 Feb 15;70(3):269-79. [PubMed Citation]
-
H 1-6 (1 -2-hydroxyiminomethyl-I -pyridino- 3-(4-carbamoyl- 1 -pyridino)-2-oxapropane dichloride) has been evaluated as an oxime alternative to pralidoxime, and toxogonin in the treatment of organophosphorus (OP) poisoning. The dose response effects of atropine (ATR) and HI-6 were investigated to more fully explore the interaction of these compounds in the treatment of OP poisoning. ATR, HI-6 and various combinations of the two drugs were evaluated against lethal poisoning by soman (GD) and tabun (GA) in guinea pigs. The effect of adjunctive diazepam treatment on the efficacy of atropine and HI-6 against soman was also investigated. Animals of either sex were challenged S.C. with OP and treated i.m. 1 min later with ATR and/or HI-6. When used, diazepam was injected immediately after ATR+HI6. LD50s of each treatment were calculated from probit models based on 24-hour survival against 5 levels of nerve agent and 6 animals per challenge level. A protective index (PI) was calculated by dividing the nerve agent LD50 in the presence of treatment by the LD50 in the absence of treatment. Treatment with HI-6 alone had little effect on the toxicity of either OP Treatment with AIR alone was more effective than HI-6 alone and was significantly more effective against soman than against tabun. When used in combination atropine and HI-6 had a strong synergistic effect against both agents. The dose of atropine used with HI-6 was critical in determining the efficacy of HI-6 against either agent. The slopes of the dose-lethality curves were minimally affected by the dose of ATR or HI-6. Adjunctive treatment with diazepam enhanced the efficacy of HI-6 and atropine against soman. It is concluded that 1) ATR has a large effect on the efficacy of HI-6 against OP poisoning, 2) the dose of ATR must be carefully selected in studies investigating the efficacy of HI-6 against OP poisoning, 3) the effective dose of ATR in the guinea pig is approximately 16 mg/kg, and 4) diazepam is a useful adjunct to atropine and HI-6.
Koplovitz I, Menton R, Matthews C, Shutz M, Nalls C, Kelly S. Dose-response effects of atropine and HI-6 treatment of organophosphorus poisoning in guinea pigs. Drug Chem Toxicol. 1995 May-Aug;18(2-3):119-36. [PubMed Citation]
-
The reactivating and therapeutic efficacy of two salts of the oxime HI-6 (dichloride and dimethanesulphonate) against chosen nerve agents (tabun, soman and cyclosarin) was compared in rats. The potency of both salts of HI-6 to decrease the acute toxicity of tabun, soman and cyclosarin was similar in nerve agent-poisoned rats. While the potency of HI-6 dichloride and HI-6 dimethanesulphonate to counteract acute toxic effects of tabun is rather low, both salts of HI-6 were able to decrease the acute toxicity of soman two times and acute toxicity of cyclosarin more than three times. The therapeutic efficacy of both salts of the oxime HI-6 corresponds to their reactivating potency. While the reactivating efficacy of HI-6 dichloride as well as HI-6 dimethanesulphonate against tabun was negligible, their potency to reactivate soman-inhibited acetylcholinesterase and cyclosarin-inhibited acetylcholinesterase in peripheral (blood) and central (brain) compartment was relatively high. HI-6 dichloride showed a somewhat higher potency to reactivate tabun-inhibited acetylcholinesterase in brain, and soman-inhibited acetylcholinesterase in blood and brain than HI-6 dimethanesulphonate but the differences were not significant. Thus, the replacement of dichloride anion by dimethanesulphonate anion in the oxime HI-6 does not influence the therapeutic and reactivating efficacy of the oxime HI-6 against nerve agents. In addition, the higher solubility and stability of HI-6 dimethanesulphonate in comparison with HI-6 dichloride makes it possible to increase the dose and thus, the effectiveness of the oxime HI-6 in the antidotal treatment of acute nerve agent poisonings.
Kassa J, Jun D, Kuca K, Bajgar J. Comparison of reactivating and therapeutic efficacy of two salts of the oxime HI-6 against tabun, soman and cyclosarin in rats. Basic Clin Pharmacol Toxicol. 2007 Nov;101(5):328-32. [PubMed Citation]
-
The ability of three newly developed reversible inhibitors of acetylcholinesterase (AChE) (K298, K344 and K474) and currently available carbamate pyridostigmine to increase the resistance of mice against soman and the efficacy of antidotal treatment of soman-poisoned mice was compared. Neither pyridostigmine nor new reversible inhibitors of AChE were able to increase the LD(50) value of soman. Thus, the pharmacological pre-treatment with pyridostigmine or newly synthesized inhibitors of AChE was not able to protect mice against soman-induced lethal acute toxicity. The pharmacological pre-treatment with pyridostigmine alone or with K474 was able to slightly increase the efficacy of antidotal treatment (the oxime HI-6 in combination with atropine) of soman-poisoned mice, but the increase in the efficacy of antidotal treatment was not significant. The other newly developed reversible inhibitors of AChF (K298, K344) were completely ineffective. These findings demonstrate that pharmacological pre-treatment of soman-poisoned mice with tested reversible inhibitors of AChF is not promising.
Kassa J, Musilek K, Koomlova M, Bajgar J. A comparison of the efficacy of newly developed reversible inhibitors of acetylcholinesterase with commonly used pyridostigmine as pharmacological pre-treatment of soman-poisoned mice. Basic Clin Pharmacol Toxicol. 2012 Apr;110(4):322-6. [PubMed Citation]
-
The ability of three oximes, HI-6, MMB-4 and ICD-467, to reactivate cholinesterase (ChE) inhibited by the organophosphorus compound soman was compared in blood (plasma and erythrocytes), brain regions (including spinal cord) and peripheral tissues of rats. Animals were intoxicated with soman (100 micrograms/kg, SC; equivalent to 0.9 x LD50 dose) and treated 1 min later with one of these oximes (100 or 200 mumol/kg, IM). Toxic sign scores and total tissue ChE activities were determined 30 min later. Soman markedly inhibited ChE activity in blood (93-96%), brain regions (ranging from 78% to 95%), and all peripheral tissues (ranging from 48.9% to 99.8%) except liver (11.9%). In blood, treatment with HI-6 or ICD-467 resulted in significant reactivation of soman-inhibited ChE. In contrast, MMB-4 was completely ineffective. HI-6 and ICD-467 were equally effective at the high dose. At the low dose ICD-467 treatment resulted in significantly higher plasma ChE than HI-6 treatment, whereas HI-6 treatment resulted in higher erythrocyte ChE than ICD-467 treatment. However, none of these three oximes reactivated or protected soman-inhibited ChE in the brain. In all peripheral tissues (except liver) studied, MMB-4 was not effective. HI-6 reactivated soman-inhibited ChE in all tissues except lung, heart, and skeletal muscle. ICD-467 was highly effective in reactivating ChE in all tissues and afforded a complete recovery of ChE to control levels in intercostal muscle and salivary gland. Oxime treatments did not modify the toxic scores produced by soman. However, treatment with the high dose (200 mumol/kg) of ICD-467 depressed respiration and two of the six rats died in 10 min. These observations indicate that MMB-4 is completely ineffective in protecting and/or reactivating soman-inhibited ChE, HI-6 is an effective ChE reactivator as reported earlier in rats and other species, and the imidazolium oxime ICD-467 is a powerful reactivator of soman-inhibited ChE; however, its toxic interactions with soman may not be related to tissue ChE levels.
Shih TM. Comparison of several oximes on reactivation of soman-inhibited blood, brain and tissue cholinesterase activity in rats. Arch Toxicol. 1993;67(9):637-46. [PubMed Citation]
-
The influence of the combination of oximes on the reactivating and therapeutic efficacy of antidotal treament of acute tabun poisoning was evaluated. The ability of two combinations of oximes (HI-6 + obidoxime and HI-6 + K203) to reactivate tabun-inhibited acetylcholinesterase and reduce acute toxicity of tabun was compared with the reactivating and therapeutic efficacy of antidotal treatment involving single oxime (HI-6, obidoxime, K203) using in vivo methods. Studies determining percentage of reactivation of tabun-inhibited blood and tissue acetylcholinesterase in poisoned rats showed that the reactivating efficacy of both combinations of oximes is higher than the reactivating efficacy of the most effective individual oxime in blood and diaphragm and comparable with the reactivating effects of the most effective individual oxime in brain. Moreover, both combinations of oximes were found to be slightly more efficacious in the reduction of acute lethal toxic effects in tabun-poisoned mice than the antidotal treatment involving individual oxime. A comparison of reactivating and therapeutic efficacy of individual oximes showed that the newly developed oxime K203 is slightly more effective than commonly used obidoxime and both of them are markedly more effective than the oxime HI-6. Based on the obtained data, we can conclude that the antidotal treatment involving chosen combinations of oximes brings beneficial effects for the potency of antidotal treatment to reactivate tabun-inhibited acetylcholinesterase in rats and to reduce acute toxicity of tabun in mice.
Kassa J, Karasova JZ, Pavlikova R, Misik J, Caisberger F, Bajgar J. The influence of combinations of oximes on the reactivating and therapeutic efficacy of antidotal treatment of tabun poisoning in rats and mice. J Appl Toxicol. 2010 Mar;30(2):120-4. [PubMed Citation]
-
The chemical weapon nerve agent known as Russian VX (VR) is a potent organophosphorus (OP) compound that is much less studied than its VX analogue with respect to toxicity, as well as to the effectiveness of several known countermeasures against it. An anaesthetized domestic swine model was utilized to assess several approaches in mitigating its toxicity, including the utility of cooling VR treated skin to increase the therapeutic window for treatment. The 6h LD50 for VR topically applied on the ear was 100 μg/kg. Treatment of VR exposed animals (5 x LD50) with pralidoxime (2PAM) very poorly regenerated inhibited blood cholinesterase activity, but was partially effective in preventing signs of OP poisoning and increasing survival. In contrast, treatment with the Hagedorn oxime HI-6 reactivated cholinesterase, eliminated all signs of poisoning and prevented death. Decontamination with the Reactive Skin Decontaminant Lotion (RSDL) 15 min after VR exposure was completely effective in preventing death. Cooling of the VR exposure sites for 2 or 6h prevented signs of OP poisoning and death during the cooling period. However, these animals died very quickly after the cessation of cooling, unless they were treated with oxime or decontaminated with RSDL. Blood analyses showed that cooling of agent exposure sites delayed the entry of VR into the bloodstream. Medical treatment with HI-6 and to a lesser extent 2PAM, or decontamination with RSDL are effective in protecting against the toxic effects of cutaneous exposure to VR. Immobilizing this agent (and related compounds) within the dermal reservoir by cooling the exposure sites, dramatically increases the therapeutic window in which these medical countermeasures are effective.
Mikler J, Tenn C, Worek F, Reiter G, Thiermann H, Garrett M, Bohnert S, Sawyer TW. Immobilization of Russian VX skin depots by localized cooling: implications for decontamination and medical countermeasures. Toxicol Lett. 2011 Sep 25;206(1):47-53. [PubMed Citation]
-
This study forms part of a larger programme of work aimed at developing improved medical countermeasures for nerve agent poisoning with less reliance on pretreatment. Therapy with N(6)-cyclopentyladenosine (CPA), physostigmine, hyoscine and HI-6 protected guinea-pigs against the incapacitating and lethal effects of a supralethal challenge of soman (135 microg/kg) when given 1 min after poisoning. CPA, however has well-recognised side effects that are likely to preclude it being licensed for use in humans so further refinements were made to the doses of the other therapy components to improve efficacy in the absence of CPA. An immediate therapy comprising physostigmine (0.2 mg/kg), hyoscine (4 mg/kg) and HI-6 (93.6 mg/kg), when given 1 min after nerve agent, provided good protection against the lethal effects of GA, GB, GD, GF and VX poisoning and reduced the duration of the signs of incapacitation and hypothermia. In the case of GA and GB poisoning some animals exhibited a short period of substantial incapacitation. Most animals continued to gain weight over the following 6 days without the need for further medical intervention. In the case of GA poisoning further medical intervention would be needed to ensure the longer term survival of all animals and it is likely that in the battlefield situation further medical treatment would be available within 2-4 h. The drug combination described in this paper protects against supralethal doses of a range of nerve agents, with minimal incapacitation in the absence of any pretreatment. Further modification and refinement of this therapy is required for human use and it may provide a way forward for development of medical countermeasures for the treatment of organophosphate poisoning in the wider community should there be a need.
Wetherell J, Price M, Mumford H. A novel approach for medical countermeasures to nerve agent poisoning in the guinea-pig. Neurotoxicology. 2006 Jul;27(4):485-91. [PubMed Citation]
-
The therapeutic effectiveness of a new binary autoinjector containing 500 mg HI-6 and 2 mg atropine sulphate was tested in anesthetized pigs poisoned by a lethal dose of soman i.v. (9 micrograms/kg per 20 min). Pharmacokinetics and pharmacodynamics of HI-6 were studied concomitantly on administration of HI-6 alone, together with atropine sulphate, or together with atropine sulphate during soman intoxication. Cardiopulmonary parameters were monitored and serum concentrations of oxime and acetylcholinesterase (AChE) were measured in blood samples taken at intervals over a 6-h period postinjection. Five minutes after the start of soman infusion, mean AChE activity was decreased to 27 +/- 4.3% of baseline and signs of poisoning appeared. The antidotes, HI-6 and atropine sulphate, were then administered i.m. One minute after this injection there was a transient significant increase in AChE activity of 76 +/- 8.2% of baseline (p < 0.01). It then again decreased and remained suppressed throughout the experiment. Mean respiratory rate was significantly decreased (p < 0.01) to 20 +/- 3.2% of baseline after 20 min of soman infusion and remained low during the rest of the experiment. The poisoning signs were counteracted 15-20 min after antidote therapy and all pigs survived soman intoxication without ventilatory assistance. Administration of either atropine or atropine and soman had no significant effect on the pharmacokinetics of HI-6 in anesthetized pigs.
Göransson-Nyberg A, Cassel G, Jeneskog T, Karlsson L, Larsson R, Lundström M, Persson SA. Treatment of organophosphate poisoning in pigs: antidote administration by a new binary autoinjector. Arch Toxicol. 1995;70(1):20-7. [PubMed Citation]
-
More effective countermeasures against nerve-agent poisoning are needed, because current ones do not protect sufficiently, particularly the central nervous system (CNS). The purpose of the present study was to make a comparison of the antidotal capabilities of atropine/obidoxime/diazepam (termed the obidoxime regimen), atropine/HI-6 (1-[([4-(aminocarbonyl)pyridinio]methoxy)methyl]-2-[(hydroxyimino)methyl]pyridinium)/avizafone (termed the HI-6 regimen), and scopolamine/HI-6/physostigmine (termed the physostigmine regimen) against various doses of soman (2, 3, 4 x LD(50) ). The results showed that each regimen administered twice (1 min and 5min after exposure) effectively prevented or terminated epileptiform activity within 10 min. However, the regimens differed markedly in life-saving properties with the physostigmine regimen ranking highest followed in descending order by the HI-6 and obidoxime regimens. Pretreatment with pyridostigmine increased the potency of the HI-6 regimen, but not the obidoxime regimen. The latter regimen administered thrice (1 min, 5 min, and 9 min after exposure) did not compensate for the insufficiency. In half of the rats that lived for 7 days, neuropathology was unexpectedly observed predominantly in the left hemisphere unrelated to whether they seized or not. Local glutamatergic excitotoxic activity may occur even if manifest toxic signs are absent. The physostigmine regimen has excellent antidotal capacity, but the very narrow therapeutic window (< 10 min) makes it unsuitable for use in the field. The HI-6 regimen appears to constitute an efficacious therapy against lower doses of soman (2 and 3 x LD(50) ).
Myhrer T, Enger S, Aas P. Determination of anti-convulsant and life-preserving capacities of three types of auto-injector therapies against soman intoxication in rats. Drug Test Anal.Drug Test Anal. 2013 Aug;5(8):693-701. [PubMed Citation]
-
The purpose of the present study was to examine the efficacy of a triple combination of drugs with adequate anticonvulsant effects and a dual combination with inadequate anticonvulsant effects followed by adjunct therapy. The results showed that combined intramuscular injections of HI-6 (42 mg/kg), atropine (14 mg/kg), and avizafone (3 mg/kg) administered 1, 16, and 31 min. after exposure to a soman dose of 4 x LD(50) completely terminated seizures with a moderate mortality rate (25%). When the soman dose was lowered to 3 x LD(50) the anticonvulsant effect was complete, and no rats died within 24 hr. Rats challenged with 5 x LD(50) of soman all died within 10 min. Without avizafone in the combination, seizures induced by 3 or 4 x LD(50) of soman could not be terminated unless an adjunct therapy consisting of procyclidine (6 mg/kg), diazepam (10 mg/kg), and pentobarbital (30 kg/kg) was given, and the mortality rate was comparatively high (78%). Administration of the adjunct therapy alone 6-16 min. after 4 x LD(50) of soman stopped the seizure activity, but all the rats died within 24 hr. Marked neuropathology was found in the piriform cortex and amygdala, whereas the hippocampal CA1 field was effectively protected when both the triple combination and the dual combination plus adjuncts had stopped seizures 35-55 min. after onset. It is concluded that termination of soman-induced seizures at an early stage (<20 min.) is crucial to avoid neuronal pathology.
Myhrer T, Enger S, Ass P. Efficacy of immediate and subsequent therapies against soman-induced seizures and lethality in rats. Basic Clin Pharmacol Toxicol. 2006 Feb;98(2):184-91. [PubMed Citation]
-
A treatment regimen consisting of HI-6, scopolamine, and physostigmine (termed the physostigmine regimen) has been based on the serendipitous discovery that it exerts powerful antidotal effects against high levels of soman poisoning if it is administered 1 min after exposure. A medical therapy with corresponding efficacy, but without the time limitation of the latter regimen, has been developed through studies of microinfusions of anticonvulsants into seizure controlling sites in the forebrain of rats. From these studies procyclidine emerged as the most potent anticonvulsant, and its potency was further enhanced when being combined with the antiepileptic levetiracetam during systemic administration. In the present study, the capacity of HI-6, levetiracetam, and procyclidine (termed the procyclidine regimen) was tested against that of the physostigmine regimen. The results showed that both regimens were very effective against supralethal doses of soman (3, 4, 5 x LD50) when given 1 and 5 min after intoxication. When the treatments were administered 10 and 14 or 20 and 24 min after soman exposure, only the procyclidine regimen was able to terminate seizures and preserve lives. When used as prophylactic therapies, both regimens protected equally well against seizures, but only the procyclidine regimen provided neuroprotection. The procyclidine regimen has apparently capacities to serve as a universal therapy against soman intoxication in rats.
Myhrer T, Enger S, Mariussen E, Aas P. Two medical therapies very effective shortly after high levels of soman poisoning in rats, but only one with universal utility. Toxicology 2013 Dec 15;314(2-3):221-8..
-
The reactivating and therapeutic efficacy of two combinations ofoximes (HI-6 + trimedoxime and HI-6 + K203) was compared with the effectiveness of antidotal treatment involving single oxime (HI-6, trimedoxime, K203) using in vivo methods. In vivo determined percentage of reactivation of cyclosarin-inhibited blood and tissue acetylcholinesterase in poisoned rats showed that the reactivating efficacy of both combinations of oximes is slightly higher than the reactivating efficacy of the most effective individual oxime in blood, diaphragm as well as in brain. Moreover, both combinations of oximes were found to be slightly more efficacious in the reduction of acute lethal toxic effects in cyclosarin-poisoned mice than the antidotal treatment involving single oxime. Based on the obtained data, we can conclude that the antidotal treatment involving chosen combinations of oximes brings a beneficial effect for its ability to counteract the acute poisoning with cyclosarin.
Kassa J, Karasová JZ, Pavlíková R, Caisberger F, Bajgar J. The ability of oxime mixtures to increase the reactivating and therapeutic efficacy of antidotal treatment of cyclosarin poisoning in rats and mice. Acta Medica (Hradec Kralove). 2012;55(1):27-31. [PubMed Citation]
-
Oxime reactivator HI-6 (asoxime, in some sources) is a potent antidote suitable for treatment of intoxication by nerve agents. Despite the fact that HI-6 is considered for practical application in emergency situations, the impact of HI-6 on patients' bodies has not been established yet. The present experiment was carried out in order to estimate whether HI-6 would be able to trigger or protect from oxidative stress in a BALB/c mice model. HI-6 was applied in doses ranging from 0.2 to 20% of LD50. Ferric-reducing antioxidant power (FRAP), thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH), and glutathione reductase (GR) were assayed in the blood, liver, kidney, and brain of treated animals. It was found that HI-6 does not increase GR or TBARS. On the contrary, TBARS levels in the brain and liver were found to be significantly decreased in HI-6-treated animals. Pertinent antioxidant properties of HI-6 were excluded by the FRAP method. Endogenous antioxidants were unchanged, with the exception of the kidney. Low-molecular-weight antioxidants assayed by the FRAP method were significantly decreased in kidneys of animals treated with HI-6. However, GSH partially recovered the loss of the other low-molecular-weight antioxidants and was significantly increased in the kidney of HI-6-exposed mice. HI-6 potential to produce nephropathy is hypothesized. The achieved conclusions were quite surprising and showed a complex impact of HI-6 on the body.
Pohanka M, Sobotka J, Svobodova H, Stetina R. Investigation of oxidative stress in blood, brain, kidney, and liver after oxime antidote HI-6 application in a mouse experimental model. Drug Chem Toxicol. 2011 Jul;34(3):255-60 [PubMed Citation]
-
The potency of the oxime HI-6 and two combinations of oximes (HI-6 + trimedoxime, HI-6 + K203) to reduce sarin-induced acute neurotoxic signs and symptoms was evaluated in this study. Sarin-induced neurotoxicity and the neuroprotective effects of atropine alone or in combination with HI-6 alone and HI-6 combined with trimedoxime or K203 in rats poisoned with sarin at a sublethal dose (108 μg/kg i.m.; 90% of LD(50) value) were monitored by a functional observatory battery (FOB) 24 h following sarin administration. The results indicate that both mixtures of oximes combined with atropine were able to survive sarin-poisoned rats 24 h following sarin administration while two non-treated sarin-poisoned rats and one sarin-poisoned rat treated with atropine alone or with atropine in combination with the oxime HI-6 died within 24 h following sarin poisoning. All types of antidotal treatment were able to decrease sarin-induced neurotoxic signs and symptoms but not completely. While atropine alone and atropine in combination with the oxime HI-6 were able to eliminate some sarin-induced neurotoxic signs and symptoms, the neuroprotective efficacy of both combinations of oximes with atropine was slightly higher. Thus, both tested combinations of oximes in combination with atropine bring a small benefit for the neuroprotective efficacy of antidotal treatment of acute sarin poisonings.
Kassa J, Kunesova G. The benefit of combination of oximes for the neuroprotective efficacy of antidotal treatment of sarin-poisoned rats. Toxicol Mech Methods. 2012 May;22(4):260-7. [PubMed Citation]
-
Asoxime (HI-6) is a well known oxime reactivator used for counteracting intoxication by nerve agents. It is able to reactivate acetylcholinesterase (AChE) inhibited even by sarin or soman. The present experiment was aimed to determine markers of oxidative stress represented by thiobarbituric acid reactive substances and antioxidants represented by ferric reducing antioxidant power, reduced and oxidized glutathione in a Beagle dog model. Two groups of dogs were intramuscularly exposed to single (11.4 mg/kg.b.wt.) or tenfold (114 mg/kg.b.wt.) human therapeutically doses of HI-6. HI-6 affinity for AChE in vitro was evaluated in a separate experiment. Complete serum biochemistry and pharmacokinetics were also performed with significant alteration in blood urea nitrogen, creatine phosphokinase, glucose and triglycerides. Blood samples were collected before HI-6 application and after 30, 60, and 120 min. The overall HI-6 impact on organism is discussed.
Pohanka M, Novotny L, Zdarova-Karasova J, Bandouchova H, Zemek F, Hrabinova M, Misik J, Kuca K, Bajgar J, Zitka O, Cernei N, Kizek R, Pikula J. Asoxime (HI-6) impact on dogs after one and tenfold therapeutic doses: assessment of adverse effects, distribution, and oxidative stress. Environ Toxicol Pharmacol. 2011 Jul;32(1):75-81. [PubMed Citation]
-
Purpose of the present study was to show that therapies for nerve agent poisoning based on specific neuropharmacological approaches can have greater probability for being successful than treatment regimens based on fragmental research or serendipitous discoveries. By following the guidelines for research in experimental epilepsy, neuronal target areas for nerve agents have been identified through lesion studies, and critical receptors for pharmacological treatment have been specified through microinfusion studies of rats. Subsequent experimentations have shown that the results achieved from microinfusion studies are transferable to systemic administration. It is demonstrated that a treatment regimen developed through the novel approach is more efficacious than regimens derived from conventional research on countermeasures. A therapy consisting of HI-6, levetiracetam, and procyclidine that has been worked out along the new lines, exerts powerful anticonvulsant capacity and appears to have universal utility as a stand-alone therapy against soman intoxication in rats. It would be of great interest to examine whether the latter findings can be expanded to other animal species than rats and other classical nerve agents than soman.
Myher T, Aas P. Choice of approaches in developing novel medical countermeasures for nerve agent poisoning. Neurotoxicology 2014 May:44C:27-38. [PubMed Citation]
-
Eye exposure to the organophosphorus (OP) irreversible cholinesterase inhibitor sarin results in long-term miosis and impaired visual function. We have previously shown that tropicamide is better at ameliorating this insult than topical atropine or cyclopentolate. However, to minimize side effects associated with repeated tropicamide applications and high treatment doses, we evaluated the effects of oximes (ChE re-activators) alone and combined with tropicamide at ameliorating OP-induced ocular impairments. Rats were topically exposed to sarin, followed by topical treatment with various oximes alone or in combination with tropicamide. Pupil width and light reflex were measured by an infrared-based digital photograph system, while visual performance was assessed by employing the cueing version of the Morris water maze (MWM). KEY RESULTS: Oxime treatment following sarin ocular exposure induced a slow persistent pupil widening with efficacy in the order of HLö-7 > HI-6 > obidoxime = TMB-4 = MMB-4. In the light reflex test, the ability of the iris to contract following oxime treatment was mostly impaired at 1 h and was back to normal at 4 h following sarin exposure. All oxime treatments ameliorated the sarin-induced visual impairment as tested in the visual task (MWM). The combined topical treatment of tropicamide with an oxime induced a rapid improvement in pupil widening, light reflex and visual performance, and enabled a reduction in tropicamide dose. CONCLUSIONS AND IMPLICATIONS: The use of tropicamide combined with an oxime should be considered as the topical treatment of choice against the toxic effects of ocular OP exposure.
Gore A, Bloch-Shilderman E, Egoz I, Turetz J, Brandeis R. Efficacy assessment of a combined anticholinergic and oxime treatment against topical sarin-induced miosis and visual impairment in rats. Br J Pharmacol 2014 May;171(9):2364-74.
Other non-clinical studies
Human non-clinical studies-
Acetylcholinesterase was purified from human caudate nucleus and skeletal muscle. The enzyme preparations were used to study aging and reactivation by HI-4 and obidoxime after inhibition by soman and its isomers. HI-6 was found to be the most potent reactivator, for both enzyme preparations a higher reactivatability and a higher rate of aging were observed after inhibition by C(+)- soman than after inhibition by C(-)-soman. Aging was retarded by propidium diiodide. Reactivation by the two oximes was also studied after inhibition by tabun, sarin and VX. Tissue homogenates were used for this part of the work. Our conclusion is that HI-6 is superior to obidoxime for human acetylcholinesterases inhibited by soman and sarin, while obidoxime is better towards tabun-inhibited enzyme. (Class IV)
Puu G, Artursson E, Bucht G. Reactivation Of Nerve Agent Inhibited Human Acetylcholinesterases by Hi-6 and Obidoxime. Biochem Pharmacol. 1986 May 1;35(9):1505-10. [PubMed Citation].
-
The treatment of poisoning by highly toxic organophosphorus compounds (nerve agents) is unsatisfactory. Until now, the efficacy of new potential antidotes has primarily been evaluated in animals. However, the extrapolation of these results to humans is hampered by species differences. Since oximes are believed to act primarily through reactivation of inhibited acetylcholinesterase (AChE) and erythrocyte AChE is regarded to be a good marker for the synaptic enzyme, the reactivating potency can be investigated with human erythrocyte AChE in vitro. The present study was undertaken to evaluate the ability of various oximes at concentrations therapeutically relevant in humans to reactivate human erythrocyte AChE inhibited by different nerve agents. Isolated human erythrocyte AChE was inhibited with soman, sarin, cyclosarin, tabun or VX for 30 min and reactivated in the absence of inhibitory activity over 5 to 60 min by obidoxime, pralidoxime, HI 6 or HLo 7 (10 and 30 microM). The AChE activity was determined photometrically. The reactivation of human AChE by oximes was dependent on the organophosphate used. After soman, sarin, cyclosarin, or VX the reactivating potency decreased in the order HLo 7 > HI 6 > obidoxime >pralidoxime. Obidoxime and pralidoxime were weak reactivators of cyclosarin-inhibited AChE. Only obidoxime and HLo 7 reactivated tabun-inhibited AChE partially (20%), while pralidoxime and HI 6 were almost ineffective (5%). Therefore, HLo 7 may serve as a broad-spectrum reactivator in nerve agent poisoning at doses therapeutically relevant in humans. (Class IV)
Worek F, Widmann R, Knopff O, Szinicz L. Reactivating potency of obidoxime, pralidoxime, HI 6 and HLö 7 in human erythrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch Toxicol. 1998 Mar;72(4):237-43. [PubMed Citation]
-
Acetylcholinesterase (AChE) reactivators are employed for the prophylaxis and treatment of intoxications with organophosphorus AChE inhibitors, including nerve agents and pesticides. For the recovery of inhibited enzyme, derivatives from the group of pyridinium or bispyridinium aldoximes (called oximes) are used. Adverse effects of these substances are not well elucidated, because of their narrow and one-shot usage. Owing to this fact, the study evaluated the influence of some currently applied oximes on human platelet aggregation in vitro. The antiplatelet activity of pralidoxime, obidoxime, HI-6, methoxime and HLo 7 was assayed in human platelet rich plasma (2.5 x 10(8) platelets per ml) at a concentration of 1.35 mM. Arachidonic acid (AA), adenosine diphosphate (ADP), collagen (COL) and thrombin (TR) were used as agonists of platelet aggregation. All tested substances, except pralidoxime and methoxime, caused a significant inhibition of the aggregation process induced by AA, ADP and COL. Of the oximes assayed, none was found to influence TR triggered aggregation. Since reduced platelet aggregation can play an important role as an adverse effect in reactivator administration, further evaluation is needed for the estimation of the real impact of active oximes to the aggregation process in humans. (Class IV)
Jun D, Kuca K, Hronek M, Opletal L. Effect of some acetylcholinesterase reactivators on human platelet aggregation in vitro. J Appl Toxicol. 2006 May-Jun;26(3):258-61 [PubMed Citation]
-
Organophosphorus pesticides (e.g. chlorpyrifos, malathion, and parathion) and nerve agents (sarin, tabun, and VX) are highly toxic organophosphorus compounds with strong inhibition potency against two key enzymes in the human body-acetylcholinesterase (AChE; EC 3.1.1.7) and butyrylcholinesterase (BuChE; EC 3.1.1.8). Subsequent accumulation of acetylcholine at synaptic clefts can result in cholinergic crisis and possible death of intoxicated organism. For the recovery of inhibited AChE, derivatives from the group of pyridinium or bispyridinium aldoximes (called oximes) are used. Their efficacy depends on their chemical structure and also type of organophosphorus inhibitor. In this study, we have tested potency of selected cholinesterase reactivators (pralidoxime, obidoxime, trimedoxime, methoxime and H-oxime HI-6) to reactivate human erythrocyte AChE and human plasma BuChE inhibited by pesticide paraoxon. For this purpose, modified Ellman's method was used and two different concentrations of oximes (10 and 100 microM), attainable in the plasma within antidotal treatment of pesticide intoxication were tested. Results demonstrated that obidoxime (96.8%) and trimedoxime (86%) only reached sufficient reactivation efficacy in case of paraoxon-inhibited AChE. Other oximes evaluated did not surpassed more than 25% of reactivation. In the case of BuChE reactivation, none of tested oximes surpassed 12.5% of reactivation. The highest reactivation efficacy was achieved for trimedoxime (12.4%) at the concentration 100 microM. From the data obtained, it is clear that only two from currently available oximes (obidoxime and trimedoxime) are good reactivators of paraoxon-inhibited AChE. In the case of BuChE, none of these reactivators could be used for its reactivation. (Class IV)
Jun D, Musilova L, Kuca K, Kassa J. Potency of several oximes to reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon in vitro. Chem Biol Interact. 2008 Sep 25;175(1-3):421-4. [PubMed Citation]
-
We have in vitro tested the ability of common, commercially available, cholinesterase reactivators (pralidoxime, obidoxime, methoxime, trimedoxime and HI-6) to reactivate human acetylcholinesterase (AChE), inhibited by five structurally different organophosphate pesticides and inhibitors (paraoxon, dichlorvos, DFP, leptophos-oxon and methamidophos). We also tested reactivation of human butyrylcholinesterase (BChE) with the aim of finding a potent oxime, suitable to serve as a pseudocatalytic bioscavenger in combination with this enzyme. Such a combination could allow an increase of prophylactic and therapeutic efficacy of the administered enzyme. According to our results, the best broad-spectrum AChE reactivators were trimedoxime and obidoxime in the case of paraoxon, leptophos-oxon, and methamidophos-inhibited AChE. Methamidophos and leptophos-oxon were quite easily reactivatable by all tested reactivators. In the case of methamidophos-inhibited AChE, the lower oxime concentration (10(-5) M) had higher reactivation ability than the 10(-4) M concentration. Therefore, we evaluated the reactivation ability of obidoxime in a concentration range of 10(-3)-10(-7) M. The reactivation of methamidophos-inhibited AChE with different obidoxime concentrations resulted in a bell shaped curve with maximum reactivation at 10(-5) M. In the case of BChE, no reactivator exceeded 15% reactivation ability and therefore none of the oximes can be recommended as a candidate for "pseudocatalytic" bioscavengers with BChE. (Class IV)
Jun, D, Musilova L, Musilek K, Kuca K. In vitro ability of currently available oximes to reactivate organophosphate pesticide-inhibited human acetylcholinesterase and butyrylcholinesterase. Int J Mol Sci. 2011;12(3):2077-2087. [PubMed Citation]
-
The high number of fatalities due to poisoning by organophosphorus compound-based (OP) pesticides and the availability of highly toxic OP-type chemical warfare agents (nerve agents) emphasize the necessity for an effective medical treatment. Acute OP toxicity is mainly caused by inhibition of acetylcholinesterase (AChE, EC 3.1.1.7). Reactivators (oximes) of inhibited AChE are a mainstay of treatment. However, human AChE inhibited by certain OP, e.g. the phosphoramidates tabun and fenamiphos, is rather resistant towards reactivation by oximes while AChE inhibited by others, e.g. the phosphoramidate methamidophos is easily reactivated by oximes. To get more insight into a potential structure-activity relationship human AChE was inhibited by 16 different tabun analogues and the time-dependent reactivation by 1mM obidoxime, TMB-4, MMB-4, HI 6 or HLo 7, the reactivation kinetics of obidoxime and the kinetics of aging and spontaneous reactivation were investigated. A clear structure-activity relationship of aging, spontaneous and oxime-induced reactivation kinetics could be determined with AChE inhibited by N-monoalkyl tabun analogues depending on the chain length of the N-alkyl residue. N,N-dialkyl analogues bearing ethyl and n-propyl residues were completely resistant towards reactivation while N,N-di-i-propyl tabun was highly susceptible towards reactivation by oximes. AChE inhibited by phosphonoamidate analogues of tabun, bearing a N,N-dimethyl and N,N-diethyl group, could be reactivated and had comparable reactivation kinetics with obidoxime. These results in conjunction with previous data with organophosphates and organophosphonates emphasizes the necessity for kinetic studies as basis for future work on structural analysis with human AChE and for the development of effective broad-spectrum oximes. (Class IV)
Worek F, Aurbek N, Koller M, Becker C, Eyer P, Thiermann H. Kinetic analysis of reactivation and aging of human acetylcholinesterase inhibited by different phosphoramidates. Biochem Pharmacol. 2007 Jun 1;73(11):1807-1817. [PubMed Citation]
Comparative human and animal in vitro studies-
Organophosphorus compounds such as nerve agents inhibit, practically irreversibly, cholinesterases by their phosphorylation in the active site of these enzymes. Current antidotal treatment used in the case of acute nerve agent intoxications consists of combined administration of anticholinergic drug (usually atropine) and acetylcholinesterase (AChE, EC 3.1.1.7) reactivator (HI-6, obidoxime, pralidoxime), which from a chemical view is a derivative from the group of pyridinium or bispyridinium aldoximes (commonly called oxime). Oximes counteract acetylcholine increase, resulting from AChE inhibition. In the human body environment these compounds are powerful nucleophiles and are able to break down the bond between AChE and nerve agent molecule. This process leads to renewal of enzyme functionality -- to its reactivation. The usefulness of oxime in the reactivation process depends on its chemical structure and on the nerve agent whereby AChE is inhibited. Due to this fact, selection of suitable reactivator in the treatment of intoxications is very important. In our work, we have compared differences in the in vitro inhibition potency of VX and Russian VX on rat, pig and human brain, and subsequently we have tested reactivation of rat brain cholinesterase inhibited by these agents using oxime HI-6, obidoxime, pralidoxime, trimedoxime and methoxime. The results showed that no major differences in the reactivation process of both VX and Russian VX-inhibited cholinesterase. The similarity in reactivation was caused by analogous chemical structure of either nerve agent; and that oxime HI-6 seems to be the most effective reactivator tested, which confirms that HI-6 is currently the most potent reactivator of AChE inhibited by nerve agents. The results obtained in our study should be considered in the future development of new AChE reactivators.
Kuca K, Jun D, Cabal J, Hrabinova M, Bartosova L, Opletalova V. Russian VX: inhibition and reactivation of acetylcholinesterase compared with VX agent. Basic Clin Pharmacol Toxicol. 2006 Apr;98(4):389-94. [PubMed Citation]
-
Oxime HI-6 is an efficient reactivator of the acetylcholinesterase inhibited by organophosphorous nerve agents. In this study we have estimated cytotoxicity of HI-6 by the colony forming assay and genotoxicity by the comet assay on human and rodent cell lines. IC50 of HI-6 assessed by the colony forming capacity was 3.59 mM for HeLa cells and 5.18 mM for a mouse cell line L929. Small difference in cytotoxicity was found among other cell lines tested: IC50 was 1.61 mM for human A549 cells, 1.14 mM for UROtse line, 1.96 mM and 1.71 mM for Chinese hamster cells AA8 and UV-20, respectively. The A549 cell viability measured with the MTT test was 5 times decreased comparing 2 and 24 hours of HI-6 oxime treatment. The 5 mM HI-6 concentration reduced the viability within 2 hours to 95% only, however, it induced a significant number of DNA breaks in mouse cells L929, and also in human UROtse and HepG2 cells. 1-β-D-arabinofuranosylcytosine (10(-4) M) and hydroxyurea (10(-2) M), supplemented to the cultivation medium, did not cause any significant accumulation of DNA breaks during treatment, which indicated that the nucleotide excision repair was not acting on the induced DNA damage.
Svobodova H, Jost P, Stetina R. Cytotoxicity and genotoxicity evaluation of antidote oxime HI-6 tested on eight cell lines of human and rodent origin. Gen Physiol Biophys. 2012 Mar;31(1):77-84. [PubMed Citation]
Animal in vitro studies-
In vitro comparison of reactivation efficacy of five currently used oximes — pralidoxime, obidoxime, trimedoxime, methoxime, and HI-6 (at two concentrations: 10-5 and 10-3 M) — against acetylcholinesterase (AChE; E.C. 3.1.1.7) inhibited by six different nerve agents (VX, Russian VX, sarin, cyclosarin, tabun, soman) and organophosphorus insecticide chlorpyrifos was the aim of this study. As a source of AChE in the experiments, rat brain homogenate was used. According to the results obtained, no AChE reactivator was able to reach sufficient potency for AChE inhibited by all nerve agents used. Moreover, oxime HI-6 (the most effective one) was not able to reactivate tabun- and soman-inhibited AChE. Due to this fact, it could be designated as a partially broadspectrum reactivator.
Kuca K, Jun D, Bajgar J. Currently used cholinesterase reactivators against nerve agent intoxication: comparison of their effectivity in vitro. Drug Chem Toxicol. 2007;30(1):31-40. [PubMed Citation]
-
A comparison of one mono- and seven bisquaternary acetylcholinesterase (AChE) reactivators of acetylcholinesterase inhibited by VX agent was performed. As a source of the acetylcholinesterase, a rat brain homogenate was taken. There were significant differences in reactivation potency of all tested oximes. The oxime TO205 seems to be the most efficacious followed by TO046, HI-6, HS-6, K027, obidoxime, MMC and 2-PAM. In addition, the results of this study showed that the reactivation potency of the tested reactivators depends on many factors--such as the number of pyridinium rings, the number of oxime groups and their position, as well as the length and the shape of linkage bridge between two pyridinium rings.
Kuca, K, Kassa J. Oximes-induced reactivation of rat brain acetylcholinesterase inhibited by VX agent. Hum Exp Toxicol. 2004 Apr;23(4):167-71. [PubMed Citation]
-
In our study, we have tested six acetylcholinesterase (AChE) reactivators (pralidoxime, obidoxime, HI-6, trimedoxime, BI-6 and Hlo-7) for reactivation of sarin- and cyclosarin-inhibited AChE using an in vitro reactivation test. We have used rat brain homogenate as the suitable source of enzyme. All oximes are able to reactivate sarin-inhibited AChE. On the other hand, only HI-6 is able to reactivate satisfactorily cyclosarin-inhibited AChE.
Kuca K, Cabal J, Jun D, Kassa J, Bartosová L, Kunesová G. In vitro reactivation potency of some acetylcholinesterase reactivators against sarin- and cyclosarin-induced inhibitions. J Appl Toxicol. 2005 Jul-Aug;25(4):296-300. [PubMed Citation]
-
The efficacy of H oximes (HI-6, HLo-7), the oxime BI-6, and currently used oximes (pralidoxime, obidoxime, trimedoxime) to reactivate acetylcholinesterase inhibited by two nerve agents (tabun, VX agent) was tested in vitro. Both H oximes (HI-6, HLo-7) and the oxime BI-6 were found to be more efficacious reactivators of VX-inhibited acetylcholinesterase than pralidoxime and obidoxime. On the other hand, their potency to reactivate tabun-inhibited acetylcholinesterase was low and did not reach the reactivating efficacy of trimedoxime and obidoxime. Thus, none of these compounds can be considered to be a broad-spectrum reactivator of nerve agent-inhibited acetylcholinesterase in spite of high potency to reactivate acetylcholinesterase inhibited by some nerve agents. More than one oxime may be necessary for the antidotal treatment of nerve agent-exposed individuals.
Kuca K, Cabal J, Kassa J, Jun D, Hrabinova M. In vitro potency of H oximes (HI-6, HLo-7), the oxime BI-6, and currently used oximes (pralidoxime, obidoxime, trimedoxime) to reactivate nerve agent-inhibited rat brain acetylcholinesterase. J Toxicol Environ Health A. 2006 Aug;69(15):1431-1440. [PubMed Citation]
-
The mechanism of intoxication with organophosphorus compounds, including highly toxic nerve agents and less toxic pesticides, is based on the formation of irreversibly inhibited acetylcholinesterase, which causes cumulation of neuromediator acetylcholine in synaptic clefts and subsequent overstimulation of cholinergic receptors, that is followed by a generalized cholinergic crisis. Nerve agent poisoning is conventionally treated using a combination of a cholinolytic (atropine mostly) to counteract the accumulation of acetylcholine and acetylcholinesterase reactivators (pralidoxime or obidoxime) to reactivate inhibited acetylcholinesterase. In this study of cyclosarin poisoning treatment, oximes of different chemical structures (obidoxime, HI-6, BI-6, and HS-6) were tested in vitro on rat brain acetylcholinesterase (enzyme source: rat brain homogenate), and afterwards, they were tested in vivo in equimolar doses, in mice and rats. The HI-6 oxime appeared to be the most effective oxime in vitro and in vivo.
Bartosova L, Kuca K, Jun D, Kunesova G. Bispyridinium oximes as antidotal treatment of cyclosarin poisoning-in vitro and in vivo testing. Int J Toxicol. 2005 Nov-Dec;24(6):399-402. [PubMed Citation]
-
Administration of acetylcholinesterase (AChE) reactivators (oximes) is usually used in order to counteract the poisoning effects of nerve agents. The possibility was suggested that oximes may show some therapeutic and/or adverse effects through their action in central nervous system. There are no sufficient data about interaction of oximes with monoaminergic neurotransmitter's systems in the brain. Oxime-type AChE reactivators pralidoxime, obidoxime, trimedoxime, methoxime and HI-6 were tested for their potential to affect the activity of monoamine oxidase of type A (MAO-A) and type B (MAO-B) in crude mitochondrial fraction of pig brains. The compounds were found to inhibit fully MAO-A with half maximal inhibitory concentration (IC50) of 0.375 mmol/l (pralidoxime), 1.53 mmol/l (HI-6), 2.31 mmol/l (methoxime), 2.42 mmol/l (obidoxime) and 4.98 mmol/l (trimedoxime). Activity of MAO-B was fully inhibited by HI-6 and pralidoxime only with IC50 4.81 mmol/l and 11.01 mmol/l, respectively. Methoxime, obidoxime and trimedoxime displayed non-monotonic concentration dependent effect on MAO-B activity. Because oximes concentrations effective for MAO inhibition could not be achieved in vivo at the cerebral level, we suppose that oximes investigated do not interfere with brain MAO at therapeutically relevant concentrations.
Fišar Z, Hroudová J, Korábečný J, Musílek K, Kuča K. In vitro effects of acetylcholinesterase reactivators on monoamine oxidase activity. Curr Med Chem. 2009;16(17):176-180. [PubMed Citation]
-
Phrenic nerve diaphragm muscles of young adult rats were used to study the ability of the oximes 2-PAM and HI-6 to recover muscle function depressed by organophosphate (OP) agents. The single twitch of diaphragm muscles which were exposed to soman (0.2 microM) recovered after washing with saline for 3 hr, but the muscles pretreated with sarin (0.4 microM), VX (0.2 microM), or tabun (0.4 microM) showed only partial recovery. In addition, after 3 hr washing, the muscles pretreated with soman as well as with tabun did not recover the tetanus sustaining ability (TSA), yet complete recovery was observed with muscles pretreated with sarin and VX. These results indicate that the OPs have different effects on muscle contractile properties and that VX- and sarin-pretreated muscles recover equally well after wash with physiological solution. The recovery of twitch tension of diaphragm muscles by 2-PAM and HI-6 was similar to that achieved by washing with saline for 3 hr for sarin- and soman-exposed muscles. The most remarkable differences were seen in the recovery of TSA. Both 2-PAM and HI-6 recovered the TSA of muscles that were pretreated with sarin and VX. Although 2-PAM recovered the TSA after tabun pretreatment, HI-6 had no discernible effect. On the other hand, HI-6 recovered the TSA of soman-pretreated muscles but 2-PAM did not. The effectiveness of muscle function recovery was not related to the oximes' ability to reactivate AChE, thus indicating that the recovery of muscle contractility may be attributed to a direct effect of these compounds on the muscle.
Reddy VK, Deshpande SS, Cintra WM, Scoble GT, Albuquerque EX. Effectiveness of oximes 2-PAM and HI-6 in recovery of muscle function depressed by organophosphate agents in the rat hemidiaphragm: an in vitro study. Fundam Appl Toxicol. 1991 Nov;17(4):746-60. [PubMed Citation]
Non-clinical reviews
-
Highly toxic organophosphorus inhibitors of acetylcholinesterase referred as nerve agents are considered to be among the most dangerous chemical warfare agents. The oximes represent very important part of medical countermeasures of nerve agent poisonings. They are used to reactivate the nerve agent-inhibited acetylcholinesterase. Despite long-term research activities, there is no single, broad-spectrum oxime suitable for the antidotal treatment of poisoning with all organophosphorus agents. There are two approaches how to increase and broaden the effectiveness of antidotal treatment of poisoning with nerve agents - to develop new structural analogues of currently available oximes and/or to combine currently available or newly developed oximes. The review describes the evaluation of the potency of newly developed oximes (especially the oxime K203) or combinations of oximes to reactivate nerve agent-inhibited acetylcholinesterase and to counteract the acute toxicity of nerve agents in comparison with single commonly used oxime (obidoxime, trimedoxime or HI-6).
Kassa J, Musilek K, Karasova JZ, Kuca K, Bajgar J. Two possibilities how to increase the efficacy of antidotal treatment of nerve agent poisonings. Mini Rev Med Chem. 2012 Jan;12(1):24-34. [PubMed Citation]
-
Since the September 11, 2001, terrorist attacks in the United States, the specter of a chemical threat against civilian populations has renewed research interest in chemical warfare agents, their mechanisms of action, and treatments that reverse their effects. In this Account, we focus specifically on organophosphorus nerve agents (OPNAs). Although some OPNAs are used as pest control, the most toxic chemicals in this class are used as chemical warfare agents in armed conflicts. The acute toxicity of OPNAs results from the irreversible inhibition of acetylcholinesterase (AChE, EC 3.1.1.7) via the formation of a covalent P-O bond at the serine hydroxyl group in the enzyme active site. AChE breaks down the neurotransmitter acetylcholine at neuronal synapses and neuromuscular junctions. The irreversible inhibition of AChE causes the neurotransmitter to accumulate in the synaptic cleft, leading to overstimulation of cholinergic receptors, seizures, respiratory arrest, and death. The current treatment for OPNA poisoning combines an antimuscarinic drug (e.g., atropine), an anticonvulsant drug (e.g., diazepam), and an AChE reactivator of the pyridinium aldoxime family (pralidoxime, trimedoxime, obidoxime, HI-6, HLö-7). Because of their high nucleophilicity, oximes can displace the phosphyl group from the catalytic serine, thus restoring the enzyme's catalytic activity. During 50 years of research in the reactivator field, researchers have synthesized and tested numerous structural modifications of monopyridinium oximes and bispyridinium oximes. In the past decade, medicinal chemists have focused their research on the more efficient bispyridinium reactivators, but all known reactivators have several drawbacks. First, due to their permanent positive charge, they do not cross the blood-brain barrier (BBB) efficiently and do not readily reactivate AChE in the central nervous system. Second, no single oxime is efficient against a wide variety of OPNAs. Third, oximes cannot reactivate "aged" AChE. This Account summarizes recent strategies for the development of AChE reactivators capable of crossing the BBB. The use of nanoparticulate transport and inhibition of P-glycoprotein efflux pumps improves BBB transport of these AChE reactivators. Chemical modifications that increased the lipophilicity of the pyridinium aldoximes, the addition of a fluorine atom and the replacement of a pyridyl ring with a dihydropyridyl moiety, enhances BBB permeability. The glycosylation of pyridine aldoximes facilitates increased BBB penetration via the GLUT-1 transport system. The development of novel uncharged reactivators that can move efficiently across the BBB represents one of the most promising of these new strategies.
Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc Chem Res. 2012 May 15;45(5):756-66. [PubMed Citation]
-
During more than five decades, pyridinium oximes have been developed as therapeutic agents used in the medical treatment of poisoning with organophosphorus compounds. Their mechanism of action is reactivation of acetylcholinesterase (AChE) inhibited by organophosphorus agents. Organophosphorus compounds (OPC) are used as pesticides and developed as warfare nerve agents such as tabun, soman, sarin, VX and others. Exposure to even small amounts of an OPC can be fatal and death is usually caused by respiratory failure resulting from paralysis of the diaphragm and intercostal muscles, depression of the brain respiratory center, bronchospasm, and excessive bronchial secretions. The mechanism of OPC poisoning involves phosphorylation of the serine hydroxyl group at the active site of AChE leading to the inactivation of this essential enzyme, which has an important role in neurotransmission. AChE inhibition results in the accumulation of acetylcholine at cholinergic receptor sites, producing continuous stimulation of cholinergic fibers throughout the central and peripheral nervous systems. Presently, a combination of an antimuscarinic agent, e.g. atropine, AChE reactivator such as one of the standard pyridinium oximes (pralidoxime, trimedoxime, obidoxime, HI-6) and diazepam has been used for the treatment of organophosphate poisoning in humans. Despite enormous efforts devoted to synthesis and development of new pyridinium oximes as potential antidotes against poisoning with OPC, only four compounds have found their application in human medicine so far. However, they differ in their activity in poisoning with warfare nerve agents and pesticides and there is still no universal broad-spectrum oxime capable of protecting against all known OPC. In this article, we review data on structure-activity relationship of pyridinium oximes and discuss their pharmacological and toxicological significance.
Jokanović M, Prostran M. Pyridinium oximes as cholinesterase reactivators. Structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds. Curr Med Chem. 2009;16(17):2177-88. [PubMed Citation]
-
This paper reviews the mechanisms of interaction of organophosphorus compounds with cholinesterases and clinical signs of acute poisoning. Further, we describe the current understanding of the mechanisms of action of pyridinium oximes pralidoxime (PAM-2), trimedoxime (TMB-4), obidoxime (LüH-6, Toxogonin), HI-6 and HLö-7 which are used as cholinesterase reactivators in the treatment of poisoning with organophosphorus compounds. We also review the most important literature data related to the efficacy of these oximes in the treatment of poisoning with warfare nerve agents soman, sarin, tabun, VX and cyclosarin and organophosphorus insecticides. Finally, we discuss the criteria for selection of oximes intended for further development as antidotes in poisoning with organophosphorus compounds and auto-injectors for their application in urgent situations.
Jokanović M, Stojiljković MP. Current understanding of the application of pyridinium oximes as cholinesterase reactivators in treatment of organophosphate poisoning. Eur J Pharmacol. 2006 Dec 28;553(1-3):10-7. [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
In a double-blind, placebo-controlled, single-dose ascending pharmacokinetics and tolerance study, we evaluated the bispyridinium oxime HI-6 dichloride monohydrate (62.5, 125, 250, and 500 mg), administered intramuscularly with atropine sulphate, 2 mg, in 24 healthy male volunteers. The plasma HI-6 peak concentration (Cmax) and area under the concentration-time curve (AUC) demonstrated linear pharmacokinetics with low intradose variability, suggestive of uniformity of effect among subjects. HI-6 (500 mg) attained plasma drug concentrations that appeared adequate for practical use as an antidote. The mean +/- SD time to maximum plasma HI-6 concentration (tmax = 0.69 +/- 0.21 h, n = 16), and absorption half-life (t/2a = 0.17 +/- 0.05 h) indicated rapid onset of effect. The volume of distribution (Vd = 0.25 +/- 0.04 L kg-1 TBW) approximated the extracellular fluid volume. A high total body clearance (CL = 252 +/- 52 mL min-1) and short apparent elimination half-life (t/2e = 1.15 +/- 0.19 h) were expected for this polar quaternary ammonium drug. The renal clearance CLr = 137 +/- 33 mL min-1), which approximated the expected glomerular filtration rate, and 24 h urinary excretion of unchanged drug (55 +/- 10%) indicated substantial non-renal elimination. Blood pressure, heart rate, respiratory rate, electrocardiographic parameters, mental acuity, and vision were not altered. Adverse events and changes in serum, urine, and semen laboratory tests were mild. The pharmacokinetics, safety, and apparent efficacy of HI-6 suggest it may be a superior oxime antidote against nerve agent poisoning.
Clement JG, Bailey DG, Madill HD, Tran LT, Spence JD. The acetylcholinesterase oxime reactivator HI-6 in man: pharmacokinetics and tolerability in combination with atropine. Biopharm Drug Dispos. 1995 Jul;16(5):415-25. [PubMed Citation]
-
New pharmaceutical formulations of the oxime HI-6 as sustained-release and conventional tablets were studied in healthy volunteers. Twenty-six subjects, divided into 3 groups, received 3784 mg or 7568 mg doses of HI-6 conventional tablets or 4027 mg of the oxime in the form of sustained-release tablets. Peak plasma concentrations of HI-6 were reached within 0.6 h (10.2 mumol/l) and 1.6 h (21.4 mumol/l) following the ingestion of conventional tablets. Elimination half-lives were similar (1.7 h and 1.3 h) and the respective urinary recoveries amounted to 3.2% and 2.9%. After the administration of sustained-release tablets of HI-6, maximal concentration (8.8 mumol/l) was attained in 2.2 h, elimination half-life was 1.9 h and 4.2% of the dose was excreted unchanged in urine. Undesirable side effects were not reported by the subjects or revealed by clinical or laboratory tests. The results indicate low bioavailability of the oral formulations of HI-6 in man.
Jovanovic D, Maksimovic M, Joksovic D, Kovacevic V. Oral forms of the oxime HI-6: a study of pharmacokinetics and tolerance after administration to healthy volunteers. Vet Hum Toxicol. 1990 Oct;32(5):419-21. [PubMed Citation]
-
After intramuscular administration of graded doses of HI-6 (62.5, 125, 250, and 500 mg) to 22 healthy men, it has been established that therapeutic concentrations of the oxime in plasma, arbitrarily taken as 4 micrograms/ml, were achieved by doses of 250 and 500 mg in about 5 min, and maintained from 2 to 3 hr. The two lowest doses have not been satisfactory in this respect. Of the total doses injected, from 56.3 to 62% of HI-6 was excreted into urine unchanged during the first 6 hr. No side-effects were reported by the subjects, nor revealed by clinical or laboratory tests during the study. Exceptional tolerance of HI-6 in man found in this study, along with its high efficiency proven in experimental poisoning by sarin, VX, and soman, make it the most promising oxime aimed at the treatment of human poisoning by known chemical warfare nerve agents.
Kusić R, Bosković B, Vojvodić V, Jovanović D. HI-6 in man: blood levels, urinary excretion, and tolerance after intramuscular administration of the oxime to healthy volunteers. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 2):S89-97 [PubMed Citation]
Animal
-
The absorption and disposition kinetics of HI-6 were determined in Beagle dogs given single doses (25 mg kg-1) of the drug by the intravenous, intramuscular, and oral routes. Concentrations of the oxime in plasma and urine were measured by HPLC. A two-compartment open model was used to describe the disposition curve following intravenous drug administration while a one-compartment open model with first-order absorption adequately described the data following intramuscular or oral administration of the dose. Extravascular distribution of HI-6 was limited (Vss 203 ml kg-1) and the drug was eliminated rapidly after intravenous administration (t1/2 46.5 min, MAT 55.4 min). Systemic clearance was 3.68 ml min-1 x kg. A major fraction of the dose (63.7 per cent) was excreted in urine over a 24-h collection period. Following intramuscular drug administration, the absorption half-life (t1/2(a), 5.3 min), MAT (17.1 min), Cmax (70.37 micrograms ml-1) and tmax (15.9 min) indicate that the drug was rapidly absorbed. Systemic availability was 83.43 per cent after oral drug administration, absorption was preceded by a lag time (23.2 min). The t1/2(a) (41.5 min), MAT (81.6 min), Cmax (4.30 micrograms ml-1) and Tmax (90.6 min) indicate somewhat delayed absorption. Systemic availability (11.38 per cent) and the fraction of dose excreted unchanged in the urine (9.3 per cent) show that the drug was poorly absorbed. The apparent half-life (58.0 min) and MRT (137.6 min) following oral administration were significantly longer (p < 0.05) than following intravenous or intramuscular administration suggesting that the rate of absorption from the gastrointestinal tract decreases the elimination rate of the drug. In conclusion, HI-6 has limited distribution within the body, is rapidly eliminated mainly by renal excretion unchanged in the urine, and the bioavailability (i.e. rate and extent of absorption) of the drug varies with the route of administration.
Baggot JD, Buckpitt A, Johnson D, Brennan P, Chung H. Bioavailability and disposition kinetics of HI-6 in Beagle dogs. Biopharm Drug Dispos. 1993 Mar;14(2):93-105. [PubMed Citation]
-
Anesthetized pigs were injected i.m. with 500 mg HI-6 dichloride (HI-6 2Cl) (1-[[[4-(aminocarbonyl)-pyridinio]methoxy]methyl]-2[(hydroxyimino)methyl]pyridinium dichloride; CAS 34433-31-3)) or the molar equivalent of HI-6 dimethanesulphonate (HI-6 DMS) 633 mg. Plasma HI-6 concentrations were measured by HPLC (1, 3, 5, 10, 15, 30, 60 min and every 30 min until 4h or 6h following the i.v. or i.m. dose respectively) while a variety of physiological responses were continuously examined. HI-6 (500 mg 2Cl or 633 mg DMS) resulted in an identical pharmacokinetic profile unaffected by atropine co-administration. Neither HI-6 salt resulted in clinically significant changes in cardiovascular or respiratory function. HI-6 DMS (1899 mg i.v.) resulted in plasma HI-6 concentrations about 10 times higher than measured following i.m. 500 mg 2Cl or 633 mg DMS and resulted in small transitory effect on mean arterial pressure. Atropine plus HI-6 DMS (1-9 mg/kg or 127-172 mg/kg i.m.) protected up to 100% of guinea pigs exposed to 5 x LD50 of GF (cyclohexyl methyl phosphonoflouridate) or soman (pinacolyl methylphosphonofluoridate) (GD) respectively. The results suggest that the two HI-6 salts have a similar pharmacokinetic profile while HI-6 DMS appears extremely safe and effective against nerve agents and may be as suitable for human use.
Lundy PM, Hill I, Lecavalier P, Hamilton MG, Vair C, Davidson C, Weatherby KL, Berger BJ. The pharmacokinetics and pharmacodynamics of two HI-6 salts in swine and efficacy in the treatment of GF and soman poisoning. Toxicology. 2005 Mar 30;208(3):399-409. [PubMed Citation]
-
The effect of fasting, atropine, and poisoning by an organophosphate anticholinesterase soman (pinacolyl methylphosphonofluoridate) on the pharmacokinetics of the acetylcholinesterase oxime reactivator HI-6 (CAS Reg. No. 34433-31-3; 1-[(4-(aminocarbonyl)pyridinio)methoxy)methyl)-2-(hydroxy imino)methyl) pyridinium dichloride) was investigated. Pharmacokinetic parameters (elimination half-life, volume of distribution, clearance rate) were determined for the following groups: (1) a 20 and 50 mg kg-1 dose of HI-6; (2) a 50 mg kg-1 dose of HI-6 after fasting for 18 h (water ad lib); (3) a 50 mg kg-1 dose of HI-6 at 0, 4, and 24 h after atropine (17.4 mg kg-1, i.p.) and soman (287 micrograms kg-1, s.c.); and (4) a 50 mg kg-1 dose of HI-6 at 0 and 4 h after soman (100 micrograms kg-1, s.c.). Fasting increased significantly (p less than 0.05) the elimination of half-life (t1/2) and tended to increase the volume of distribution (Vd) and decrease the clearance rate (CL). Following soman (287 micrograms kg-1) poisoning the t1/2 of HI-6 increased from 8.6 min to 21.6 min and the Vd increased to 0.731 kg-1. At the lower soman dose (100 micrograms kg-1) no significant effect on HI-6 pharmacokinetics was found. Atropine (17.4 mg kg-1: i.p.) pretreatment increased the t1/2 and CL while having no effect on the Vd. By 24 h the pharmacokinetic parameters of HI-6 in the various treatment groups were not significantly different from the control group. The changes in the pharmacokinetics of HI-6 following soman and atropine are probably the result of haemodynamic changes.
Clement JG, Simons KJ, Briggs CJ. Effect of poisoning by soman (pinacolyl methylphosphonofluoridate) on the serum half-life of the cholinesterase reactivator HI-6 in mice. Biopharm Drug Dispos. 1988 Mar-Apr;9(2):177-86. [PubMed Citation]
-
The intravenous pharmacokinetics of the oximes HI-6 (pyridinium-1-(((4-carbamoil-pyridinio)metoxy)methyl)2 -(hydroxyiminomethyl)dichloride monohydrate), (132.54 mu mol/kg) and trimedoxime (1,1'-(1,3'-propanedyl)bis((4-hydroxyimino) methyl)-pyridinium dibromide), (55.98 mu mol/kg) in mice was investigated. The concentrations of oximes in plasma determined by high pressure liquid chromatography (HPLC) corresponded to a two-compartment pharmacokinetic open model. The oximes were rapidly eliminated from mice plasma, with half-times of 57.93 min. for HI-6 and 108.08 min. for trimedoxime. Although the oximes passed from circulation into the tissues at approximately the same rate, their transport back to the central compartment was two-times slower in the case of trimedoxime: t(1/2k21) was 77.9 min. for trimedoxime and 41.7 min. for HI-6. The total body clearance (CI(tot)) of HI-6 was about 25% higher than that of trimedoxime. The central compartment volume of HI-6 distribution (V(1)) was greater, whereas the volume of distribution of the peripheral compartment (V(2)) was lower for about 35% with respect to the corresponding parameters of trimedoxime. The calculated pharmacokinetic parameters for the oxime HI-6 and trimedoxime show that trimedoxime is eliminated more slowly in mice, and penetrates better into the peripheral compartment where it remains longer.
Milic B, Maksimovic M, Nedelijkovic M. Trimedoxime and HI-6: kinetic comparison after intravenous administration to mice. Pharmacol Toxicol. 1996 Apr;78(4):269-72. [PubMed Citation]
-
A newly developed autoinjector (Astra Tech, Sweden) containing 500 mg HI-6 and 2 mg atropine sulphate was tested in anaesthetized normal pigs. The pharmacokinetics and pharmacodynamics of the drugs after administration by the autoinjector were compared with those after conventional needle and syringe delivery intramuscularly and intravenously. Cardiopulmonary parameters were monitored and serum concentrations of oxime, atropine, and acetylcholinesterase were determined in blood samples taken at intervals over a 6 h period postinjection. After injection in anaesthetized pigs, both HI-6 and atropine were absorbed rapidly and completely from the injection site. Therapeutic serum concentrations of HI-6, arbitrarily taken as 4 micrograms mL-1, were reached within 1 min of intravenous and autoinjector administration, and within 5 min of intramuscular injection. The concentrations remained above this level for 3-4 h. There were no significant changes in acetylcholinesterase activity, mean arterial blood pressure, or respiration frequency after injection of HI-6 and atropine sulphate. The heart rates increased significantly after administration of the two drugs (cardioacceleration defined as > or = 5% increase in heart rate), regardless of the technique employed. Our results show that HI-6 and atropine sulphate can be given intramuscularly by the new autoinjector with the same effectiveness and speed as when given intravenously. Irrespective of the injection technique, no overt signs of toxicity were observed at the drug concentrations used.
Nyberg AG, Cassel G, Jeneskog T, Karlsson L, Larsson R, Lundström M, Palmer L, Persson SA. Pharmacokinetics of HI-6 and atropine in anaesthetized pigs after administration by a new autoinjector. Biopharm Drug Dispos. 1995 Nov;16(8):635-51. [PubMed Ciation]
-
The pharmacokinetics of 2-PAM, a component of the current nerve agent antidote therapy for U.S. military forces was compared to the pharmacokinetics of another acetylcholinesterase reactivator HI-6. Additionally, the effects of these compounds on muscle tissue following intramuscular injection was examined. Plasma concentrations of the oximes were determined by HPLC. Plasma concentration-time profiles for both oximes fit a one-compartment open model with first-order absorption and elimination. The results demonstrate that the half-time of absorption of HI-6 was significantly higher than that for 2-PAM. Musculoirritancy was assessed on the basis of quantitative histological examinations of the injection sites and by the measurement of serum creatinine phosphokinase. Comparison of the scores from the histological sections demonstrate no difference between the two oximes. Serum creatinine phosphokinase values were elevated following injections of HI-6, but were not consistently elevated following the 2-PAM injections.
Moore DH, Hayward IJ, Tucker FS, Lukey B. HI-6 and 2-PAM in sheep: pharmacokinetics and effects on muscle tissue following intramuscular injection. Biopharm Drug Dispos. 1991 Apr;12(3):223-32. [PubMed Citation]
-
Female rats poisoned with multiple LD50s of soman or tabun have been shown previously to respond to the protective effects of HI-6 more positively than male rats. This present study was designed first to determine the distribution pattern and concentration of [14C] HI-6 in rats, and secondly, to determine the possibility that HI-6 might be located in high concentrations in critical tissues in the female as opposed to the male. To these ends, [14C] HI-6 was administered to groups of male and female rats and its radiolabelled distribution determined by whole body autoradiography and/or by measurement of its actual concentration, by scintillation spectrometry. The experiments were repeated in the presence of 2 x LD50 soman and supporting therapy with atropine. In both sexes, HI-6 levels were highest in the kidney, followed in order by cartilage greater than plasma greater than liver greater than heart greater than or equal to lung greater than or equal to diaphragm greater than brain and spinal cord. The relative distribution in the two sexes was confirmed by both methods and was not significantly altered in the presence of soman and atropine. The lack of a measurable difference in tissue distribution of [14C] HI-6 derived radioactivity between males and females suggested that the hormone-dependent difference in the protective effects previously observed was not due to selective accumulation of [14C] HI-6 in organs believed to be important in its therapeutic activity, such as brain or diaphragm.
Lundy PM, Hand BT, Broxup BR, Yipchuck G, Hamilton MG. Distribution of the bispyridinium oxime [14C] HI-6 in male and female rats. Arch Toxicol. 1990;64(5):377-82. [PubMed Citation]
-
The pharmacokinetics of HI-6 ((4-carboxamidopyridinium (1) methyl)-(2'-hydroxyiminomethyl-pyridinium (1') methyl) ether dichloride) have been studied in rabbits receiving an intramuscular (50 micrograms kg-1) or intravenous (12.5 micrograms kg-1) dose. The plasma concentration-time profile for the intramuscular dose (n = 8) fits a one-compartment open model with first-order absorption and elimination. The absorption half-life was 2 min and maximum concentration (51 micrograms mL-1) was reached in 9 min. The pharmacokinetics for the intravenous dose (n = 8) was described by a two-compartment open model with first-order distribution and elimination. The apparent volume of distribution was 0.1 L kg-1. Half-lives of distribution and elimination were 5 and 38 min, respectively. The results indicate HI-6 is rapidly absorbed, distributed and eliminated in rabbits receiving an intramuscular dose.
Lukey BJ, Woodard CL, Clark CR, McCluskey MP. HI-6 pharmacokinetics in rabbits after intravenous and intramuscular administration. J Pharm Pharmacol. 1992 Aug;44(8):690-2. [PubMed Citation]
-
One of the shortcomings of current treatment of nerve agent poisoning is that oximes hardly penetrate the blood-brain barrier (BBB), whereas nerve agents easily do. Increasing the concentration of oximes in the brain, would therefore provide an attractive approach to improve medical countermeasures. An explanation for limited penetration might be that oximes are substrates for the active P-glycoprotein (Pgp) efflux transporter located in the BBB. Using quantitative brain microdialysis in rats, the effect of i.v. injected tariquidar, a non-competitive, specific Pgp-inhibitor, on HI-6 levels in blood and brain was investigated. It appeared that tariquidar enhanced HI-6 levels in the brain approximately 2-fold during the first hour after HI-6 administration, whereas plasma levels did not differ between the treatment groups. A subsequent proof-of-concept study in rats showed that soman-induced seizures and convulsions were prevented almost completely when they were, in addition to HI-6 and atropine, pretreated with tariquidar. Moreover, twice as much AChE activity was present in their brains as compared to control rats. These results in rats indicate that modulation of the BBB by a drug like tariquidar, which is non-toxic by itself, is of great value in enhancing the efficacy of oximes.
Joosen MJ, van der Schans MJ, van Dijk CG, Kuijpers WC, Wortelboer HM, van Helden HP. Increasing oxime efficacy by blood-brain barrier modulation. Toxicol Lett. 2011 Sep 25;206(1):67-71. [PubMed Citation]
-
A one-compartment open model with first-order absorption was used for comparing new oral formulations of the potent acetylcholinesterase reactivating oxime HI-6. Although mean peak plasma levels did not differ between retard and conventional tablets (21.38 and 20.74 mumol/l), the time for reaching peak levels was significantly longer (5.5 h) with retard than with conventional tablets (2.86 h). Among other pharmacokinetic estimates only absorption half-lives and areas under the concentration-time curve (AUC) were significantly different (P less than 0.05). The AUC with retard tablets was 8.07% and that of conventional tablets 5.42% of intravenous AUC, indicating low bioavailability of oral HI-6 formulations. Potential therapeutic use of HI-6 requires, therefore, further investigations in order to improve its gastrointestinal absorption.
Maksimović M, Jovanović D, Kovacević V, Bokonjić D. Oral kinetics and bioavailability of the cholinesterase reactivator HI-6 after administration of 2 different formulations of tablets to dogs. Toxicol Lett. 1987 Nov;39(1):85-91. [PubMed Citation]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for HI-6 has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (Public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Shelf life
Stability
-
Commercially manufactured wet/dry autoinjectors containing atropine in solution and powdered HI-6 were evaluated using HPLC for consistency of drug delivery with various solvation times and stability of drugs postsolvation at a temperature of 40 degrees C. Three configurations of autoinjector were tested. System A (SYS A), with a specified mixing time of 5 sec, delivered a volume of 3.0 ml containing 1.86 mg of atropine sulfate and 443 mg of the bispyridinium oxime HI-6 dichloride. System B1 (SYS B1) and System B2 (SYS B2), with specified mixing times of 40 sec, delivered volumes of 2.3 ml containing 2.13 and 2.06 mg atropine citrate and 424 and 545 mg HI-6 dichloride, respectively. Average coefficients of variation for SYS A were 3.4% for atropine and 5.8% for HI-6 and for SYS B1 and B2 were 5.2% for atropine and 7.0% for HI-6 determinations. Stored from 3 to 14 days at 40 degrees C after the autoinjector contents were mixed, SYS A delivered 1.77 mg atropine sulfate and SYS B1 and B2 delivered 2.02 mg atropine citrate. The delivery of HI-6 dichloride decreased with a half-life of 34 days for SYS A, 39 days for SYS B1, and 32 days for SYS B2. This resulted in a decrease to 90% of the respective day 0 amount after 4 (SYS A) or 5 (SYS B1 or B2) days.
Schlager JW, Dolzine TW, Stewart JR, Wannarka GL, Shih ML. Operational evaluation of three commercial configurations of atropine/HI-6 wet/dry autoinjectors. Pharm Res. 1991 Sep;8(9):1191-4. [PubMed Citation]
-
HI-6 exhibits superior efficacy in the therapy of intoxication by different highly toxic organophosphorus nerve agents. Therefore HI-6 is a promising candidate for the development of new antidotes against nerve agents. For ethical and safety reasons antidotes containing HI-6 should get marketing authorization. Active pharmaceutical ingredients of medicinal products have to fulfil regulatory conditions in terms of purity and stability. Photostability is an essential parameter in this testing strategy. HI-6 was tested under conditions of ICH Q1B Photostability testing of new drug substances and products. The data showed a marked degradation of HI-6 after exposure to daylight. The mechanism of degradation could be detected as photoisomerism. The light burden dependent rate of photoisomerism was followed quantitatively. Based on these quantitative results on the amount of light induced isomeric product a pharmacological qualification was made. A standardized in vitro test showed a decreased ability of light exposed HI-6 to reactivate sarin- and paraoxon-inhibited human acetylcholinesterase. These results have an impact on the further development of antidotes containing HI-6, as light protection will probably be necessary during handling, packaging, storage and application.
Bogan R, Worek F, Koller M, Klaubert B. Photostability of antidotal oxime HI-6, impact on drug development. Drug Test Anal. 2012 Mar-Apr;4(3-4):208-14. [PubMed Citation]
-
HI 6 (Pyridinium, 1-[[[4-(aminocarbonyl)pyridinio]methoxy]methyl]-2-[(hydro xyimino) methyl]-dichloride is an effective antidote against poisoning with extremely toxic organophosphates. Because of conflicting reports on the stability of HI 6 in aqueous solutions, we studied the factors influencing its stability. HI 6 has been shown to be most stable in acidic solution between pH 2 and 3. At that pH, HI 6 decomposes probably by attack of nucleophiles on the methylene carbon atom of the animal-acetal bond of the ether bridge. HI 6 decomposition follows first order kinetics. From Arrhenius plots of the decay of HI 6 at various concentrations it became obvious that the rate of decomposition increased with increasing HI 6 concentration with simultaneous decrease in the energy of activation. To decide whether the pyridinium compound itself or its anions are responsible for the enhanced decomposition, we studied the influence of chloride, phosphate and iodide. These anions stimulated the decay of HI 6 at increasing strength; their effect, however, was small as compared to that brought about by the pyridinium oxime itself. Since 1-methylisonicotinamide chloride had virtually no effect in contrast to 1-methylpyridinium-2-aldoxime chloride, we conclude that the oximate anion is responsible for the intermolecular attack on HI 6. At present, we recommend storage of HI 6 at concentrations not exceeding 0.1 M in aqueous solution at pH 2.5 and low temperatures. Under these conditions an apparent shelf-life of 20 years is calculated when HI 6 is stored at 8 degrees C.
Eyer P, Hagedorn I, Ladstetter B. Study on the stability of the oxime HI 6 in aqueous solution. Arch Toxicol. 1988;62(2-3):224-6. [PubMed Citation]
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataNo data available at this time.
8. Route of Administration/Monitoring
-
During the last five decades, five pyridinium oximes were found to be worthy of use as antidotes against nerve agents in humans: pralidoxime, in a form of chloride or PAM-2 Cl and mesylate or P2S (against sarin, cyclosarin and VX), trimedoxime or TMB-4 and obidoxime or LüH-6 (both against tabun, sarin and VX), HI-6 (against sarin, soman, cyclosarin and VX) and HLö-7 (against all the five nerve agents). In order to provide the auto-injector with the best and most potent acetylcholinesterase reactivator, the Defence Research and Development Canada (DRDC) received in the 1990s a core funding from the federal government's CBRN research and Technology Initiative (CRTI). Its ultimate result should be three products: (1) 3-in-1 auto-injector (atropine, HI-6 dimethanesulphonate and avizafone, as anticonvulsant), (2) 2-in-1 auto-injector (atropine and HI-6 dimethanesulphonate) and (3) HI-6 dimethanesulphonate in a vial for administration by the medically trained personnel. Previous experimental and clinical experience suggests that, among the oximes mentioned, only trimedoxime and obidoxime can be used for acetylcholinesterase reactivation and antidotal protection against most of the organophosphorus insecticides. The search for an "omnipotent" oxime, effective in reactivation of AChE inhibited with both nerve agents and organophosphorus insecticides, is still ongoing.
Stojiljković MP, Jokanović M. Pyridinium oximes: rationale for their selection as causal antidotes against organophosphate poisonings and current solutions for auto-injectors. Arh Hig Rada Toksikol. 2006 Dec;57(4):435-43. [PubMed Citation]
-
To counteract organophosphate poisoning, the combined administration of atropine and a cholinesterase reactivator has been a standard therapy. Because of potential large-scale emergencies that might occur upon dissemination of nerve agents, administration of life-saving drugs by autoinjectors for self- and buddy-aid can be mandatory. HI 6 and HLO 7 belong to the newer generation of 'H-oximes' with a broader antidotal spectrum, including soman, and are therefore considered as possible candidates to replace the currently marketed oximes, pralidoxime and obidoxime. Since HI 6 and HLO 7 are unstable in solution they must be administered by the newly developed binary wet/dry autoinjectors that allow rapid dissolution of solid compounds prior to injection. The purpose of this in vitro study was to evaluate the performance of two commercial autoinjector systems, containing solid HI 6 or HLO 7 together with atropine in solution, and to determine the delivery of the oximes. The Astra Tech HI 6 autoinjectors Meditec contained 500 mg HI 6 dichloride and delivered 426 mg HI 6 (coefficient of variation, CV 5.7%). The HI 6 autoinjectors from STI Binaject contained 600 mg HI 6 and delivered 533 mg (CV 1.0%). The somewhat large variation of the HI 6 remaining in the Meditec autoinjectors was markedly increased when the device was fired against an increasing back pressure, such as during i.m. administration. At a pressure of 0.6 kg/cm2 the Meditec autoinjectors delivered only 273 mg HI 6 (CV 13.4%), whereas the Binaject autoinjectors still delivered 511 mg (CV 2.7%) against 1 kg/cm2. Incomplete delivery from the Meditec autoinjectors was also found during administration of HI 6 to dogs, where the Binaject autoinjectors functioned reliably. Malfunctions of the Meditec autoinjectors were detected neither in vitro nor in vivo when they were filled with 225 mg HLO 7 dimethanesulfonate and atropine or with atropine only. The same holds true for the Binaject injectors. Special care has to be taken with regard to the appropriate shaking procedure during dissolution. In this respect, the manufacturers should improve the user's instructions.
Thiermann H, Spohrer U, Klimmek R, Eyer P. Operational evaluation of wet / dry autoinjectors containing atropine in solution and powdered HI 6 or HLO 7. International Journal of Pharmaceutics. 1994: 109: 35-43.
9. Adverse effects
-
HI6 chloride was tested in a double-blind, placebo controlled, ascending dose-tolerance study (HI6 + 2 mg atropine sulfate) in 24 healthy male volunteers. Doses from 62.5 up to 500 mg were well tolerated by the subjects without serious complaints. There were no clinically significant changes in heart rate or ECG, respiration or blood pressure or visual and mental acuity following 500 mg HI 6 plus atropine IM. The changes in aspartate aminotransferase (AST) creatinine phosphokinase (CPK), creatinine and gamma-glutamyl transferase (gamma-GT) following the highest HI 6 dose were considered clinically insignificant.
Marrs TC, Maynard RL, Sidell FR, eds. Chemical Warfare Agents Toxicology and Treatment, 2nd Edition. West Sussex, England: John Wiley & Sons Ltd, 2007 p.305-329
10. Contraindication(s)
No data available at this time.
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesNo data available at this time.
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues-
Title: Next generation oxime therapeutic for chemical agent inhibited CNS (brain) ChEs
Current treatment of acute pesticide or organophosphate (OP) poisoning includes a combined administration of a cholinesterase reactivator (oxime), a muscarinic receptor antagonist (atropine) and an anticonvulsant (diazepam). Since the oxime does not penetrate the blood brain barrier (BBS), removal of excessive accumulation of acetylcholine (ACh) is not accomplished by acetylcholinesterase (AChE). Pesticides and OPs are significant terrorist threats to civilian populations. The chemical agent sarin was used in the Tokyo subway terrorist event in 1995 and resulted in long-term neuronal sequelae. Clearly, a new formulation of oxime is required to improve CNS therapy. Because of their chemical structure, the positively charged small molecule oximes do not penetrate the BBB and therefore can not treat pesticide- and OP- induced toxicity in the brain. The long term goal is to transport oxime (2-PAM, MMB-4) quickly and non-invasively into the CNS. CNS-penetrating oxime therapy will reactivate the brain AChE in a timely manner, reduce the requirement for anticonvulsant drug regimens, and improve the long term recovery of the exposed individual by reducing or eliminating CNS neuronal damage, (a) The investigators will synthesize and evaluate more lipoidal forms (pro-oximes), that can be converted to their active (charged) form in the brain, (b) In addition, the investigators will develop carrier(s) of oximes that will come from a class of FDA approved/phase 1 trial Pharmaceuticals that pass the BBB... including Stealth liposomes, nanoparticles, or cyclodextrins. (c) Two additional advantages to the carriers: should permit a sustained delivery of oxime into the systemic circulation through the skin (via skin patch) and prolong the circulatory time of the oxime(s), which are rapidly cleared by the renal system (T1/2<2 hr). (d) For formulations that are successful in in vitro BBB tissue culture and pig skin penetration models, the investigators will determine reactivation kinetics of the encapsulated oximes with pesticide- and OP-inhibited AChE and BuChE, the release rates of the oxime from the carrier in the peripheral and CNS of animal model (guinea pig), and the pharmacokinetics of the pro-oxime and oxime carrier and distribution in diaphragm, blood, and brain, (d) The most efficacious formulation will be validated in a status epilepticus guinea pig model as a therapy for CNS chemical agent OP-induced toxicity of DFP, GB, GD, and VX using EEC radiotelemetry probe techniques. This program will address an important and potential threat to civilian populations, for post-exposure CNS treatment in response to exposure to chemical threat agents. Further, the outcome of this work could provide a non-invasive means of administrating appropriate therapeutics for deleterious effects of OP poisoning, directly relevant to emergency and preparedness response to chemical threats for Public Health.
RePORTER. NIH. Next generation oxime therapeutic for chemical agent inhibited CNS (brain) ChEs.
-
Title: Rapid development of in vivo models for countermeasure discovery: organophosphate
A major problem in chemical countermeasure discovery is the potential emergence of novel agents for which there are no known antidotes or post exposure therapies. Classic countermeasures have often been discovered only serendipitously or have taken years to develop. As novel chemical threats emerge (in the form of novel chemical warfare agents or environmental pollutants), the speed with which we are able to understand and counteract each threat will determine the magnitude of its societal impact. One promising approach for rapidly identifying and manipulating the molecular pathways underlying the response to any chemical threat is the use of phenotype-based chemical screens. Models can be developed in which specific toxicants result in reproducible phenotypes in cells or whole organisms. By subjecting these models to high-throughput screening (HTS), small molecules could be identified that reverse the phenotype through a variety of novel mechanisms. Small molecules discovered by these screens would be excellent lead compounds for novel countermeasures and powerful tools for dissecting toxicity pathways, which may in turn point to additional countermeasure targets. In this application, the investigators outline a process for rapid development of in vivo organophosphate toxicity models and their use in discovery of novel organophosphate countermeasures. Although the investigators expect the proposed project will lead directly to development of novel organophosphate countermeasures, they also expect it to serve as a model for rapid countermeasure development that can be applied broadly to other existing and emerging chemical threats. Specifically, the investigators propose: Aim 1. To develop and validate in vivo assays for organophosphate toxicity in the zebrafish. They will seek to identify physiological responses that are surrogates for known human responses to organophosphates and that can be scaled for high throughput in vivo screening. Aim 2. To scale these novel assays for automated high-throughput screening in multiwell plates. The investigators will develop assays for organophosphate toxicity that can be performed automatically in 96-well format. Aim 3. To identify novel compounds that counteract the effects of prior organophosphate exposure. High-throughput screening will identify compounds that facilitate recovery from organophosphate exposure
RePORTER. NIH. Rapid development of in vivo models for countermeasure discovery: organophosphate.
-
Title: Oxime-assisted catalysis of organophosphates and reactivation of AChE
The proposed project is directed to improving prophylaxis and therapy for organophosphate nerve agent organophosphate exposure through the design of mutant human acetylcholinesterases (AChEs), novel oximes and related reactivating nucleophiles. This strategy of oxime-assisted catalysis modifies AChE such that, when placed in the circulation, the oxime and AChE become a catalytic, rather than a stoichiometric, scavenger of organophosphates. Proof of principle has already been established for hydrolysis of a series of organophosphates, and the investigators will refine AChE mutations and oxime structure to enhance catalytic rates for hydrolysis and retention in plasma space. Since oxime therapy, 2-PAM and HI-6, also reactivates inhibited AChE at the target site and the therapeutic benefits of long term oxime therapy have become fully appreciated recently, the investigators propose to design new reactivators based on contemporary knowledge of AChE structure to which they and other groups have contributed to over the past two decades. This approach considers impaction of the active center gorge and angle of access of the oxime as limiting constraints in the design strategy. Moreover, the /research/ groups have applied a novel freeze-frame, click chemistry to the development of reactivating agents using AChE-phosphonate conjugates as the template for synthesis of the triazoles. In addition to oxime assisted catalysis in plasma, new oximes and other nucleophiles will be developed as novel reactivating agents at the target site. This chemistry also enjoys the advantage of combinatorial screening for medium to high throughput in the synthesis-screening paradigm. Nucleophiles and nucleophile AChE combinations will be optimized for enhancing therapeutic efficacy to the particular offending organophosphate. Hence, the investigators approach will both augment catalytic scavenging of organophosphates in the circulation and enhance reactivation at the target tissues.
RePORTER. NIH. Oxime-assisted catalysis of organophosphates and reactivation of AChE.
-
Title: Synthesis of methylating ligands that reactivate aged acetylcholinesterase
Organophosphorus (OP) chemical warfare agents, such as sarin and soman, are acutely toxic compounds that act by inhibiting the activity of the enzyme acetylcholinesterase (AChE) at nerve-nerve and neuromuscular junctions in the central and peripheral nervous systems, respectively. Inhibition of the enzyme occurs by phosphylation of the active site serine nucleophile that is normally involved in catalysis. Prompt administration of oximes can lead to dephosphylation of the phosphyl-AChE adduct and hence is an antidote strategy. However, and particularly with the chemical warfare agent soman, the initial phosphyl-AChE adduct undergoes a dealkylation reaction that leads to what is called the aged adduct, for which there is no known antidote. This application proposes to address this perplexing problem by synthesis and evaluation of AChE ligands that can bind in the active site of the aged enzyme adduct, and subsequently serve as methyl transfer agents to realkylate the aged enzyme. This in turn will resurrect the susceptibility of the adduct to nucleophilic dephosphylation by oximes and hence lead to recovery of enzyme activity. Success in this endeavor should therefore open the door to the development of efficacious drugs for antidote therapy against currently intractable OP chemical warfare agents. PUBLIC HEALTH RELEVANCE: This application endeavors to synthesize and evaluate ligands of the enzyme acetylcholinesterase that can serve as antidotes against organophosphorus (OP) chemical warfare agents. This work is motivated by the concern that terrorist organizations may aspire to inflict mass casualties by use of these agents. It is anticipated that as OP agent antidote therapy improves, the health and security risks that these agents pose to our society will be ameliorated.
RePORTER. NIH. Synthesis of methylating ligands that reactivate aged acetylcholinesterase.
-
Title: Optimization of nonpyridinium oximes for BChE hydrolysis of OPs in plasma
The principal aim of this study is to optimize the efficient and affordable butyrylcholinesterase (BChE) based catalytic scavenger system developed in vitro for treatment of acute organophosphate (OP) intoxication. Currently used oxime and atropine combination therapy is inefficient for treatment of higher exposure OP poisoning due to constant acetylcholinesterase (AChE) reinhibition by excess OP. Novel approaches in therapy based on BChE as stoichiometric scavenger appear prohibitively expensive with a four figure price tag per single application. Conversion of BChE from stoichiometric to catalytic scavenger by combining it with an oxime reactivator has been hampered by lack of efficient BChE reactivators. In the investigators preliminary studies they were able to identify a novel class of superior specific BChE reactivators of distinct nontraditional structural scaffold. The proposed study deals with optimization of this novel class of reactivators using six step optimization iterative cycle that include detailed kinetic characterization of both reactivation and OP hydrolytic properties of oxime/BChE catalytic scavenger systems in buffer medium, extracorporeal human blood and in vivo in mouse animal model. Results of preliminary studies indicate that BChE in combination with optimized oxime reactivators should completely and rapidly, in a several minute timeframe, hydrolyze OPs in plasma space following exposure to an order of magnitude higher than LD50 OP doses at a fraction of cost of currently developed BChE based stoichiometric scavenger system. The proposed optimized catalytic scavenger system technology will thus enable an effective treatment of large OP intoxicated populations and serve as deterrent to the use of OP based nerve agents as terrorist or combat weapons in closed ventilation systems. PUBLIC HEALTH RELEVANCE: This project will develop optimized, efficient and affordable butyrylcholinesterase based catalytic scavenger system for rapid degradation of toxicants in the plasma space of patients exposed to OP agents.
RePORTER. NIH. Optimization of nonpyridinium oximes for BChE hydrolysis of OPs in plasma.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations-
The development of promising scavenger molecules effective against a wide variety of chemical nerve agents should be pursued.
-
The development of oximes that are broader acting than 2-PAM, and have the ability to reverse the permanent binding that can occur with some nerve agents should be pursued.
-
Critical nerve agent antidotes should be evaluated to determine if they can be safely and effectively administered to infants, children and adults via the intravenous and aerosol routes.
-
The accelerated development of promising antidotes for chemical nerve agents should be pursued.
-
Expanded labeling of currently licensed anticonvulsants for use against chemical nerve agents and their utilization in autoinjectors should be pursued.
-
Objective screening tests that can evaluate behavior and cognitive ability, and can differentiate chemically-induced neurological injury from pre-existing neurological illness need to be developed.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendationsNo data available at this time.
15. Study-related ethical concerns
— including review panel recommendationsNo data available at this time.
16. Global regulatory status
U.S.
-
Alternative oximes, such as trimedoxime (TMB4), Toxogonin, and HI-6 (an H-series oxime), are available in other countries for the treatment of nerve agent-induced injuries. Some of these have been or are in the process of being evaluated for use by the U.S. military, but none have been evaluated for possible use in U.S. civilian populations. Several promising new oxime candidates also have been identified and will require further investigation.
Medical Countermeasures Against Chemical Threats: Nervous System, Potential Medical Countermeasures (NIAID)
E.U.
-
Following are the recommendations for HI-6 dichloride monohydrate or dimethane sulphonate:
-
Adults: Initiate as soon as possible; slow IV 500 mg. Repeat after 2 hours. Continuation up to 24 hours 1500-2000 mg. Treatment may be needed for several days, especially for highly toxic OP.
EMEA/CPMP Guidance Document on the Use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
Other
-
Asoxime chloride is a cholinesterase reactivator that has been tried in the treatment of poisoning by organophosphorus pesticides and related compounds, including nerve agents.
Sweetman SC (ed). Martindale, The Complete Drug Reference. London: Pharmaceutical Press (2009) p.1438
17. Other potentially useful information
-
Searching for new potent acetylcholinesterase (AChE; E.C. 3.1.1.7) reactivators (oximes) is a very time-consuming process. At our department, we are able to synthesize more than 50 new AChE reactivators per year. Owing to this fact, we have to select promising reactivators using our in vitro method (potentiometric titration, pH 8 and temperature 25 degrees C; source of cholinesterases, rat brain homogenate; time of inhibition by nerve agents, 30 min; time of reactivation, 10 min) prior to in vivo experiments. For this purpose, we are using two-phase in vitro evaluation of reactivator potency. In the first phase, reactivation potency of all newly synthesized AChE reactivators is tested at two concentrations: 10(-3) M and 10(-5) M. Afterwards, all reactivators achieving reactivation potency over 15% (especially at the concentration 10(-5) M) are tested. The second phase consists of the measurement of the relationship between concentration of the oxime and its reactivation ability. In most cases, the reactivation bell-shaped curve is obtained. The most potent AChE reactivators are selected and provided for further experiments during our development process.
Kuca K, Cabal J, Jun D, Hrabinova M. In vitro evaluation of acetylcholinesterase reactivators as potential antidotes against tabun nerve agent poisonings. Drug Chem Toxicol. 2006;29(4):443-9. [PubMed Citation]
-
The pertinent threat of using organophosphorus compound (OP)-type chemical warfare agents (nerve agents) during military conflicts and by non-state actors requires the continuous search for more effective medical countermeasures. OP inhibit acetylcholinesterase (AChE) and therefore standard treatment of respective poisoning includes AChE reactivators (oximes) in combination with antimuscarinic agents. Hereby, standard oximes, 2-PAM and obidoxime, are considered to be rather insufficient against various nerve agents. Numerous experimental oximes have been investigated in the last decades by in vitro and in vivo models. Recently, we studied the reactivating potency of several oximes with human AChE inhibited by structurally different OP and observed remarkable differences depending on the OP and oxime. In order to investigate structure-activity relationships we determined the various kinetic constants (inhibition, reactivation, aging) for a series of sarin analogues bearing a methyl, ethyl, n-propyl, n-butyl, i-propyl, i-butyl, cyclohexyl or pinacolyl group with human AChE and BChE. The rate constants for the inhibition of human erythrocyte AChE and plasma BChE by these OP (k(i)), for the spontaneous dealkylation (k(a)) and reactivation (k(s)) of OP-inhibited AChE and BChE as well as for the oxime-induced reactivation of OP-inhibited AChE and BChE by the oximes obidoxime, 2-PAM, HI 6, HLö 7 and MMB-4 were determined. With compounds bearing a n-alkyl group the inhibition rate constant increased with chain length. A relation between chain length and spontaneous reactivation velocity was also observed. In contrast, no structure-activity dependence could be observed for the oxime-induced reactivation of AChE and BChE inhibited by the compounds tested. In general, OP-inhibited AChE and BChE were susceptible towards reactivation by oximes. HLö 7 was the most potent reactivator followed by HI 6 and obidoxime while 2-PAM and MMB-4 were rather weak reactivators. These data indicate a potential structure-activity relationship concerning inhibition and spontaneous reactivation but not for oxime-induced reactivation.
Bartling A, Worek F, Szinicz L, Thiermann H. Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology. 2007 Apr 20;233(1-3):166-72. [PubMed Citation]
-
Organophosphorus compound-based (OP) chemical warfare agents (nerve agents) represent a continuing threat to military forces and the civilian population. OPs act primarily by inhibiting acetylcholinesterase (AChE), the standard treatment for which includes AChE reactivators (oximes) in combination with antimuscarinic drugs. In the last decades, the efficacy of oximes has been investigated mostly in small animal models. In order to increase the predictive value of animal studies it is desirable to measure numerous physiological and biochemical parameters. This is difficult in small animals. Large animal models fulfil these requirements and swine are increasingly being used in toxicology studies. Swine breeds generally show considerable variability in different characteristics which may be minimised by the use of specially bred minipigs which have a known genetic background and health status. A comparative study was, therefore, initiated to investigate the kinetic properties of the White Landrace pig and Göttingen minipig AChE in respect of inhibition by the pesticide paraoxon; the nerve agents cyclosarin, VX and VR; the reactivation of inhibited AChE by oximes (obidoxime, pralidoxime and HI 6); and the aging and spontaneous reactivation of inhibited AChE. The determination of the respective kinetic constants found similarities between pig and minipig AChE which showed marked differences in comparison with human AChE values. This has to be considered in designing meaningful models for the investigation of oxime efficacy in pig or minipig experiments. The generated data indicate comparable kinetic properties of pig and minipig AChE and may provide a kinetic basis for extrapolation of data from pig studies to humans.
Worek F, Aurbek N, Wetherell J, Pearce P, Mann T, Thiermann H. Inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds: pig versus minipig acetylcholinesterase. Toxicology. 2008 Feb 3;244(1):35-41. [PubMed Citation]
-
The aim of our study was to evaluate the impact of acetylcholinesterase reactivators--K027 [1-(4-carbamoyl pyridinium)-3-(4-hydroxyiminomethyl pyridinium) propane dibromide], HI-6 [1-(4-carbamoylpyridinium)-3-(2-hydroxyimino methylpyridinium) oxapropane dichloride] and obidoxime [1,3-bis(4-hydroxyiminomethyl pyridinium)oxapropane dichloride] on hepatic functions in vivo. Male Wistar rats were randomly divided to seven groups and intramuscularly administered with saline and acetylcholinesterase reactivators (K027, HI-6 and obidoxime) at doses of 5% LD(50) and 50% LD(50). Liver tissue samples were taken 24 hr after administration. Histochemical detection of lipid droplets and immunohistochemical detection of multidrug resistance protein 2 (Mrp2) were provided. Lipid droplet count in rat liver did not show any significant differences in animals administered with K027, HI-6 and obidoxime in comparison with the control group. Mrp2 protein expression significantly decreased when animals were administered with K027 at a dose of 50% LD(50) and HI-6 and obidoxime at doses of 5% LD(50) and 50% LD(50), when compared to the controls. No statistical differences of Mrp2 expression were measured when animals were administered with K027 at a dose of 5% LD(50) in comparison with control animals. We found impaired hepatic transporter function after administration of HI-6, obidoxime and higher concentration of K027, which might be the underlying mechanism of acetylcholinesterase reactivators' hepatotoxicity.
Pejchal J, Osterreicher J, Kuca K, Jun D, Bajgar J, Kassa J. The influence of acetylcholinesterase reactivators on selected hepatic functions in rats. Basic Clin Pharmacol Toxicol. 2008 Aug;103(2):119-23. [PubMed Citation]
-
Administration of oxime therapy is currently the standard approach used to reverse the acute toxicity of organophosphorus (OP) compounds, which is usually attributed to OP inhibition of acetylcholinesterase (AChE). Rate constants for reactivation of OP-inhibited AChE by even the best oximes, such as HI-6 and obidoxime, can vary >100-fold between OP-AChE conjugates that are easily reactivated and those that are difficult to reactivate. To gain a better understanding of this oxime specificity problem for future design of improved reactivators, we conducted a QSAR analysis for oxime reactivation of AChE inhibited by OP agents and their analogues. Our objective was to identify common mechanism(s) among OP-AChE conjugates of phosphates, phosphonates and phosphoramidates that result in resistance to oxime reactivation. Our evaluation of oxime reactivation of AChE inhibited by a sarin analogue, O-methyl isopropylphosphonofluoridate, or a cyclosarin analogue, O-methyl cyclohexylphosphono fluoridate, indicated that AChE inhibited by these analogues was at least 70-fold more difficult to reactivate than AChE inhibited by sarin or cyclosarin. In addition, AChE inhibited by an analogue of tabun (i.e., O-ethyl isopropylphosphonofluoridate) was nearly as resistant to reactivation as tabun-inhibited AChE. QSAR analysis of oxime reactivation of AChE inhibited by these OP compounds and others suggested that the presence of both a large substituent (i.e., the size of dimethylamine) and an alkoxy substituent in the structure of OP compounds is the common feature that results in resistance to oxime reactivation of OP-AChE conjugates whether the OP is a phosphate, phosphonate or phosphoramidate.
Maxwell DM, Brecht KM, Sweeney RE. A common mechanism for resistance to oxime reactivation of acetylcholinesterase inhibited by organophosphorus compounds. Chem Biol Interact.2013 Mar 25;203(1):72-6. [PubMed Citation]
-
The standard treatment of intoxication with organophosphorus (OP) compounds includes the administration of oximes acting as acetylcholinesterase (AChE) reactivating antidotes. However, the blood-brain barrier (BBB) restricts the rapid transport of these drugs from the blood into the brain in therapeutically relevant concentrations. Since human serum albumin (HSA) nanoparticles enable the delivery of a variety of drugs across the BBB into the brain, HI 6 dimethanesulfonate and HI 6 dichloride monohydrate were bound to these nanoparticles in the present study. The resulting sorption isotherms showed a better fit to Freundlich's empirical adsorption isotherm than to Langmuir's adsorption isotherm. At the pH of 8.3 maximum drug binding capacities of 344.8 μg and 322.6 μg per mg of nanoparticles were calculated for HI 6 dimethanesulfonate and HI 6 dichloride monohydrate, respectively. These calculated values are higher than the adsorption capacity of 93.5 μg/mg for obidoxime onto HSA nanoparticles determined in a previous study. In vitro testing of the nanoparticulate oxime formulations in primary porcine brain capillary endothelial cells (pBCEC) demonstrated an up to two times higher reactivation of OP-inhibited AChE than the free oximes. These findings show that nanoparticles made of HSA may enable a sufficient antidote OP-poisoning therapy with HI 6 derivatives even within the central nervous system (CNS).
Dadparvar M, Wagner S, Wien S, Kufleitner J, Worek F, von Briesen H, Kreuter J. HI 6 human serum albumin nanoparticles--development and transport over an in vitro blood-brain barrier model. Toxicol Lett. 2011 Sep 25;206(1):60-6. [PubMed Citation]
-
logP (octanol-water) = -3.110 (est)
US NLM. ChemIDplus Lite. HI-6
18. Publications
Aas P. In vitro effects of toxogonin, HI-6 and HLö-7 on the release of [3H]acetylcholine from peripheral cholinergic nerves in rat airway smooth muscle. Eur J Pharmacol. 1996 Apr 22;301(1-3):59-66. [PubMed Citation]
Baggot JD, Buckpitt A, Johnson D, Brennan P, Chung H. Bioavailability and disposition kinetics of HI-6 in Beagle dogs. Biopharm Drug Dispos. 1993 Mar;14(2):93-105. [PubMed Citation]
Bartling A, Worek F, Szinicz L, Thiermann H. Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology. 2007 Apr 20;233(1-3):166-72. [PubMed Citation]
Bartosova L, Kuca K, Jun D, Kunesova G. Bispyridinium oximes as antidotal treatment of cyclosarin poisoning-in vitro and in vivo testing. Int J Toxicol. 2005 Nov-Dec;24(6):399-402. [PubMed Citation]
Bajgar J, Fusek J, Kassa J, Kuca K, Jun D. Chemical aspects of pharmacological prophylaxis against nerve agent poisoning. Curr Med Chem. 2009;16(23):2977-86. [PubMed Citation]
Bogan R, Worek F, Koller M, Klaubert B. Photostability of antidotal oxime HI-6, impact on drug development. Drug Test Anal. 2012 Mar-Apr;4(3-4):208-14. [PubMed Citation]
Clement JG, Simons KJ, Briggs CJ. Effect of poisoning by soman (pinacolyl methylphosphonofluoridate) on the serum half-life of the cholinesterase reactivator HI-6 in mice. Biopharm Drug Dispos. 1988 Mar-Apr;9(2):177-86. [PubMed Citation]
Clement JG, Rosario S, Bessette E, Erhardt N. Soman and sarin inhibition of molecular forms of acetylcholinesterase in mice. Time course of recovery and reactivation by the oxime HI-6. Biochem Pharmacol. 1991 Jul 5;42(2):329-35.
Clement JG, Bailey DG, Madill HD, Tran LT, Spence JD. The acetylcholinesterase oxime reactivator HI-6 in man: pharmacokinetics and tolerability in combination with atropine. Biopharm Drug Dispos. 1995 Jul;16(5):415-25. [PubMed Citation]
Dadparvar M, Wagner S, Wien S, Kufleitner J, Worek F, von Briesen H, Kreuter J. HI 6 human serum albumin nanoparticles--development and transport over an in vitro blood-brain barrier model. Toxicol Lett. 2011 Sep 25;206(1):60-6. [PubMed Citation]
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
EMEA/CPMP Guidance Document on the Use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
Eyer P, Hagedorn I, Ladstetter B. Study on the stability of the oxime HI 6 in aqueous solution. Arch Toxicol. 1988;62(2-3):224-6. [PubMed Citation]
Fišar Z, Hroudová J, Korábečný J, Musílek K, Kuča K. In vitro effects of acetylcholinesterase reactivators on monoamine oxidase activity. Curr Med Chem. 2009;16(17):176-180. [PubMed Citation]
Göransson-Nyberg A, Cassel G, Jeneskog T, Karlsson L, Larsson R, Lundstrom M, Perrson SA. Treatment of organophosphate poisoning in pigs: antidote administration by a new binary autoinjector. Arch Toxicol. 1995;70(1):20-7. [PubMed Citation]
Gore A, Bloch-Shilderman E, Egoz I, Turetz J, Brandeis R. Efficacy assessment of a combined anticholinergic and oxime treatment against topical sarin-induced miosis and visual impairment in rats. Br J Pharmacol 2014 May;171(9):2364-74. [PubMed Citation]
Herkert NM, Freude G, Kunz U, Thiermann H, Worek F. Comparative kinetics of organophosphates and oximes with erythrocyte, muscle and brain acetylcholinesterase. Toxicol Lett. 2012 Mar 7;209(2):173-8. [PubMed Citation]
Joosen MJ, van der Schans MJ, van Dijk CG, Kuijpers WC, Wortelboer HM, van Helden HP. Increasing oxime efficacy by blood-brain barrier modulation. Toxicol Lett. 2011 Sep 25;206(1):67-71. [PubMed Citation]
Jovanović D, Randjelović S, Joksović D. A case of unusual suicidal poisoning by the organophosphorus insecticide dimethoate. Hum Exp Toxicol. 1990 Jan;9(1):49-51. [PubMed Citation]
Jovanovic D, Maksimovic M, Joksovic D, Kovacevic V. Oral forms of the oxime HI-6: a study of pharmacokinetics and tolerance after administration to healthy volunteers. Vet Hum Toxicol. 1990 Oct;32(5):419-21. [PubMed Citation]
Jokanović M, Stojiljković MP. Current understanding of the application of pyridinium oximes as cholinesterase reactivators in treatment of organophosphate poisoning. Eur J Pharmacol. 2006 Dec 28;553(1-3):10-7. [PubMed Citation]
Jokanović M, Prostran M. Pyridinium oximes as cholinesterase reactivators. Structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds. Curr Med Chem. 2009;16(17):2177-88. [PubMed Citation]
Jun D, Kuca K, Hronek M, Opletal L. Effect of some acetylcholinesterase reactivators on human platelet aggregation in vitro. J Appl Toxicol. 2006 May-Jun;26(3):258-61 [PubMed Citation]
Jun D, Musilova L, Kuca K, Kassa J. Potency of several oximes to reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon in vitro. Chem Biol Interact. 2008 Sep 25;175(1-3):421-4. [PubMed Citation]
Jun, D, Musilova L, Musilek K, Kuca K. In vitro ability of currently available oximes to reactivate organophosphate pesticide-inhibited human acetylcholinesterase and butyrylcholinesterase. Int J Mol Sci. 2011;12(3):2077-2087. [PubMed Citation]
Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. J Toxicol Clin Toxicol. 2002;40(6):803-16. [PubMed Citation]
Kassa J, Jun D, Kuca K, Bajgar J. Comparison of reactivating and therapeutic efficacy of two salts of the oxime HI-6 against tabun, soman and cyclosarin in rats. Basic Clin Pharmacol Toxicol. 2007 Nov;101(5):328-32. [PubMed Citation]
Kassa J, Karasova JZ, Pavlikova R, Misik J, Caisberger F, Bajgar J. The influence of combinations of oximes on the reactivating and therapeutic efficacy of antidotal treatment of tabun poisoning in rats and mice. J Appl Toxicol. 2010 Mar;30(2):120-4. [PubMed Citation]
Kassa J, Kunesova G. The benefit of combination of oximes for the neuroprotective efficacy of antidotal treatment of sarin-poisoned rats. Toxicol Mech Methods. 2012 May;22(4):260-7. [PubMed Citation]
Kassa J, Musilek K, Karasova JZ, Kuca K, Bajgar J. Two possibilities how to increase the efficacy of antidotal treatment of nerve agent poisonings. Mini Rev Med Chem. 2012 Jan;12(1):24-34. [PubMed Citation]
Kassa J, Karasová JZ, Pavlíková R, Caisberger F, Bajgar J. The ability of oxime mixtures to increase the reactivating and therapeutic efficacy of antidotal treatment of cyclosarin poisoning in rats and mice. Acta Medica (Hradec Kralove). 2012;55(1):27-31. [PubMed Citation]
Kassa J, Musilek K, Koomlova M, Bajgar J. A comparison of the efficacy of newly developed reversible inhibitors of acetylcholinesterase with commonly used pyridostigmine as pharmacological pre-treatment of soman-poisoned mice. Basic Clin Pharmacol Toxicol. 2012 Apr;110(4):322-6. [PubMed Citation]
Koplivitz I, Stewart JR. A comparison of the efficacy of HI6 and 2-PAM against soman, tabun, sarin, and VX in the rabbit. Toxicol Lett. 1994 Feb 15;70(3):269-79. [PubMed Citation]
Koplovitz I, Menton R, Matthews C, Shutz M, Nalls C, Kelly S. Dose-response effects of atropine and HI-6 treatment of organophosphorus poisoning in guinea pigs. Drug Chem Toxicol. 1995 May-Aug;18(2-3):119-36. [PubMed Citation]
Kuca, K, Kassa J. Oximes-induced reactivation of rat brain acetylcholinesterase inhibited by VX agent. Hum Exp Toxicol. 2004 Apr;23(4):167-71. [PubMed Citation]
Kuca K, Cabal J, Jun D, Kassa J, Bartosová L, Kunesová G. In vitro reactivation potency of some acetylcholinesterase reactivators against sarin- and cyclosarin-induced inhibitions. J Appl Toxicol. 2005 Jul-Aug;25(4):296-300. [PubMed Citation]
Kuca K, Cabal J, Kassa J, Jun D, Hrabinova M. In vitro potency of H oximes (HI-6, HLo-7), the oxime BI-6, and currently used oximes (pralidoxime, obidoxime, trimedoxime) to reactivate nerve agent-inhibited rat brain acetylcholinesterase. J Toxicol Environ Health A. 2006 Aug;69(15):1431-1440. [PubMed Citation]
Kuca K, Jun D, Cabal J, Hrabinova M, Bartosova L, Opletalova V. Russian VX: inhibition and reactivation of acetylcholinesterase compared with VX agent. Basic Clin Pharmacol Toxicol. 2006 Apr;98(4):389-94. [PubMed Citation]
Kuca K, Jun D, Bajgar J. Currently used cholinesterase reactivators against nerve agent intoxication: comparison of their effectivity in vitro. Drug Chem Toxicol. 2007;30(1):31-40. [PubMed Citation]
Kuca K, Cabal J, Jun D, Hrabinova M. In vitro evaluation of acetylcholinesterase reactivators as potential antidotes against tabun nerve agent poisonings. Drug Chem Toxicol. 2006;29(4):443-9. [PubMed Citation]
Kusić R, Bosković B, Vojvodić V, Jovanović D. HI-6 in man: blood levels, urinary excretion, and tolerance after intramuscular administration of the oxime to healthy volunteers. Fundam Appl Toxicol. 1985 Dec;5(6 Pt 2):S89-97 [PubMed Citation]
Kusić R, Jovanović D, Randjelović S, Joksović D, Todorovic V, Bosković B, Jokanović M, Vojvodić V. HI-6 in man: efficacy of the oxime in poisoning by organophosphorus insecticides. Hum Exp Toxicol. 1991 Mar;10(2):113-8. [PubMed Citation]
Lukey BJ, Woodard CL, Clark CR, McCluskey MP. HI-6 pharmacokinetics in rabbits after intravenous and intramuscular administration. J Pharm Pharmacol. 1992 Aug;44(8):690-2. [PubMed Citation]
Lundy PM, Hand BT, Broxup BR, Yipchuck G, Hamilton MG. Distribution of the bispyridinium oxime [14C] HI-6 in male and female rats. Arch Toxicol. 1990;64(5):377-82. [PubMed Citation]
Lundy PM, Hansen AS, Hand BT, Boulet CA. Comparison of several oximes against poisoning by soman, tabun and GF. Toxicology. 1992;72(1):99-105. [PubMed Citation]
Lundy PM, Hill I, Lecavalier P, Hamilton MG, Vair C, Davidson C, Weatherby KL, Berger BJ. The pharmacokinetics and pharmacodynamics of two HI-6 salts in swine and efficacy in the treatment of GF and soman poisoning. Toxicology. 2005 Mar 30;208(3):399-409. [PubMed Citation]
Lundy PM, Raveh L, Amitai G. Development of the bisquaternary oxime HI-6 toward clinical use in the treatment of organophosphate nerve agent poisoning. Toxicol Rev. 2006;25(4):231-43. [PubMed Citation]
Lundy PM, Hamilton MG, Sawyer TW, Mikler J. Comparative protective effects of HI-6 and MMB-4 against organophosphorous nerve agent poisoning. Toxicology 2011 Jul 29;285(3):90-6. [PubMed Citation]
Luo C, Tong M, Chilukuri N, Brecht K, Maxwell DM, Saxena A. An in vitro comparative study on the reactivation of nerve agent-inhibited guinea pig and human acetylcholinesterases by oximes. Biochemistry. 2007 Oct 23;46(42):11771-9 [PubMed Citation]
Luo C, Tong M, Maxwell DM, Saxena A. Comparison of oxime reactivation and aging of nerve agent-inhibited monkey and human acetylcholinesterases. Chem Biol Interact. 2008 Sep 25;175(1-3):261-6. [PubMed Citation]
Luo C, Chambers C, Pattabiraman N, Tong M, Tipparaju P, Saxena A. Y124 at the peripheral anionic site is important for the reactivation of nerve agent-inhibited acetylcholinesterase by H oximes. Biochem Pharmacol. 2010 Nov 1;80(9):1427-36. [PubMed Citation]
Luo C, Chambers C, Yang Y, Saxena A. Mechanism for potent reactivation ability of H oximes analyzed by reactivation kinetic studies with cholinesterases from different species. Chem Biol Interact. 2010 Sep 6;187(1-3):185-90. [PubMed Citation]
Maksimović M, Jovanović D, Kovacević V, Bokonjić D. Oral kinetics and bioavailability of the cholinesterase reactivator HI-6 after administration of 2 different formulations of tablets to dogs. Toxicol Lett. 1987 Nov;39(1):85-91. [PubMed Citation]
Marrs TC, Rice P, Vale JA. The role of oximes in the treatment of nerve agent poisoning in civilian casualties. Toxicol Rev. 2006;25(4): 297-323. [PubMed Citation]
Marrs TC, Maynard RL, Sidell FR, eds. Chemical Warfare Agents Toxicology and Treatment, 2nd Edition. West Sussex, England: John Wiley & Sons Ltd, 2007 p.305-329
Maxwell DM, Brecht KM, Sweeney RE. A common mechanism for resistance to oxime reactivation of acetylcholinesterase inhibited by organophosphorus compounds. Chem Biol Interact.2013 Mar 25;203(1):72-6. [PubMed Citation]
Medical Countermeasures Against Chemical Threats: Nervous System, Potential Medical Countermeasures (NIAID)
Mercey G, Verdelet T, Renou J, Kliachyna M, Baati R, Nachon F, Jean L, Renard PY. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc Chem Res. 2012 May 15;45(5):756-66. [PubMed Citation]
Mikler J, Tenn C, Worek F, Reiter G, Thiermann H, Garrett M, Bohnert S, Sawyer TW. Immobilization of Russian VX skin depots by localized cooling: implications for decontamination and medical countermeasures. Toxicol Lett. 2011 Sep 25;206(1):47-53. [PubMed Citation]
Milic B, Maksimovic M, Nedelijkovic M. Trimedoxime and HI-6: kinetic comparison after intravenous administration to mice. Pharmacol Toxicol. 1996 Apr;78(4):269-72. [PubMed Citation]
Moore DH, Hayward IJ, Tucker FS, Lukey B. HI-6 and 2-PAM in sheep: pharmacokinetics and effects on muscle tissue following intramuscular injection. Biopharm Drug Dispos. 1991 Apr;12(3):223-32. [PubMed Citation]
Myhrer T, Enger S, Ass P. Efficacy of immediate and subsequent therapies against soman-induced seizures and lethality in rats. Basic Clin Pharmacol Toxicol. 2006 Feb;98(2):184-91. [PubMed Citation]
Myhrer T, Enger S, Aas P. Determination of anti-convulsant and life-preserving capacities of three types of auto-injector therapies against soman intoxication in rats. Drug Test Anal.Drug Test Anal. 2013 Aug;5(8):693-701. [PubMed Citation]
Myhrer T, Enger S, Mariussen E, Aas P. Two medical therapies very effective shortly after high levels of soman poisoning in rats, but only one with universal utility. Toxicology 2013 Dec 15;314(2-3):221-8. [PubMed Citation]
Myhrer T, Aas P. Choice of approaches in developing novel medical countermeasures for nerve agent poisoning. Neurotoxicology 2014 May:44C:27-38. [PubMed Citation]
Nyberg AG, Cassel G, Jeneskog T, Karlsson L, Larsson R, Lundström M, Palmer L, Persson SA. Pharmacokinetics of HI-6 and atropine in anaesthetized pigs after administration by a new autoinjector. Biopharm Drug Dispos. 1995 Nov;16(8):635-51. [PubMed Ciation]
Pejchal J, Osterreicher J, Kuca K, Jun D, Bajgar J, Kassa J. The influence of acetylcholinesterase reactivators on selected hepatic functions in rats. Basic Clin Pharmacol Toxicol. 2008 Aug;103(2):119-23. [PubMed Citation]
Pohanka M, Sobotka J, Svobodova H, Stetina R. Sulfur mustard induced oxidative stress and its alteration using asoxime (HI-6). Interdiscip Toxicol. 2013 Dec;6(4):198-202.
Pohanka M, Sobotka J, Svobodova H, Stetina R. Investigation of oxidative stress in blood, brain, kidney, and liver after oxime antidote HI-6 application in a mouse experimental model. Drug Chem Toxicol. 2011 Jul;34(3):255-60 [PubMed Citation]
Pohanka M, Novotny L, Zdarova-Karasova J, Bandouchova H, Zemek F, Hrabinova M, Misik J, Kuca K, Bajgar J, Zitka O, Cernei N, Kizek R, Pikula J. Asoxime (HI-6) impact on dogs after one and tenfold therapeutic doses: assessment of adverse effects, distribution, and oxidative stress. Environ Toxicol Pharmacol. 2011 Jul;32(1):75-81. [PubMed Citation]
Puu G, Artursson E, Bucht G. Reactivation Of Nerve Agent Inhibited Human Acetylcholinesterases by Hi-6 and Obidoxime. Biochem Pharmacol. 1986 May 1;35(9):1505-10. [PubMed Citation].
RamaRao G, Afley P, Acharya J, Bhattacharya BK. Efficacy of antidotes (midazolam, atropine and HI-6) on nerve agent induced molecular and neuropathological changes. BMC Neurosci. 2014 Apr 4;15:47. [PubMed Citation].
Reddy VK, Deshpande SS, Cintra WM, Scoble GT, Albuquerque EX. Effectiveness of oximes 2-PAM and HI-6 in recovery of muscle function depressed by organophosphate agents in the rat hemidiaphragm: an in vitro study. Fundam Appl Toxicol. 1991 Nov;17(4):746-60. [PubMed Citation]
RePORTER. NIH. Next generation oxime therapeutic for chemical agent inhibited CNS (brain) ChEs.
RePORTER. NIH. Optimization of nonpyridinium oximes for BChE hydrolysis of OPs in plasma.
RePORTER. NIH. Oxime-assisted catalysis of organophosphates and reactivation of AChE.
RePORTER. NIH. Rapid development of in vivo models for countermeasure discovery: organophosphate.
RePORTER. NIH. Synthesis of methylating ligands that reactivate aged acetylcholinesterase.
Schlager JW, Dolzine TW, Stewart JR, Wannarka GL, Shih ML. Operational evaluation of three commercial configurations of atropine/HI-6 wet/dry autoinjectors. Pharm Res. 1991 Sep;8(9):1191-4. [PubMed Citation]
Shih TM. Comparison of several oximes on reactivation of soman-inhibited blood, brain and tissue cholinesterase activity in rats. Arch Toxicol. 1993;67(9):637-46. [PubMed Citation]
Shrot S, Markel G, Dushnitsky T, Krivoy A. Neurotoxicology. The possible use of oximes as antidotal therapy in organophosphate-induced brain damage. 2009 Mar;30(2):167-73. [PubMed Citation]
Soukup O, Krůšek J, Kaniaková M, Kumar UK, Oz M, Jun D, Fusek J, Kuča K, Tobin G. Oxime reactivators and their in vivo and in vitro effects on nicotinic receptors. Physiol Res. 2011;60(4):679-86. [PubMed Citation]
Soukup O, Kumar UK, Proska J, Bratova L, Adem A, Jun D, Fusek J, Kuca K, Tobin G. Environ Toxicol Pharmacol. The effect of oxime reactivators on muscarinic receptors: functional and binding examinations. 2011 May;31(3):364-70. [PubMed Citation]
Soukup O, Kristofikova Z, Jun D, Tambor V, Ripova D, Kuca K. The interaction of standard oxime reactivators with hemicholinium-3 sensitive choline carriers. Toxicol Lett. 2012 Aug 3;212(3):315-9. [PubMed Citation]
Stojiljković MP, Jokanović M. Pyridinium oximes: rationale for their selection as causal antidotes against organophosphate poisonings and current solutions for auto-injectors. Arh Hig Rada Toksikol. 2006 Dec;57(4):435-43. [PubMed Citation]
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
Svobodova H, Jost P, Stetina R. Cytotoxicity and genotoxicity evaluation of antidote oxime HI-6 tested on eight cell lines of human and rodent origin. Gen Physiol Biophys. 2012 Mar;31(1):77-84. [PubMed Citation]
Sweetman SC (ed). Martindale, The Complete Drug Reference. London: Pharmaceutical Press (2009) p.1456
Thiermann H, Spohrer U, Klimmek R, Eyer P. Operational evaluation of wet / dry autoinjectors containing atropine in solution and powdered HI 6 or HLO 7. International Journal of Pharmaceutics. 1994: 109: 35-43.
Thiermann H, Szinicz L, Eyer P, Felgenhauer N, Zilker T, Worek F. Lessons to be learnt from organophosphorus pesticide poisoning for the treatment of nerve agent poisoning. Toxicology. 2007 Apr 20;233(1-3):145-54. [PubMed Citation]
US NLM. ChemIDplus Lite. HI-6
Wetherell J, Price M, Mumford H. A novel approach for medical countermeasures to nerve agent poisoning in the guinea-pig. Neurotoxicology. 2006 Jul;27(4):485-91. [PubMed Citation]
Worek F, Widmann R, Knopff O, Szinicz L. Reactivating potency of obidoxime, pralidoxime, HI 6 and HLö 7 in human erythrocyte acetylcholinesterase inhibited by highly toxic organophosphorus compounds. Arch Toxicol. 1998 Mar;72(4):237-43. [PubMed Citation]
Worek F, Aurbek N, Koller M, Becker C, Eyer P, Thiermann H. Kinetic analysis of reactivation and aging of human acetylcholinesterase inhibited by different phosphoramidates. Biochem Pharmacol. 2007 Jun 1;73(11):1807-1817. [PubMed Citation]
Worek F, Aurbek N, Wetherell J, Pearce P, Mann T, Thiermann H. Inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds: pig versus minipig acetylcholinesterase. Toxicology. 2008 Feb 3;244(1):35-41. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 9/18/2014
