You are here: Home > Medical Countermeasures Database > Hydroxocobalamin
Hydroxocobalamin - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Hydroxocobalamin
2. Chemical Defense therapeutic area(s)
— including key possible usesHydroxocobalamin is indicated as an antidote in patients with known or suspected cyanide poisoning.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
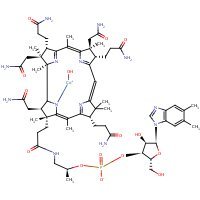
HSDB. Hydroxocobalamin
Mechanism of action
-
Cyanide is an extremely toxic poison. In the absence of rapid and adequate treatment, exposure to a high dose of cyanide can result in death within minutes due to the inhibition of cytochrome oxidase resulting in arrest of cellular respiration. Specifically, cyanide binds rapidly with cytochrome a3, a component of the cytochrome c oxidase complex in mitochondria. Inhibition of cytochrome a3 prevents the cell from using oxygen and forces anaerobic metabolism, resulting in lactate production, cellular hypoxia and metabolic acidosis. In massive acute cyanide poisoning, the mechanism of toxicity may involve other enzyme systems as well. Signs and symptoms of acute systemic cyanide poisoning may develop rapidly within minutes, depending on the route and extent of cyanide exposure.
The action of Cyanokit in the treatment of cyanide poisoning is based on its ability to bind cyanide ions. Each hydroxocobalamin molecule can bind one cyanide ion by substituting it for the hydroxo ligand linked to the trivalent cobalt ion, to form cyanocobalamin, which is then excreted in the urine.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
-
The cobalt ion in hydroxocobalamin combines with cyanide to form the nontoxic cyanocobalamin. One mole of hydroxocobalamin binds 1 mole of CN. Given the molecular weights of each, 52 g of hyroxocobalamin are needed to bind 1 g of cyanide. An ex vivo study using human skin fibroblasts demonstrates that hydroxocobaslamin penetrates intracellularly to form cyanocabalamin. In the setting of cyanide poisoning, hydroxocobalamin removes cyanide from the mitochondrial electron transport chain, allowing oxidative metabolism to proceed. Hydroxocobalamin also binds nitric oxide, particularly in the absence of cyanide. The same property potentially contributes to its beneficial effects by increasing systolic and diastolic blood pressure and improving the hemodynamic status of cyanide-poisoned patients.
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1695-1697
-
The rational for administering hydroxocobalamin (OHCbl) as an antidote to cyanide poisoning is based on the high affinity of CN ion for cobalt compounds. However, only few data are available on the influence of OHCbl on the intracellular cyanide pool. 2. In human fibroblasts incubated for 10 min with 500 microM of [14C] cyanide, the accumulation ratio was 25 at 37 degrees C (10.45 +/- 1.51 mM) and 11.9 at 4 degrees C. 3. Using the monoblastic U-937 cell line, a rapid uptake of radioactive cyanide was observed with a maximum accumulation ratio of 1.97 at 5 min. 4. A linear relationship between cyanide uptake by U-937 cells and cyanide concentration in incubation medium (10-500 microM; 5 min) was found suggesting a first order process (k = 0.25 min-1). 5. After incubation of fibroblasts with 500 microM of OHCbl, a 75% decrease of intracellular cyanide was observed, with concomittant formation of intracellular cyanocobalamin CNCbl (intracellular/extracellular ratio: 158). 6. These findings suggest that OHCbl is able to penetrate into heavily cyanide loaded cells and to complex cyanide to the non-toxic CNCbl form.
Astier A, Baud FJ. Complexation of intracellular cyanide by hydroxocobalamin using a human cellular model. Hum Exp Toxicol. 1996 Jan;15(1):19-25. [PubMed Citation]
Summary of clinical and non-clinical studies
Hydroxocobalamin, a precursor of vitamin B12, was approved by the Food and Drug Administration (FDA) as an antidote to cyanide poisoning in the United States (Shepherd et al., 2008) and has a history as a treatment of smoke inhalation in France (Fortin, 2010). Cyanide exists as the gas vapor of hydrogen cyanide (HCN) and cyanogen chloride (CNCl), which are liquid at room temperature; and as a solid in the form of cyanide salts containing sodium, potassium or calcium. It inhibits mitochondrial cytochrome oxidase primarily in the brain and heart, which is necessary for oxidative respiration (Riou et al., 1990; Guidotti, 2006). Symptoms of inhalation cyanide poisoning are lactic acidosis, restlessness, increased respiratory rate, increased blood pressure, heart palpitations, vomiting, convulsions, cardiac arrest, loss of consciousness and even death. Although cyanide poisoning is a major source of morbidity and mortality from smoke exposure in structural fires (Fortin et al., 2011), it has also been utilized in various forms to inflict intentional harm, and is considered a chemical weapon of concern for use by terrorists (Megarbane et al., 2003; Sauer et al., 2001). Preclinically, hydroxocobalamin has been tested in dogs given a lethal dose of cyanide and subsequently assigned to 150 mg/kg, 75 mg/kg, or vehicle (0.9% saline) drug treatments. Hydroxocobalamin reduced whole blood cyanide concentrations by 50%, and two weeks after exposure and treatment, 100% of dogs in the 150 mg/kg treated group survived compared to 79% in the 75 mg/kg group and only 18% in the vehicle treated group (Borron et al., 2006). Clinical review of hospital reports, excluding smoke inhalation patients, indicated that ten of 14 patients (71%) survived when treated with hydroxocobalamin (5-20 g) beginning a median 2.1 hours after cyanide ingestion or inhalation (Borron et al., 2007). Timely hydroxocobalamin administration has also contributed to full recovery from cardiorespiratory arrest secondary to cyanide poisoning from smoke inhalation (Fortin et al., 2006; Fortin et al., 2011). The Paris Fire Brigade reported that between 1995 and 2003, there was a 41.7% survival rate for 72 patients exposed to cyanide-poisoning through smoke inhalation, and within this group 9 of the the 12 patients with increased systolic blood pressure (bp) treated with hydroxocobalamin demonstrated a recovery of systolic bp to normal (Fortin, 2006). Little is known about the effect of the drug in pregnant women, but a case report of a 32 year old woman at 36 weeks gestation presenting with burns and concomitant smoke inhalation injury treated with hydroxocobalamin demonstrated that both the woman and her child fared well with no significant complications due to the smoke inhalation at 6 months of follow-up (Roderique et al., 2012). The most common drug related adverse events reported in adult subjects treated with 2.5-10 g hydroxocobalamin intravenously were reddening of the urine or skin, cutaneous or pustular/papular rash, and an increase in blood pressure (Fortin et al., 2006; Uhl et al., 2006; Forsyth et al., 1993) and methemoglobinemia in pediatrics (Geller et al., 2006).
B. Link to clinical studies
Studies involving multiple populations
Adult
-
Cyanide poisoning is an important source of morbidity and mortality from smoke exposure in structural fires. This case involved administration of a cyanide antidote to a prisoner (male, 23 years) in France, discovered in cardiorespiratory arrest after about 30 minutes exposure to smoke from a burning mattress during an apparent suicide attempt. Smoke exposure, circulatory failure during initial resuscitation, and elevated blood cyanide and lactate led to the diagnosis of cyanide poisoning. Hydroxocobalamin (Cyanokit, 5 g intravenous) was given immediately and on arrival at the hospital. Cardiopulmonary resuscitation restored cardiovascular function after 33 minutes. There were no neurological or other sequelae. Timely hydroxocobalamin administration contributed to full recovery from cardiorespiratory arrest secondary to cyanide poisoning from smoke inhalation. Hydroxocobalamin should be available to emergency medical teams attending fire scenes (Class IV).
Fortin JL, Baud FJ, Astier A, Barriot P, Lecarpentier Y. Hydrogen cyanide poisoning in a prison environment: a case report. J Correct Health Care. 2011 Jan;17(1):29-33. [PubMed Citation]
-
Hydroxocobalamin, a precursor of vitamin B12, has a history of use in the prehospital setting in France for cyanide poisoning, particularly that associated with smoke inhalation. Because cyanide poisoning by ingestion is less common than smoke inhalation-associated cyanide poisoning, less information is available on prehospital use of hydroxocobalamin to treat cyanide poisoning by ingestion. This report describes a case of prehospital use of hydroxocobalamin for poisoning by ingestion of cyanide. The case supports the efficacy of hydroxocobalamin for acute cyanide poisoning caused by ingestion of a cyanide salt. No adverse events attributed to hydroxocobalamin were observed (Class IV).
Fortin JL, Waroux S, Giocanti JP, Capellier G, Ruttimann M, Kowalski JJ. Hydroxocobalamin for poisoning caused by ingestion of potassium cyanide: a case study. J Emerg Med 2010 Sep;39(3):320-4. [PubMed Citation]
-
This chart review was undertaken to assess efficacy and safety of hydroxocobalamin for acute cyanide poisoning. Hospital records of the Fernand Widal and Lariboisiere Hospitals were reviewed for intensive care unit admissions with cyanide poisoning for which hydroxocobalamin was used as first-line treatment from 1988 to 2003. Smoke inhalation cases were excluded. Hydroxocobalamin (5-20 g) was administered to 14 consecutive patients beginning a median 2.1 hours after cyanide ingestion or inhalation. Ten patients (71%) survived and were discharged. Of the 11 patients with blood cyanide exceeding the typically lethal threshold of 100 micromol/L, 7 survived. The most common hydroxocobalamin-attributed adverse events were chromaturia and pink skin discoloration. Severe cyanide poisoning of the nature observed in most patients in this study is frequently fatal. That 71% of patients survived after treatment with hydroxocobalamin suggests that hydroxocobalamin as first-line antidotal therapy is effective and safe in acute cyanide poisoning (Class IV).
Borron SW, Baud FJ, Megarbane B, Bismuth C. Hydroxocobalamin for Severe Acute Cyanide Poisoning by Ingestion or Inhalation; Am J Emerg Med. 2007 Jun;25(5):551-8. [PubMed Citation]
-
INTRODUCTION: This article reports the results of a retrospective study of 8 years of experience of the Paris Fire Brigade with the prehospital use of hydroxocobalamin. METHODS: The head physician at the Paris Fire Brigade extracted and summarized data from standardized forms completed at the fire scene and, when available, hospital reports to assess survival status and clinical parameters associated with the use of hydroxocobalamin for each patient who received it for smoke inhalation-associated cyanide poisoning from 1995 to 2003. RESULTS: Of the 101 patients administered hydroxocobalamin, 30 survived, 42 died (17 at the fire scene and 25 at the intensive-care unit), and survival status was not known in the remaining 29 patients. Among the 72 patients for whom survival status was known, survival rate was 41.7% after the administration of hydroxocobalamin. Of the 38 patients found in cardiac arrest, 21 had a return of spontaneous circulation during prehospital care. Of the 12 patients who were initially hemodynamically unstable (systolic blood pressure 0 to < or =90 mmHg), 9 recovered systolic blood pressure an average of 30.6 minutes after the start of hydroxocobalamin infusion. Among nonsedated patients in the sample as a whole (n = 52), mean (SD) Glasgow coma scale score improved from 7.9 (5.4) initially to 8.5 (5.7) after administration of hydroxocobalamin. Among nonsedated patients who were initially neurologically impaired (n = 18), Glasgow coma scale score improved in 9 patients, did not change in 8 patients, and worsened in 1 patient. Two adverse events--red or pink coloration of urine or skin (n = 5) and cutaneous rash (n = 1)--were assessed as being possibly related to hydroxocobalamin. CONCLUSION: Hydroxocobalamin has a risk:benefit ratio rendering it suitable for prehospital use in the management of acute cyanide poisoning caused by smoke inhalation (Class IV).
Fortin JL, Giocanti JP, Ruttimann M, Kowalski JJ. Prehospital administration of hydroxocobalamin for smoke inhalation - associated cyanide poisoning: 8 years of experience in the Paris Fire Brigade; Clin Toxicol 2006; 44 (Suppl 1): 37-44. [PubMed Citation]
-
INTRODUCTION: This randomized, double-blind, placebo-controlled, ascending-dose study was conducted in healthy volunteers to evaluate the safety of the investigational cyanide antidote hydroxocobalamin. METHODS: Four ascending dosing groups received intravenous doses of 2.5, 5, 7.5 or 10 g hydroxocobalamin over 7.5 to 30 minutes at a constant infusion rate. Volunteers (n = 136) randomized 3:1 to receive hydroxocobalamin or placebo underwent a 4-day in-house observation after infusion on Day 1 and follow-up visits on Days 8, 15, and 28. RESULTS: The most common drug-related adverse events were asymptomatic and self-limiting chromaturia and reddening of the skin, which are attributed to the red color of hydroxocobalamin. Other adverse events included pustular/papular rash, headache, erythema at the injection site, decrease in lymphocyte percentage, nausea, pruritus, chest discomfort, and dysphagia. Hydroxocobalamin was associated with an increase in blood pressure in some volunteers. Blood pressure changes peaked toward the end of hydroxocobalamin infusion and typically returned to baseline levels by 4 hours postinfusion. Maximum mean changes from baseline in systolic blood pressure ranged from 22.6 to 27.0 mmHg across hydroxocobalamin doses compared with 0.2 to 6.7 mmHg in the corresponding placebo groups. Maximum mean change from baseline in diastolic blood pressure ranged from 14.3 to 25.4 mmHg across hydroxocobalamin doses compared with -3.0 to 3.8 mmHg in the corresponding placebo groups. Two allergic reactions that occurred within minutes after start of the 5- and 10-g hydroxocobalamin infusions were successfully managed with dexamethasone and/or dimethindene maleate. CONCLUSION: Timely intervention for acute cyanide poisoning could entail administration of an antidote in the prehospital setting based on a presumptive diagnosis. Results of this placebo-controlled study in healthy volunteers corroborate previous studies and French postmarketing experience in cyanide-exposed patients in suggesting that the safety profile of hydroxocobalamin is consistent with prehospital or hospital use (Class II).
Uhl W, Nolting A, Golor G, Rost KL, Kovar A. Safety of hydroxocobalamin in healthy volunteers in a randomized, placebo-controlled study; ClinToxicol 2006 (Phil) 44 Suppl 1: 17-28. [PubMed Citation]
-
The safety, efficacy and pharmacokinetic parameters of 5 g of hydroxocobalamin given intravenously, alone or in combination with 12.5 g of sodium thiosulfate, were evaluated in healthy adult men who were heavy smokers. Sodium thiosulfate caused nausea, vomiting, and localized burning, muscle cramping, or twitching at the infusion site. Hydroxocobalamin was associated with a transient reddish discoloration of the skin, mucous membranes, and urine, and when administered alone produced mean elevations of 13.6% in systolic and 25.9% in diastolic blood pressure, with a concomitant 16.3% decrease in heart rate. No other clinically significant adverse effects were noted. Hydroxocobalamin alone decreased whole blood cyanide levels by 59% and increased urinary cyanide excretion. Pharmacokinetic parameters of hydroxocobalamin were best defined in the group who received both antidotes: t1/2 (alpha), 0.52 h; t1/2 (beta), 2.83 h; Vd (beta), 0.24 L/kg; and mean peak serum concentration 753 mcg/mL (560 mumol/L) at 0-50 minutes after completion of infusion. Hydroxocobalamin is safe when administered in a 5 gram intravenous dose, and effectively decreases the low whole blood cyanide levels found in heavy smokers (Class III).
Forsyth JC, Mueller PD, Becker CE, Osterloh J, Benowitz NL, Rumack BH, Hall AH. Hydroxocobalamin as a cyanide antidote: safety, efficacy and pharmacokinetics in heavily smoking normal volunteers J Toxicol Clin 1993; Toxicol 31: 277-94. [PubMed Citation]
-
Limited work has focused on occupational exposures that may increase the risk of cyanide poisoning by ingestion. A retrospective chart review of all admissions for acute cyanide poisoning by ingestion for the years 1988 to 2008 was conducted in a tertiary university hospital serving the largest population in the country working in jewelry and textile facilities. Of the 9 patients admitted to the hospital during the study period, 8 (7 males, 1 female; age 36 ± 11 years, mean ± SD) attempted suicide by ingestion of potassium cyanide used in their profession as goldsmiths or textile industry workers. Five patients had severe neurologic impairment and severe metabolic acidosis (pH 7.02 ± 0.08, mean ± SD) with high anion gap (23 ± 4 mmol/L, mean ± SD). Of the 5 severely intoxicated patients, 3 received antidote therapy (sodium thiosulfate or hydroxocobalamin) and resumed full consciousness in less than 8 hours. All patients survived without major sequelae. Cyanide intoxication by ingestion in our patients was mainly suicidal and occurred in specific jobs where potassium cyanide is used. Metabolic acidosis with high anion is a good surrogated marker of severe cyanide poisoning. Sodium thiosulfate and hydroxocobalamin are both safe and effective antidotes (Class IV).
Coentrão L, Moura D. Acute cyanide poisoning among jewelry and textile industry workers. Am J Emerg Med. 2011 Jan;29(1):78-81. [PubMed Citation]
-
Hydroxocobalamin is a new antidote approved by the FDA for the treatment of cyanide poisoning. Our report describes a patient with cyanide poisoning who survived after treatment with hydroxocobalamin and complications we encountered with hemodialysis. A 34-year-old female presented to the emergency department after a syncopal event and seizures. Her systolic blood pressure was 75 mmHg, her QRS complex progressively widened, and pulses were lost. She was intubated and resuscitated with fluids, sodium bicarbonate for her QRS widening and vasopressors. Venous blood gas demonstrated a pH of 6.36 with an O₂ saturation of 99%. Due to the acidemia with a normal pulse oximetry, simultaneous venous and arterial blood gases were obtained. Venous gas demonstrated a pH of 6.80 with a PO₂ of 222 mmHg, an O₂ saturation of 99%. The arterial blood gas showed a pH of 6.82, a PO₂ 518 mmHg, an O₂ saturation of 100%. Cyanide was suspected and hydroxocobalamin and sodium thiosulfate were given. Within 40 min of hydroxocobalamin administration, vasopressors were discontinued. Initially, nephrology attempted dialysis for metabolic acidosis; however, the dialysis machine repeatedly shut down due to a "blood leak". This was an unforeseen effect attributed to hydroxocobalamin. Cyanide level, drawn 20 min after the antidote was completed, was elevated at 22 mcg/dL. Her urinary thiocyanate level could not be analyzed due to an "interfering substance". Hydroxocobalamin is an effective antidote. However, clinicians must be aware of its effects on hemodialysis machines which could delay the initiation of this important treatment modality in the severely acidemic patient (Class IV).
Sutter M, Tereshchenko N, Rafii R, Daubert GP. Hemodialysis complications of hydroxocobalamin: a case report; J Med Toxicol 2010 Jun; 6 (2):165-7 [PubMed Citation]
-
Clinical experience with hydroxocobalamin in acute cyanide poisoning via ingestion remains limited. This case concerns a 35-year-old mentally ill woman who consumed more than 20 apricot kernels. Published literature suggests each kernel would have contained cyanide concentrations ranging from 0.122 to 4.09 mg/g (average 2.92 mg/g). On arrival, the woman appeared asymptomatic with a raised pulse rate and slight metabolic acidosis. Forty minutes after admission (approximately 70 min postingestion), the patient experienced headache, nausea and dyspnoea, and was hypotensive, hypoxic and tachypnoeic. Following treatment with amyl nitrite and sodium thiosulphate, her methaemoglobin level was 10%. This prompted the administration of oxygen, which evoked a slight improvement in her vital signs. Hydroxocobalamin was then administered. After 24 h, she was completely asymptomatic with normalised blood pressure and other haemodynamic parameters. This case reinforces the safety and effectiveness of hydroxocobalamin in acute cyanide poisoning by ingestion (Class IV).
Cigolini D, Ricci G, Zannoni M, Codogni R, De Luca M, Perfetti P, Rocca G. Hydroxocobalamin treatment of acute cyanide poisoning from apricot kernels. Emerg Med J. 2011 Sep;28(9):804-5. [PubMed Citation]
-
OBJECTIVE: To assess the relationship between blood pressure changes following infusion of antidotal doses of hydroxocobalamin and plasma concentrations of total and free cobalamins-(III). METHODS: Independent groups of healthy volunteers received single intravenous doses of 2.5, 5, 7.5, or 10 g hydroxocobalamin over 7.5 to 30 minutes. RESULTS: In the pharmacokinetic population (n = 41), hydroxocobalamin caused short-lived mean blood pressure increases. Blood pressure increased shortly after initiation of infusion and returned nearly to baseline by 4 hours post-infusion. The time course of blood pressure changes coincided with that of changes in plasma total and free cobalamins-(III). Change in mean arterial pressure (MAP) was strongly correlated with plasma area-under-the-concentration-time curves (AUCs) of total and free cobalamins-(III) during infusion (r > 0.7) but not through 24 hours post-infusion (r < or = 0.36). CONCLUSION: The short-lived increase in mean blood pressure during administration of antidotal doses of hydroxocobalamin is closely linked to initial exposure to total and free cobalamins-(III) (Class III).
Uhl W, Nolting A, Gallemann D, Hecht S, Kovar A. Changes in blood pressure after administration of hydroxocobalamin: relationship to changes in plasma cobalamins-(III) concentrations in healthy volunteers. Clin Toxicol (Phila). 2008 Jul;46(6):551-9. [PubMed Citation]
-
STUDY OBJECTIVE: To assess outcomes in patients treated with hydroxocobalamin at the fire scene or in the ICU for suspected smoke inhalation-associated cyanide poisoning. METHODS: Adult smoke inhalation victims with suspected cyanide poisoning as determined by soot in the face, mouth, or nose or expectorations and neurologic impairment received an intravenous infusion of hydroxocobalamin 5 g (maximum 15 g) at the fire scene or in the ICU in this observational case series conducted from 1987 to 1994. Blood cyanide specimens were collected before administration of hydroxocobalamin. The threshold for cyanide toxicity was predefined as greater than or equal to 39 micromol/L. RESULTS: The sample included 69 patients (mean age 49.6 years; 33 men), of whom 39 were comatose. Out-of-hospital deaths were excluded. Fifty of the 69 patients (72%) admitted to the ICU survived after administration of hydroxocobalamin. In the group in which cyanide poisoning was confirmed a posteriori (n=42), 67% (28/42) survived after administration of hydroxocobalamin. The most common adverse events were chromaturia (n=6), pink or red skin discoloration (n=4), hypertension (n=3), erythema (n=2), and increased blood pressure (n=2). No serious adverse events were attributed to hydroxocobalamin. Laboratory tests revealed transient alterations in renal and hepatic function consistent with the critical condition of the patients and mild anemia consistent with progressive hemodilution. CONCLUSION: Empiric administration of hydroxocobalamin was associated with survival among 67% of patients confirmed a posteriori to have had cyanide poisoning. Hydroxocobalamin was well tolerated irrespective of the presence of cyanide poisoning. Hydroxocobalamin appears to be safe for the out-of-hospital treatment of presumptive cyanide poisoning from smoke inhalation (Class IV).
Borron SW, Baud FJ, Barriot P, Imbert M, Bismuth C. Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med. 2007 Jun;49(6):794-801. [PubMed Citation]
-
BACKGROUND: Inhalation of hydrogen cyanide from smoke in structural fires is common, but cardiovascular function in these patients is poorly documented. OBJECTIVE: The objective was to study the cardiac complications of cyanide poisoning in patients who received early administration of a cyanide antidote, hydroxocobalamin (Cyanokit; Merck KGaA, Darmstadt, Germany [in the United States, marketed by Meridian Medical Technologies, Bristol, TN]). METHODS: The medical records of 161 fire survivors with suspected or confirmed cyanide poisoning were reviewed in an open, multicenter, retrospective review of cases from the Emergency Medical Assistance Unit (Service d'Aide Médical d'Urgence) in France. RESULTS: Cardiac arrest (61/161, 58 asystole, 3 ventricular fibrillation), cardiac rhythm disorders (57/161, 56 supraventricular tachycardia), repolarization disorders (12/161), and intracardiac conduction disorders (5/161) were observed. Of the total 161 patients studied, 26 displayed no cardiac disorder. All patients were given an initial dose of 5 g of hydroxocobalamin. Non-responders received a second dose of 5 g of hydroxocobalamin. Of the patients initially in cardiac arrest, 30 died at the scene, 24 died in hospital, and 5 survived without cardiovascular sequelae. Cardiac disorders improved with increasing doses of hydroxocobalamin, and higher doses of the antidote seem to be associated with a superior outcome in patients with initial cardiac arrest. CONCLUSIONS: Cardiac complications are common in cyanide poisoning in fire survivors (Class IV).
Fortin JL, Desmettre T, Manzon C, Judic-Peureux V, Peugeot-Mortier C, Giocanti JP, Hachelaf M, Grangeon M, Hostalek U, Crouzet J, Capellier G. Cyanide poisoning and cardiac disorders: 161 cases. J Emerg Med. 2010 May;38(4):467-76. [PubMed Citation]
-
The stones and seeds of some plants such as apples, apricots, and peaches contain significant amounts of cyanide glycosides. Apricot pits are more toxic as they contain higher amounts of cyanogens and release hydrogen cyanide more easily. A previously healthy 27-month old male patient was admitted to our emergency department as intubated. His history revealed that he was intubated in the hospital where he was taken to with the complaint of fainting after having eaten numerous apricot pits with other family members. His general status was poor and he was unconscious. Both of his pupils were reactive. His deep tendon reflexes were increased and his plantar reflex was extensor bilaterally. The case was diagnosed as cyanide intoxication and the patient was admitted to the intensive care unit. Oxygen was constinuosly given under observation. After administration of hydroxocobalamine and sodium bicarbonate and correction of sodium deficit the patient regained consciousness and his general health improved. On the second day of admittance, he was discharged with a stable condition. This case was presented to emphasize that parents should not feed small children with apricot pits (Class IV).
Kaya A, Okur M, Üstyol L, Temel H, Çaksen H. Acute cyanide poisoning after eating apricot pits: A case report. Turk Pediatri Arsivi. 2012 47(2):141-2.
-
A 54-year-old woman was brought to hospital from an apartment fire. She had altered mental status, hypotension and evidence of inhalational injury, but no burns. Her carboxyhemoglobin level was 29%, and her lactate level was 16 mmol/L. She was treated with supplemental and hyperbaric oxygen for carbon monoxide intoxication. Hydroxocobalamin 5 g was administered intravenously in the intensive care unit for presumed cyanide poisoning. Subsequently, the patient's skin and urine became bright red. Cyanide poisoning often occurs in victims of smoke inhalation. Tests that can confirm a diagnosis of cyanide poisoning are rarely available. However, treatment should not be delayed. The diagnosis is clinical and characterized by altered mental status, cardiovascular instability and lactic acidosis. Hydroxocobalamin has been approved recently as a therapy for cyanide poisoning. It is relatively safe and is better tolerated than the ingredients of the traditional cyanide antidote kit (amyl nitrite, sodium nitrite and sodium thiosulfate). The nitrites induce methemoglobinemia, which can worsen hypotension and reduce the oxygen content of the blood, an important consideration in patients with concomitant carbon monoxide poisoning. Hydroxocobalamin should be administered as soon as cyanide poisoning is suspected — ideally in the prehospital setting. By combining with cyanide, hydroxocobalamin forms cyanocobalamin (vitamin B12), restoring mitochondrial function. Hydroxocobalamin imparts a harmless and transient reddish colour to the skin and urine. It may also cause transient hypertension, which can be beneficial in patients with cyanide poisoning (Class IV).
Cescon DW, Juurlink DN. Discoloration of skin and urine after treatment with hydroxocobalamin for cyanide poisoning. CMAJ. 2009 Jan 20;180(2):251. [PubMed Citation]
Pediatric studies
-
Confirmed cases of childhood exposure to cyanide are rare despite multiple potential sources including inhalation of fire smoke, ingestion of toxic household and workplace substances, and ingestion of cyanogenic foods. Because of its infrequent occurrence, medical professionals may have difficulty recognizing cyanide poisoning, confirming its presence, and treating it in pediatric patients. The sources and manifestations of acute cyanide poisoning seem to be qualitatively similar between children and adults, but children may be more vulnerable than adults to poisoning from some sources. The only currently available antidote in the United States (the cyanide antidote kit) has been used successfully in children but has particular risks associated with its use in pediatric patients. Because hemoglobin kinetics vary with age, methemoglobinemia associated with nitrite-based antidotes may be excessive at standard adult dosing in children. A cyanide antidote with a better risk/benefit ratio than the current agent available in the United States is desirable. The vitamin B12 precursor hydroxocobalamin, which has been used in Europe, may prove to be an attractive alternative to the cyanide antidote kit for pediatric patients. In this article we review the available data on the sources, manifestations, and treatment of acute cyanide poisoning in children and discuss unmet needs in the management of pediatric cyanide poisoning (Class IV).
Geller RJ, Barthold C, Saiers JA, Hall AH. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006 Nov; 118 (5): 2146-58. [PubMed Citation]
-
AIM: To report diagnostic, clinical and therapeutic aspects of cyanide intoxication resulting from ingestion of cyanogenic glucoside-containing apricot seeds. METHODS: Thirteen patients admitted to the Pediatric Intensive Care Unit (PICU) of Erciyes University between 2005 and 2009 with cyanide intoxication associated with ingestion of apricot seeds were reviewed retrospectively. RESULTS: Of the 13 patients, four were male. The mean time of onset of symptoms was 60 minutes (range 20 minutes to 3 hours). On admission, all patients underwent gastric lavage and received activated charcoal. In addition to signs of mild poisoning related to cyanide intoxication, there was severe intoxication requiring mechanical ventilation (in four cases), hypotension (in two), coma (in two) and convulsions (in one). Metabolic acidosis (lactic acidosis) was detected in nine patients and these were treated with sodium bicarbonate. Hyperglycaemia occurred in nine patients and blood glucose levels normalised spontaneously in six but three required insulin therapy for 3-6 hours. Six patients received antidote treatment: high-dose hydroxocobalamin in four and two were treated with a cyanide antidote kit in addition to high-dose hydroxocobalamin. One patient required anticonvulsive therapy. All patients recovered and were discharged from the PICU within a mean (SD, range) 3.1 (1.7, 2-6) days. CONCLUSION: Cyanide poisoning associated with ingestion of apricot seeds is an important poison in children, many of whom require intensive care (Class IV).
Akyildiz BN, Kurtoğlu S, Kondolot M, Tunç A. Cyanide poisoning caused by ingestion of apricot seeds. Ann Trop Paediatr. 2010;30(1):39-43. [PubMed Citation]
-
A five-year old child, after ingestion of bitter almonds, was reported to have altered consciousness, polypnea, tachycardia and dilated pupils, associated with metabolic acidosis. Bitter almonds contain cyanogenic glycosides which are responsible for these symptoms. The affinity of cyanide for cytochrome-oxydase accounts for the pathophysiology and signs of this poisoning. Their binding leads to cytotoxic hypoxia due to blockade of the cellular respiratory chain. Other aspects of their metabolism are used for treatment of acute poisoning, which is based on early oxygen therapy, gastric lavage and specific antidotes, essentially hydroxocobalamin (Class IV).
Kovalevsky P. Bengler C. Cyanide intoxication after ingesting bitter almond. Case report and review. Reanimation Soins Intensifs Medecine d'Urgence. 1996 12(3):149-153.
Pregnancy, breastfeeding studies
-
For smoke inhalation injury of a pregnant woman, one must treat two patients and be aware of the potential effects of carbon monoxide (CO) and cyanide (CN) poisoning on both the mother and the fetus. In a pregnant woman, the size and age of the fetus and the degree of poisoning allow for tremendous variability in the toxicity of CO and CN and their respective treatment options. The authors will review a case of a 32-year-old woman who was at 37 weeks of gestation and admitted to the Evans-Haynes Burn Center after a house fire and received hydroxocobalamin (Cyanokit) for suspected CN poisoning. In addition, a review of the literature, current guidelines, and treatment options of inhalation injury during pregnancy will be discussed. The authors will focus only on the toxic components of smoke inhalation injury rather than the mechanical components from heat and particulate damage. Literature review clearly identifies that the treatment of pregnant women with inhalation injury remains a controversial subject. The use of hydroxocobalamin (Cyanokit) as a treatment modality for potential CN poisoning in a pregnant patient has not been reported in the literature. Animal studies have shown that combined CO and CN poisoning are more lethal than either one alone and at lower concentrations. Due to the synergistic effects of CO and CN, and because these two toxins concentrate at even higher levels in the fetus than the mother, the authors will clarify the urgent seriousness of prompt administration of hydroxocobalamin in a pregnant patient with suspected smoke inhalation injury. This case review details the treatment of a 32-year-old woman who was at 36 weeks of gestation on admission to the Evans-Haynes Burn Center. The authors will report her injuries and the course of treatment. Although burned and presenting with concomitant smoke inhalation injury, both the woman and her child fared well with no significant complications due to the smoke inhalation at 6 months of follow-up. Smoke inhaled from modern structural fires potentially contains both CN and CO gases. This makes the prompt recognition of this injury and selection of appropriate therapy an emergent priority. In October 2010, the Food and Drug Administration approved hydroxocobalamin for use in pregnant patients in the acute setting when CN toxicity is suspected. Because CO and CN have additive effects when both are present in the body, the prompt administration of hydroxocobalamin not only eliminates the effects of CN but also potentially attenuates its synergistic effects on CO. It is only through a better understanding of the details of smoke inhalation injury, the current treatment options, and the considerations regarding their use that new research and strong guidelines can be developed to better serve our patients (Class IV).
Roderique EJ, Gebre-Giorgis AA, Stewart DH, Feldman MJ, Pozez Al. Smoke Inhalation Injury in a Pregnant Patient: A Literature Review of the Evidence and Current Best Practices in the Setting of a Classic Case; J Burn Care Res 2012 Jan 30 [Epub ahead of print] [PubMed Citation]
-
There are no adequate and well controlled studies of Cyanokit in pregnant women. In animal studies, hydroxocobalamin caused skeletal and visceral (soft tissue) abnormalities at exposures (based on AUC) similar to human exposures at the therapeutic dose. Cyanokit should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Because cyanide readily crosses the placenta, maternal cyanide poisoning results in fetal cyanide poisoning. Timely treatment of the pregnant mother may be lifesaving for both mother and fetus (Class IV).
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Geriatric
-
An 80-year-old diabetic patient was admitted to the hospital because of sudden unconsciousness and severe metabolic acidosis. His son reported the possibility of cyanide poisoning. Clinical data and the detection of cyanide in blood and gastric material confirmed this possibility. Supportive therapy and the following antidotes--sodium nitrite two doses 300 mg i.v., sodium thiosulfate 3 g i.v., and hydroxocobalamin 4 g in 24 hours--were administered immediately and the patient completely recovered in 48 hours. Our observations suggest that timely and appropriate use of antidotes for cyanide intoxication may prevent death, even in aged diabetic patients (Class IV).
Mannaioni G, Vannacci A, Marzocca C, Zorn AM, Peruzzi S, Moroni F. Acute cyanide intoxication treated with a combination of hydroxycobalamin, sodium nitrite, and sodium thiosulfate. J Toxicol Clin Toxicol. 2002;40(2):181-3. [PubMed Citation]
Clinical reviews
-
Cyanide poisoning may result from different exposures: residential fires, industrial accidents, drug and plant intoxication. Clinical features include coma, respiratory arrest and cardiovascular collapse. The biological hallmark is lactic acidosis. A plasma lactate concentration > or = 10 mmol/L in fire victims without severe burns and > or = 8 mmol/L in pure cyanide poisoned patients is a sensitive and specific indicator of cyanide intoxication. Many antidotes are available and efficient. However, therapeutic strategies are still debated. Our objective was to compare conventional treatments to hydroxocobalamin. This article reviews the literature on cyanide poisoning treatment. Conventional treatment of cyanide poisoning includes decontamination, supportive and specific treatment. Decontamination should be adapted to the route of poisoning and never postpone supportive treatment. Basic life support includes immediate administration of high flow of oxygen, airway protection and cardiopulmonary resuscitation. Advanced life support includes mechanical ventilation, catecholamine and sodium bicarbonate infusion. Supportive treatment is efficient but does not modify the time course or the body burden of cyanide. Numerous antidotes are available. Oxygen counteracts efficiently cyanide action at the mitochondrial level. Sodium thiosulfate, methemoglobin forming agents and cobalt compounds act efficiently by complexing or transforming cyanide into non-toxic stable derivatives. However, regarding the main clinical condition of cyanide poisoning, i.e. smoke inhalation, we should take into account not only the efficiency of antidotes but also their safety. Sodium thiosulfate is both efficient and safe, but acts with delay. Methemoglobin-forming agents are potent, but due to the transformation of hemoglobin into methemoglobin, they impair tissue delivery of oxygen. Experimental data showed increased mortality in carbon monoxide- and cyanide-poisoned rats treated with these agents. Cobalt EDTA and hydroxocobalamin are efficient and act immediately. Cobalt EDTA is more potent on a molar basis; however, numerous side effects limit its use to evidenced cyanide poisoning. In a prospective study, hydroxocobalamin appeared safe in fire victims with or without cyanide poisoning. The only reported side effect was a red coloration of skin and urine. In conclusion, antidotes are beneficial in cyanide poisoning. In suspected cyanide-poisoned patients, we recommend the use of hydroxocobalamin as first-line antidote, owing to its safety. In massive cyanide poisoning, due to the limited potency of hydroxocobalamin, continuous infusion of sodium thiosulfate should be associated (Class IV).
Mégarbane B, Delahaye A, Goldgran-Tolédano D, Baud FJ. Antidotal treatment of cyanide poisoning. J Chin Med Assoc. 2003 Apr;66(4): 193-203. [PubMed Citation]
-
Effective management of cyanide poisoning from chemical terrorism, inhalation of fire smoke, and other causes constitutes a critical challenge for the prehospital care provider. The ability to meet the challenge of managing cyanide poisoning in the prehospital setting may be enhanced by the availability of the cyanide antidote hydroxocobalamin, currently under development for potential introduction in the United States. This paper discusses the causes, recognition, and management of acute cyanide poisoning in the prehospital setting with emphasis on the emerging profile of hydroxocobalamin, an antidote that may have a risk:benefit ratio suitable for empiric, out-of-hospital treatment of the range of causes of cyanide poisoning. If introduced in the U.S., hydroxocobalamin may enhance the role of the U.S. prehospital responder in providing emergency care in a cyanide incident (Class IV).
Guidotti T. Acute cyanide poisoning in prehospital care: new challenges, new tools for intervention. Prehosp Disaster Med. 2006 Mar-Apr;21(2):s40-8. [PubMed Citation]
-
OBJECTIVE: To review the recently approved cyanide antidote, hydroxocobalamin, and describe its role in therapy. DATA SOURCES: Relevant publications were identified through a systematic search of PubMed using the MeSH terms and key words hydroxocobalamin and cyanide. This search was then limited to human studies published since 2000. Systematic searches were conducted through January 2008. References from identified articles were reviewed for additional pertinent human studies. STUDY SELECTION AND DATA EXTRACTION: The literature search retrieved 7 studies on the safety and/or efficacy of hydroxocobalamin in humans. Four new studies were identified by the search and 3 studies were identified from the references. DATA SYNTHESIS: Studies of antidote efficacy in humans are ethically and logistically difficult. A preclinical study demonstrated that intravenous doses of hydroxocobalamin 5 g are well tolerated by volunteer subjects. Hydroxocobalamin has been shown to reduce cyanide concentrations in controlled studies of nitroprusside therapy and in heavy smokers. A retrospective study of 14 acute cyanide poisonings also demonstrated hydroxocobalamin's safety and efficacy. Two studies examining hydroxocobalamin for smoke inhalation-associated cyanide poisoning indicated a possible benefit, but they are insufficient to establish definitive criteria for use in this setting. Randomized controlled trials of hydroxocobalamin and traditional cyanide antidotes (nitrites/thiosulfate) are lacking. CONCLUSIONS: Cyanide poisoning can rapidly cause death. Having an effective antidote readily available is essential for facilities that provide emergency care. In cases of cyanide ingestion, both the nitrite/thiosulfate combination and hydroxocobalamin are effective antidotes. Hydroxocobalamin offers an improved safety profile for children and pregnant women. Hydroxocobalamin also appears to have a better safety profile in the setting of cyanide poisoning in conjunction with smoke inhalation. However, current data are insufficient to recommend the empiric administration of hydroxocobalamin to all victims of smoke inhalation (Class IV).
Shepherd G, Velez LI. Role of hydroxocobalamin in acute cyanide poisoning. Ann Pharmacother. 2008 May;42(5):661-9 [PubMed Citation]
-
The United States is under the constant threat of a mass casualty cyanide disaster from industrial accidents, hazardous material transportation incidents, and deliberate terrorist attacks. The current readiness for cyanide disaster by the emergency medical system in the United States is abysmal. We, as a nation, are simply not prepared for a significant cyanide-related event. The standard of care for cyanide intoxication is the cyanide antidote kit, which is based on the use of nitrites to induce methemoglobinemia. This kit is both expensive and ill suited for out-of-hospital use. It also has its own inherent toxicity that prevents rapid administration. Furthermore, our hospitals frequently fail to stock this life-saving antidote or decline to stock more than one. Hydroxocobalamin is well recognized as an efficacious, safe, and easily administered cyanide antidote. Because of its extremely low adverse effect profile, it is ideal for out-of-hospital use in suspected cyanide intoxication. To effectively prepare for a cyanide disaster, the United States must investigate, adopt, manufacture, and stockpile hydroxocobalamin to prevent needless morbidity and mortality (Class IV).
Sauer SW, Keim ME. Hydroxocobalamin: improved public health readiness for cyanide disasters. Ann Emerg Med. 2001 Jun;37(6):635-41. [PubMed Citation]
-
Cyanide poisoning is uncommon, but generates interest because of the presumed utility of an antidote immediately available in those areas with a high risk of cyanide exposure. As part of its regular review of guidelines, the Australian Resuscitation Council conducted a systematic review of the human evidence for the use of various proposed cyanide antidotes, and a narrative review of the relevant pharmacological and animal studies. There have been no relevant comparative or placebo-controlled human trials. Nine case series were identified. Treatment with hydroxocobalamin was reported in a total of 361 cases. No serious adverse effects of hydroxocobalamin were reported, and many patients with otherwise presumably fatal poisoning survived. Sodium thiosulphate use was reported in two case series, similarly with no adverse effects. Treatment with a combination of sodium nitrite, amyl nitrite and sodium thiosulphate was reported in 74 patients, with results indistinguishable from those of hydroxocobalamin and sodium thiosulphate. No case series using dicobalt edetate or 4-dimethylaminophenol were identified, but successful use in single cases has been reported. Hydroxocobalamin and sodium thiosulphate differ from alternatives in having negligible adverse effects, and on the basis of current evidence are the antidotes of choice. The indications for the use of an antidote, the requirements for supportive care and a recommended approach for workplaces where there is a risk of cyanide poisoning are presented (Class IV).
Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC; Australian Resuscitation Council. Review article: management of cyanide poisoning. Emerg Med Australas. 2012 Jun;24(3):225-38. [PubMed Citation]
-
Cyanide poisoning can present in multiple ways, given its widespread industrial use, presence in combustion products, multiple physical forms, and chemical structures. The primary target of toxicity is mitochondrial cytochrome oxidase. The onset and severity of poisoning depend on the route, dose, physicochemical structure and other variables. Common poisoning features include dyspnea, altered respiratory patterns, abnormal vital signs, altered mental status, seizures, and lactic acidosis. Our present knowledge supports cyanide poisoning treatment based on excellent supportive care with adjunctive antidotal therapy. Multiple antidotes exist and vary in regional availability. All currently marketed antidotes appear to be effective. Antidotal mechanisms include chelation, formation of stable, less toxic complexes, methemoglobin induction, and sulfane sulfur supplementation for detoxification by endogenous rhodanese. Each antidote has advantages and disadvantages. For example, hydroxocobalamin is safer than the methemoglobin inducers in patients with smoke inhalation. Research for new, safer and more effective cyanide antidotes continues (Class IV).
Borron SW, Baud FJ. Antidotes for acute cyanide poisoning. Curr Pharm Biotechnol. 2012 Aug 1;13(10):1940-8. [PubMed Citation]
-
Cyanide causes intracellular hypoxia by reversibly binding to mitochondrial cytochrome oxidase a3. Signs and symptoms of cyanide poisoning usually occur less than 1 minute after inhalation and within a few minutes after ingestion. Early manifestations include anxiety, headache, giddiness, inability to focus the eyes, and mydriasis. As hypoxia progresses, progressively lower levels of consciousness, seizures, and coma can occur. Skin may look normal or slightly ashen, and arterial oxygen saturation may be normal. Early respiratory signs include transient rapid and deep respirations. As poisoning progresses, hemodynamic status may become unstable. The key treatment is early administration of 1 of the 2 antidotes currently available in the United States: the well-known cyanide antidote kit and hydroxocobalamin. Hydroxocobalamin detoxifies cyanide by binding with it to form the renally excreted, nontoxic cyanocobalamin. Because it binds with cyanide without forming methemoglobin, hydroxocobalamin can be used to treat patients without compromising the oxygen carrying capacity of hemoglobin (Class IV).
Hamel J; A Review of acute cyanide poisoning with a treatment update. Critical Care Nurse. 2011 Feb;31(1):72-82. [PubMed Citation]
-
Cyanide has several antidotes, with differing mechanisms of action and diverse toxicological, clinical, and risk-benefit profiles. The international medical community lacks consensus about the antidote or antidotes with the best risk-benefit ratio. Critical assessment of cyanide antidotes is needed to aid in therapeutic and administrative decisions that will improve care for victims of cyanide poisoning (particularly poisoning from enclosed-space fire-smoke inhalation), and enhance readiness for cyanide toxic terrorism and other mass-casualty incidents. This paper reviews preclinical and clinical data on available cyanide antidotes and considers the profiles of these antidotes relative to properties of a hypothetical ideal cyanide antidote. Each of the antidotes shows evidence of efficacy in animal studies and clinical experience. The data available to date do not suggest obvious differences in efficacy among antidotes, with the exception of a slower onset of action of sodium thiosulfate (administered alone) than of the other antidotes. The potential for serious toxicity limits or prevents the use of the Cyanide Antidote Kit, dicobalt edetate, and 4-dimethylaminophenol in prehospital empiric treatment of suspected cyanide poisoning. Hydroxocobalamin differs from these antidotes in that it has not been associated with clinically significant toxicity in antidotal doses. Hydroxocobalamin is an antidote that seems to have many of the characteristics of the ideal cyanide antidote: rapid onset of action, neutralizes cyanide without interfering with cellular oxygen use, tolerability and safety profiles conducive to prehospital use, safe for use with smoke-inhalation victims, not harmful when administered to non-poisoned patients, easy to administer (Class IV).
Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol 2009;39(7):541-52. [PubMed Citation]
-
The potential for domestic or international terrorism involving cyanide has not diminished and in fact may have increased in recent years. This paper discusses cyanide as a terrorist weapon and the current state of readiness for a cyanide attack in the United States. Many of the factors that render cyanide appealing to terrorists are difficult to modify sufficiently to decrease the probability of a cyanide attack. For example, the relative ease with which cyanide can be used as a weapon without special training, its versatile means of delivery to intended victims, and to a large degree, its ready availability cannot be significantly modified through preparedness efforts. On the other hand, the impact of an attack can be mitigated through preparedness measures designed to minimize the physical, psychological, and social consequences of cyanide exposure. Although the nation remains ill-equipped to manage a cyanide disaster, significant progress is being realized in some aspects of preparedness. Hydroxocobalamin-a cyanide antidote that may be appropriate for use in the prehospital setting for presumptive cases of cyanide poisoning-currently is under development for potential introduction in the US. If it becomes available in the US, hydroxocobalamin could enhance the role of the prehospital emergency responder in providing care to victims of a cyanide disaster. Additional progress is required in the areas of ensuring local and regional availability of antidotal treatment and supportive interventions, educating emergency healthcare providers about cyanide poisoning and its management, and raising public awareness of the potential for a cyanide attack and how to respond (Class IV).
Keim ME. Terrorism involving cyanide: the prospect of improving preparedness in the prehospital setting. Prehosp Disaster Med. 2006 Mar-Apr;21(2):s56-60. [PubMed Citation]
-
Cyanide poisoning must be seriously considered in victims of smoke inhalation from enclosed space fires; it is also a credible terrorism threat agent. The treatment of cyanide poisoning is empiric because laboratory confirmation can take hours or days. Empiric treatment requires a safe and effective antidote that can be rapidly administered by either out-of-hospital or emergency department personnel. Among several cyanide antidotes available, sodium thiosulfate and hydroxocobalamin have been proposed for use in these circumstances. The evidence available to assess either sodium thiosulfate or hydroxocobalamin is incomplete. According to recent safety and efficacy studies in animals and human safety and uncontrolled efficacy studies, hydroxocobalamin seems to be an appropriate antidote for empiric treatment of smoke inhalation and other suspected cyanide poisoning victims in the out-of-hospital setting. Sodium thiosulfate can also be administered in the out-of-hospital setting. The efficacy of sodium thiosulfate is based on individual case studies, and there are contradictory conclusions about efficacy in animal models. The onset of antidotal action of sodium thiosulfate may be too slow for it to be the only cyanide antidote for emergency use. Hydroxocobalamin is being developed for potential introduction in the United States and may represent a new option for emergency personnel in cases of suspected or confirmed cyanide poisoning in the out-of-hospital setting (Class IV).
Hall AH, Dart R, Bogdan G. Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning? Ann Emerg Med. 2007 Jun;49(6):806-13 [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
INTRODUCTION: The efficacy of hydroxocobalamin for acute cyanide poisoning was compared with that of saline vehicle in dogs. METHODS: Anesthetized adult beagle dogs were administered potassium cyanide (0.4 mg/kg/min, IV) until 3 min after the onset of apnea. Hydroxocobalamin (75 mg/kg [n = 19] or 150 mg/kg [n = 18], IV) or saline vehicle [n = 17] was then infused over 7.5 min while animals were ventilated with 100% oxygen, which was stopped after 15 min. RESULTS: In vehicle-treated animals cyanide produced deterioration that culminated in a moribund state requiring euthanasia within 4 h in 10 of 17 animals and in neurological deficits necessitating euthanasia within 2-4 d in an additional 4 animals (mortality rate 82%). Survival through 14 d was observed in 15 of 19 animals administered hydroxocobalamin 75 mg/kg (mortality rate 21%), and 18 of 18 administered hydroxocobalamin 150 mg/kg (mortality rate 0%). CONCLUSION: Hydroxocobalamin reversed cyanide toxicity and reduced mortality in a canine model.
Borron SW, Srtonerook M, Reid F. Efficacy of hydroxocobalamin for the treatment of acute cyanide poisoning in adult beagle dogs; Clin Toxicol (Phil). 2006; 44 Suppl 1: 5-15. [PubMed Citation]
-
The efficacy of hydroxocobalamin (vitamin B12a) as a specific, nontoxic antidote in acute cyanide poisoning was tested. Guinea pigs receiving lethal intravenous NaCN injections were treated with either vitamin B12a or saline solution. There was a statistically significant antidotal effect of the vitamin. No toxic effect was observed with large doses of the vitamin.
Posner MA et al; Hydroxocobalamin Therapy of Cyanide Intoxication in Guinea Pigs. Anesthesiology 1976; 44 (2): 157-60. [PubMed Citation]
-
STUDY OBJECTIVE: To determine whether hydroxocobalamin will improve survival compared with epinephrine and saline solution controls in a model of cyanide-induced cardiac arrest. METHODS: Forty-five swine (38 to 42 kg) were tracheally intubated, anesthetized, and central venous and arterial continuous cardiovascular monitoring catheters were inserted. Potassium cyanide was infused until cardiac arrest developed, defined as mean arterial pressure less than 30 mm Hg. Animals were treated with standardized mechanical chest compressions and were randomly assigned to receive one of 3 intravenous bolus therapies: hydroxocobalamin, epinephrine, or saline solution (control). All animals were monitored for 60 minutes after cardiac arrest. Additional epinephrine infusions were used in all arms of the study after return of spontaneous circulation for systolic blood pressure less than 90 mm Hg. A sample size of 15 animals per group was determined according to a power of 80%, a survival difference of 0.5, and an α of 0.05. Repeated-measure ANOVA was used to determine statistically significant changes between groups over time. RESULTS: Baseline weight, time to arrest, and cyanide dose at cardiac arrest were similar in the 3 groups. Coronary perfusion pressures with chest compressions were greater than 15 mm Hg in both treatment groups indicating sufficient compression depth. Zero of 15 (95% confidence interval [CI] 0% to 25%) animals in the control group, 11 of 15 (73%; 95% CI 48% to 90%) in the hydroxocobalamin group, and 11 of 15 (73%; 95% CI 48% to 90%) in the epinephrine group survived to the conclusion of the study (P<.001). The proportion of animals with return of spontaneous circulation at 5 minutes was 4 of 15 (27%; 95% CI 10% to 52%), and that of return of spontaneous circulation at 10 minutes was 11 of 15 (73%; 95% CI 48% to 90%) in the 2 treatment groups. Additional epinephrine infusion after return of spontaneous circulation was administered for hypotension in 2 of 11 (18%; 95% CI 4% to 48%) hydroxocobalamin animals and in 11 of 11 (100%; 95% CI 70% to 100%) of the epinephrine animals (P<.001). At 60 minutes, serum lactate was significantly lower in the hydroxocobalamin group compared with the epinephrine group (4.9 [SD 2.2] versus 12.3 [SD 2.2] mmol/L), and the pH was significantly higher (7.34 [SD 0.03] versus 7.15 [SD 0.07]). Serial blood cyanide levels in the hydroxocobalamin group were also lower than that of the epinephrine group from cardiac arrest through the conclusion of the study. CONCLUSION: Intravenous hydroxocobalamin and epinephrine both independently improved survival compared with saline solution control in our swine model of cyanide-induced cardiac arrest. Hydroxocobalamin improved mean arterial pressure and pH, decreased blood lactate and cyanide levels, and decreased the use of rescue epinephrine therapy compared with that in the epinephrine group.
Bebarta VS, Pitotti RL, Dixon PS, Valtier S, Esquivel L, Bush A, Little CM. Hydroxocobalamin and Epinephrine Both Improve Survival in a Swine Model of Cyanide-Induced Cardiac Arrest. Ann Emerg Med. 2012 Mar 14. [Epub ahead of print] [PubMed Citation]
-
STUDY OBJECTIVE: We compare the efficacy of hydroxocobalamin to sodium thiosulfate to reverse the depressive effects on mean arterial pressure in a swine model of acute cyanide toxicity and gain a better understanding of the mechanism of action of the hydroxocobalamin in reversal of the toxicity. METHODS: Swine were intubated, anesthetized, and instrumented with central arterial and venous lines and a pulmonary artery catheter. Animals (n=36) were randomly assigned to one of 3 groups: hydroxocobalamin alone (150 mg/kg), sodium thiosulfate alone (413 mg/kg), or hydroxocobalamin (150 mg/kg)+sodium thiosulfate (413 mg/kg) and monitored for 60 minutes after the start of antidotal infusion. Cyanide was infused until severe hypotension developed, defined as blood pressure 50% of baseline mean arterial pressure. Repeated-measures ANOVA was used to determine statistically significant changes between groups over time. RESULTS: Time to hypotension (25, 28, and 33 minutes), cyanide dose at hypotension (4.7, 5.0, and 5.6 mg/kg), and mean cyanide blood levels (3.2, 3.7, and 3.8 μg/mL) and lactate levels (7, 8.2, 8.3 and mmol/L) were similar. All 12 animals in the sodium thiosulfate group died compared with 2 of 12 in the hydroxocobalamin/sodium thiosulfate group and 1 of 12 in hydroxocobalamin group. No statistically significant differences were detected between the hydroxocobalamin and hydroxocobalamin/sodium thiosulfate groups for carbon monoxide, mean arterial pressure, cyanide levels, or mortality at 60 minutes. Lactate level (2.6 versus 2.1 mmol/L), pH (7.44 versus 7.42), and bicarbonate level (25 versus 26 mEq/L) at 60 minutes were also similar between groups. CONCLUSION: Sodium thiosulfate failed to reverse cyanide-induced shock in our swine model of severe cyanide toxicity. Further, sodium thiosulfate was not found to be effective when added to hydroxocobalamin in the treatment of cyanide-induced shock. Hydroxocobalamin alone was again found to be effective for severe cyanide toxicity.
Bebarta VS, Pitotti RL, Dixon P, Lairet JR, Bush A, Tanen DA. Hydroxocobalamin versus sodium thiosulfate for the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. Ann Emerg Med. 2012 Jun;59(6):532-9. [PubMed Citation]
-
Hydroxocobalamin has been shown to be a rapid and powerful antidote in acute cyanide poisoning and to prevent cyanide poisoning during sodium nitroprusside administration. However, its hemodynamic effects remain unknown. The authors therefore investigated the effects in chronically instrumented conscious dogs (n = 8) that were randomly given hydroxocobalamin (20, 70, and 140 mg.kg-1) or saline. Determination of peak cobalt plasma concentrations showed that 20 and 70 mg.kg-1 hydroxocobalamin correspond to "therapeutic doses," whereas 140 mg.kg-1 corresponds to a supratherapeutic dose. Hydroxocobalamin did not modify heart rate, mean arterial pressure, left ventricular (LV) end-diastolic pressure, and PR and QT intervals, regardless of the dose administered. The largest dose (140 mg.kg-1) induced a decrease in the maximum increase of LV pressure (-7 +/- 3%; P less than 0.05), maximum aortic blood flow acceleration (-17 +/- 5%; P less than 0.05), and cardiac output (-19 +/- 6%; P less than 0.05), whereas systemic resistance increased (+41 +/- 9%; P less than 0.05). In six other dogs, local administration of hydroxocobalamin (0.5, 1.5, and 5.0 mg.kg-1.min-1) confirmed that, in large doses, this drug has direct vasoconstrictor properties affecting both conductance (decrease in iliac artery diameter: -2.5 +/- 0.8%) and resistance (decrease in iliac artery blood flow: -19.5 +/- 3.4%) vessels. Thus, hydroxocobalamin should be a safe cyanide antidote, considering the lack of hemodynamic effects within the therapeutic range of doses.
Riou B, Gérard JL, La Rochelle CD, Bourdon R, Berdeaux A, Giudicelli JF. Hemodynamic effects of hydroxocobalamin in conscious dogs. Anesthesiology. 1991 Mar;74(3):552-8. [PubMed Citation]
-
In dogs, in addition to single doses (150, 300 and 1200 mg/kg), the toxicity of hydroxocobalamin was studied by IV route after administration of repeated doses (300, 600 and 1200 mg/kg/day for 3 days - 75, 150 and 300 mg/kg/day for 4 weeks). Platelet count was decreased in all these studies. Liver and kidneys were the main target organs. At the biochemistry level, liver enzymes (ALT, AST, ALP) were increased in all the studies but returned to baseline levels after withdrawal of the treatment. At the histopathological level, changes were attributed to an overload phenomenon. They occurred at ≥300 mg/kg and at ≥75 mg/kg in the single dose study and in repeat-dose studies, respectively, and were possibly associated with reactive and degenerative changes mainly in repeat-dose studies. Other kidney findings observed in the single dose study at 1200 mg/kg and in the 3-day study at ≥ 600 mg/kg were related to the redistribution of plasma water from the intravascular to the extravascular space occurring after hydroxocobalamin administration. This mechanism was also probably involved in the decrease in platelet count. Single cell necroses, affecting mainly macrophages, were observed at all dose levels in the bone marrow. However, they did not impact either the cellularity of the bone marrow, or the bone marrow functioning. Moreover, histopathological examination in the 4-week toxicity study showed a trend towards recovery in terms of incidence and severity. Adverse effects were observed in other organs/tissues in 3-day and 4-week studies. In the 3-day toxicity study, heart alterations occurring at the top dose of 1200 mg/kg/day and spleen alterations observed at 600 and 1200 mg/kg/day were attributed to redistribution of plasma water. In the 4-week study, heart and spleen findings were also observed and were considered to result from a non specific inflammatory reaction and from the overload phenomenon, respectively. Taking into consideration the nature and reversibility of the effects reported in the single dose study, a single dose of 300 mg/kg is considered as well tolerated in dogs. With the exception of liver fibrosis reported at 300 mg/kg in the 4-week study, all the treatment related findings observed in repeat-dose studies were either fully reversible or showed a trend to recovery after 8 weeks of treatment-free period. Liver fibrosis likely results from the inflammatory reaction reported after 4-week treatment and as a sequel of the observed sinusoid edema related to redistribution of plasma water and overload phenomenon. Therefore, the risk of hepatic fibrosis resulting from the therapeutic use of Cyanokit seems unlikely. In view of the nature of the adverse effects reported at ≤150 mg/kg/day, of their full or on-going reversibility after a 8-week recovery period, and of the toxicological concerns in patients arising from cyanide-poisoning itself, the dose of 150 mg/kg/day can be considered as a NOAEL.
European Public Assessment Report (EPAR): Scientific Discussion (Cyanokit) Last updated May 2011 (EMA)
-
STUDY OBJECTIVES: Cyanide can cause severe hypotension with acute toxicity. To our knowledge, no study has directly compared hydroxocobalamin and sodium nitrite with sodium thiosulfate in an acute cyanide toxicity model. Our objective is to compare the return to baseline of mean arterial blood pressure between 2 groups of swine with acute cyanide toxicity and treated with hydroxocobalamin with sodium thiosulfate or sodium nitrite with sodium thiosulfate. METHODS: Twenty-four swine were intubated, anesthetized, and instrumented (continuous arterial and cardiac output monitoring) and then intoxicated with a continuous cyanide infusion until severe hypotension. The animals were divided into 2 arms of 12 each and then randomly assigned to intravenous hydroxocobalamin (150 mg/kg)+sodium thiosulfate (413 mg/kg) or sodium nitrite (10 mg/kg)+sodium thiosulfate (413 mg/kg) and monitored for 40 minutes after start of antidotal infusion. Twenty animals were needed for 80% power to detect a significant difference in outcomes (alpha 0.05). Repeated measures of analysis of covariance and post hoc t test were used for determining significance. RESULTS: Baseline mean weights, time to hypotension (31 minutes 3 seconds versus 28 minutes 6 seconds), and cyanide dose at hypotension (5.6 versus 5.9 mg/kg) were similar. One animal in the hydroxocobalamin group and 2 animals in the sodium nitrite group died during antidote infusion and were excluded from analysis. Hydroxocobalamin resulted in a faster return to baseline mean arterial pressure, with improvement beginning at 5 minutes and lasting through the conclusion of the study (P< 0.05). No statistically significant difference was detected between groups for cardiac output, pulse rate, systemic vascular resistance, or mortality at 40 minutes post intoxication. Mean cyanide blood levels (4.03 versus 4.05 microg/mL) and lactate levels (peak 7.9 versus 8.1 mmol/L) at hypotension were similar. Lactate levels (5.1 versus 4.48 mmol/L), pH (7.40 versus 7.37), and base excess (-0.75 versus 1.27) at 40 minutes were also similar. CONCLUSION: Hydroxocobalamin with sodium thiosulfate led to a faster return to baseline mean arterial pressure compared with sodium nitrite with sodium thiosulfate; however, there was no difference between the antidote combinations in mortality, serum acidosis, or serum lactate.
Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier S, Bush A. Hydroxocobalamin and Sodium Thiosulfate Versus Sodium Nitrite and Sodium Thiosulfate in the Treatment of Acute Cyanide Toxicity in a Swine (Sus scrofa) Model. Ann Emerg Med. 2010 Apr;55:345-351. [PubMed Citation]
Pregnant animal studies
-
In animal studies, pregnant rats and rabbits received Cyanokit (75, 150, or 300 mg/kg/d) during the period of organogenesis. Following intraperitoneal dosing in rats and intravenous dosing in rabbits, maternal exposures were equivalent to 0.5, 1, or 2 times the human exposure at the therapeutic dose (based on AUC). In the high dose groups for both species, maternal toxicity occurred, and there was a reduced number of live fetuses due to embryofetal resorptions. In addition, decreased live fetal weight occurred in high dose rats, but not in rabbits. Incomplete skeletal ossification occurred in both rats and rabbits. In rats, two fetuses of the high dose group and two fetuses of the mid dose group (each from a different litter) had short, rudimentary or small front or hind legs. Rabbit litters and fetuses exhibited a dose dependant increase in various gross soft tissue and skeletal anomalies. The main findings in rabbits were flexed, rigid flexor or medially rotated forelimbs or hindlimbs and domed heads at external examination; enlarged anterior or posterior fontanelles of the ventricles of the brain and flat, bowed or large ribs at skeletal examination; and dilated ventricles of the brain, and thick wall of the stomach at visceral examination.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Other non-clinical studies
Animal in vitro studies-
The effects of sodium cyanide (1 mM) and the antidotal action of hydroxocobalamin (1 mM) were studied on rat cardiac papillary muscle. A 10-min period of exposure to cyanide induced a marked decrease in inotropy as shown by a decrease in the maximum unloaded shortening velocity (Vmax: 64 +/- 11% of precyanide values, p <0.01) and active isometric force (AF/s: 35 +/- 13%, p <0.01). The impairment of contraction-relaxation coupling under low load and the nearly complete disappearance of the load sensitivity of relaxation suggested a decrease in sarcoplasmic reticulum function. The proportional acceleration in isometric relaxation suggested a decrease in myofilament calcium sensitivity. There was a nearly complete recovery from cyanide poisoning after 5 min of exposure to hydroxocobalamin, whereas in a control group receiving cyanide alone, the mechanical parameters remained unchanged or were further impaired. The effects of hydroxocobalamin developed very quickly, beat to beat. The main toxic target of cyanide is brain and heart cytochrome oxidase, and brain damage appears only a few minutes after the onset of anoxia. Because hydroxocobalamin is a rapid and powerful antidote, it may be useful in the treatment of acute cyanide poisoning.
Riou B, Baud FJ, Astier A, Barriot P, Lecarpentier Y. In vitro demonstration of the antidotal efficacy of hydroxocobalamin in cyanide poisoning. Neurosurg Anesthesiol./a> 1990 Dec; 2(4): 296-304. [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
OBJECTIVE: Hydroxocobalamin has been proposed as a cyanide antidote. Little is known, however, about its pharmacokinetics in human cyanide poisoning. METHODS: We prospectively studied the pharmacokinetics of hydroxocobalamin in 11 smoke inhalation victims of whom all but one had objective evidence of cyanide exposure. Serum hydroxocobalamin levels were followed from just before drug administration to six days after a single 5 g dose of hydroxocobalamin. RESULTS: The results (mean +/- standard error) suggest a two compartment model. Distribution half-life is on the order of 1.86 +/- 0.34 h and the elimination half-life 26.2 +/- 2.7 h. The apparent volume of distribution is 0.45 +/- 0.03 L/kg. Renal and total body clearance are 0.31 +/- 0.06 and 0.83 +/- 0.07 L/h, respectively. CONCLUSION: The apparent volume of distribution suggests a predominantly extracellular partitioning of the antidote, even in the presence of cyanide, an important factor in terms of its antidotal effect. Hydroxocobalamin's elimination half-life in these cyanide-exposed patients far exceeds those found in previous studies of dogs and minimally-exposed humans. If confirmed, this half-life suggests that a single dose of hydroxocobalamin, sufficiently large enough to bind the cyanide present, should be adequate.
Houeto P, Borron SW, Sandouk P, Imbert M, Levillain P, Baud FJ. Pharmacokinetics of hydroxocobalamin in smoke inhalation victims. J Toxicol Clin Toxicol. 1996;34(4):397-404. [PubMed Citation]
-
Following intravenous administration of hydroxocobalamin significant binding to plasma proteins and low molecular weight physiological compounds occurs, forming various cobalamin-(III) complexes by replacing the hydroxo ligand. The low molecular weight cobalamins-(III) formed, including hydroxocobalamin, are termed "free cobalamins-(III)"; the sum of free and protein-bound cobalamins is termed "total cobalamins-(III)". In order to reflect the exposure to the sum of all derivatives, pharmacokinetics of cobalamins-(III) (i.e. cobalamin-(III) entity without specific ligand) were investigated instead of hydroxocobalamin alone, using the concentration unit μg eq/mL. Dose-proportional pharmacokinetics were observed following single dose intravenous administration of 2.5 to 10 g of hydroxocobalamin in healthy volunteers. Mean free and total cobalamins-(III) Cmax values of 113 and 579 μg eq/mL, respectively, were determined following a dose of 5 g of hydroxocobalamin. Similarly, mean free and total cobalamins-(III) Cmax values of 197 and 995 μg eq/mL, respectively, were determined following the dose of 10 g of hydroxocobalamin. The predominant mean half-life of free and total cobalamins-(III) was found to be approximately 26 to 31 hours at both the 5 g and 10 g dose level. The mean total amount of cobalamins-(III) excreted in urine during the collection period of 72 hours was about 60% of a 5 g dose and about 50% of a 10 g dose of hydroxocobalamin. Overall, the total urinary excretion was calculated to be at least 60 to 70% of the administered dose. The majority of the urinary excretion occurred during the first 24 hours, but red-colored urine was observed for up to 35 days following the intravenous infusion. When normalized for body weight, male and female subjects revealed no major differences in pharmacokinetic parameters of free and total cobalamins-(III) following the administration of 5 and 10 g of hydroxocobalamin.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Animal
-
The possibility of direct transport of hydroxocobalamin from the nasal cavity into the cerebrospinal fluid (CSF) after nasal administration in rats was investigated and the results were compared with a human study. Hydroxocobalamin was given to rats (n=8) both intranasally (214 microg/rat) and intravenously (49.5 microg/rat) into the jugular vein using a Vascular Access Port (VAP). Prior to and after drug administration, blood and CSF samples were taken and analysed by radioimmunoassay. The AUCCSF/AUCplasma ratio after nasal delivery does not differ from the ratio after intravenous infusion, indicating that hydroxocobalamin enters the CSF via the blood circulation across the blood-brain barrier (BBB). This same transport route is confirmed by the cumulative AUC-time profiles in CSF and plasma, demonstrating a 30 min delay between plasma absorption and CSF uptake of hydroxocobalamin in rats and in a comparative human study. The present results in rats show that there is no additional uptake of hydroxocobalamin in the CSF after nasal delivery compared to intravenous administration, which is in accordance with the results found in humans. This indicates a predictive value of the used rat model for the human situation when studying the nose to CSF transport of drugs.
Van den Berg MP, Merkus P, Romeijn SG, Verhoef JC, Merkus FW. Hydroxocobalamin uptake into the cerebrospinal fluid after nasal and intravenous delivery in rats and humans. J Drug Target. 2003 Jul;11(6):325-31. [PubMed Citation]
-
Hydroxocobalamin is a powerful cyanide antidote that prevents sodium nitroprusside-induced cyanide toxicity. The pharmacokinetics of an i.v. bolus of hydroxocobalamin (70 and 140 mg/kg) were studied in conscious dogs (n = 6). Plasma hydroxocobalamin concentrations were measured using derivative spectrophotometry. The pharmacokinetics were compatible with a two-compartment model with a first-order distribution and elimination rate, and pharmacokinetic parameters were not different between the two doses, except for the elimination half-life. At 70 mg/kg, which is the recommended dose in acute cyanide poisoning, the elimination half-life was 7.36 +/- 0.79 h, the volume of distribution was 0.49 +/- 0.10 L/kg, and the total clearance 0.58 +/- 0.11 L/h. At high doses, hydroxocobalamin has a short elimination half-life and a limited volume of distribution that exceeds blood volume. These results could be useful in elaborating guidelines for the administration of hydroxocobalamin, when repetitive bolus and/or continuous infusion is required.
de La Coussaye JE, Houeto P, Sandouk P, Levillain P, Sassine A, Riou B. Pharmacokinetics of hydroxocobalamin in dogs. J Neurosurg Anesthesiol. 1994 Apr;6(2):111-5. [PubMed Citation]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
Cyanide poisoning: the starting dose of hydroxocobalamin for adults is 5 g administered as an intravenous infusion over 15 minutes (approximately 15 mL/min). Administration of the entire vial constitutes a complete starting dose. Depending upon the severity of the poisoning and the clinical response, a second dose of 5 g may be administered by intravenous infusion for a total dose of 10 g. The rate of infusion for the second dose may range from 15 minutes (for patients in extremis) to two hours, as clinically indicated.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Children (FDA)
Cyanide poisoning: safety and effectiveness of Cyanokit have not been established in this population. In non-US marketing experience, a dose of 70 mg/kg has been used to treat pediatric patients.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Pregnancy (FDA)
Cyanide poisoning: Cyanokit should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Because cyanide readily crosses the placenta, maternal cyanide poisoning results in fetal cyanide poisoning. Timely treatment of the pregnant mother may be lifesaving for both mother and fetus.
US FDA Pregnancy Category C
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Nursing Mothers (FDA)
Cyanide poisoning: it is not known whether hydroxocobalamin is excreted in human milk. Cyanokit may be administered in life-threatening situations, and therefore, breast-feeding is not a contraindication to its use. Because of the unknown potential for adverse reactions in nursing infants, the patient should discontinue nursing after receiving Cyanokit.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Geriatric (FDA)
Cyanide poisoning: approximately 50 known or suspected cyanide poisoning victims aged 65 or older received hydroxocobalamin in clinical studies. In general, the safety and effectiveness of hydroxocobalamin in these patients was similar to that of younger patients. No adjustment of dose is required in elderly patients.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Renal Impairment (FDA)
Cyanide poisoning: the safety and effectiveness of Cyanokit have not been studied in patients with renal impairment.
Hydroxocobalamin and cyanocobalamin are eliminated unchanged by the kidneys. Oxalate crystals have been observed in the urine of both healthy subjects given hydroxocobalamin and patients treated with hydroxocobalamin following suspected cyanide poisoning
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Hepatic Impairment (FDA)
Cyanide poisoning: the safety and effectiveness of Cyanokit have not been studied in patients with hepatic impairment.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Hydroxocobalamin has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (Public Law 108-276).
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
6. Current available formulations/shelf life
Formulation:
Cyanokit (hydroxocobalamin for injection) 5 g for intravenous infusion consists of 1 vial, containing 5 g lyophilized hydroxocobalamin dark red crystalline powder for injection. After reconstitution, the vial contains hydroxocobalamin for injection, 25 mg/mL. Administration of the entire 5 g vial constitutes a complete starting dose.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Shelf life
Shelf life
3 years.
European Public Assessment Report (EPAR): Product Information (Cyanokit). Last updated May 2011 (EMA)
Stability
For the purpose of ambulatory use, Cyanokit may be exposed during short periods to the temperature variations of usual transport (15 days submitted to temperatures ranging from 5°C to 40°C), transport in the desert (4 days submitted to temperatures ranging from 5°C to 60°C) and freezing/defrosting cycles (15 days submitted to temperatures ranging from -20°C to 40°C). If these temporary conditions have been exceeded, the product should be discarded.
Chemical and physical in-use stability of the reconstituted solution with sodium chloride 9 mg/ml (0.9%) has been demonstrated for 6 hours at a temperature between 2°C and 40°C. From a microbiological point of view, the medicinal product should be used immediately. If not used immediately, in-use storage times and conditions prior to use are the responsibility of the user and would normally not be longer than 6 hours at 2°C to 8ºC.
European Public Assessment Report (EPAR): Product Information (Cyanokit). Last updated May 2011 (EMA)
Storage
Lyophilized form: Store at 25°C (77°F); excursions permitted to 15-30°C (59 to 86°F) [see USP Controlled Room Temperature].
Cyanokit may be exposed during short periods to the temperature variations of usual transport (15 days submitted to temperatures ranging from 5 to 40°C (41 to 104°F), transport in the desert (4 days submitted to temperatures ranging from 5 to 60°C (41 to 140°F)) and freezing/defrosting cycles (15 days submitted to temperatures ranging from -20 to 40°C (-4 to 104°F)).
Reconstituted solution: Store up to 6 hours at a temperature not exceeding 40ºC (104°F). Do not freeze. Discard any unused portion after 6 hours.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Hydroxocobalamin has been used to prevent and to treat cyanide toxicity associated with sodium nitroprusside.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.29-32
-
A patient committed suicide with hydrogen sulfide (H(2)S) by combining two commercial products. The patient was given hydroxocobalamin as an antidote in addition to treatment with cardiopulmonary resuscitation, but died approximately 42 min after his arrival at the hospital. The patient's cause of death was attributed to acute hydrogen sulfide poisoning. Serum concentrations of sulfide before and after administration of hydroxocobalamin were 0.22 and 0.11 μg/mL, respectively; serum concentrations of thiosulfate before and after hydroxocobalamin administration were 0.34 and 0.04 μmol/mL, respectively. Hydroxocobalamin is believed to form a complex with H(2)S in detoxification pathways of H(2)S. Although H(2)S is rapidly metabolized and excreted, the decreased sulfide concentration may be also associated with this complex formation. The decreased sulfide concentration suggests that hydroxocobalamin therapy may be effective for acute H(2)S poisoning. The decreased thiosulfate concentration seems to be associated with formation of a thiosulfate/hydroxocobalamin complex, because hydroxocobalamin can form a complex with thiosulfate. The thiosulfate concentration decreased to a greater extent than did sulfide, suggesting that hydroxocobalamin has a higher affinity for thiosulfate than for H(2)S. Therefore, prompt administration of hydroxocobalamin after H(2)S exposure may be effective for H(2)S poisoning.
Fujita Y, Fujino Y, Onodera M, Kikuchi S, Kikkawa T, Inoue Y, Niitsu H, Takahashi K, Endo S. A fatal case of acute hydrogen sulfide poisoning caused by hydrogen sulfide: hydroxocobalamin therapy for acute hydrogen sulfide poisoning. J Anal Toxicol. 2011 Mar;35(2):119-23. [PubMed Citation]
-
OBJECTIVE: To review the mechanism of action, safety, and efficacy of hydroxocobalamin in the treatment and prevention of nitroprusside-induced cyanide toxicity. DATA SOURCES: English and foreign-language journal articles and reference texts identified from Index Medicus. Both animal and human studies were included. DATA SYNTHESIS: High-dose or prolonged therapy with nitroprusside in patients with hepatic or renal dysfunction increases the risk for nitroprusside-induced cyanide or thiocyanate toxicity, respectively. Hydroxocobalamin has been shown to significantly reduce RBC and plasma cyanide concentrations in animals and surgical patients without producing clinically important adverse effects or toxic metabolites. Thiosulfate infusions also decrease cyanide toxicity but can cause accumulation of thiocyanate resulting in clinical toxicity. Cyanocobalamin cannot effectively remove cyanide due to poor binding. CONCLUSIONS: Hydroxocobalamin is a safe and effective agent in the prevention and treatment of nitroprusside-induced cyanide toxicity. Prolonged or high-dose infusions of nitroprusside should be minimized in critically ill patients, especially if hepatic and/or renal dysfunction is present.
Zerbe NF, Wagner BK. Use of vitamin B12 in the treatment and prevention of nitroprusside-induced cyanide toxicity. Crit Care Med. 1993 Mar;21(3):465-7. [PubMed Citation]
Children
-
Cyanide poisoning: usual dose - 70 mg/kg IV over 30 minutes as a single infusion; maximum dose - 5 g.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.29-32
8. Route of Administration/Monitoring
-
Intravenous infusion
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
The 5 g vial of hydroxocobalamin for injection is to be reconstituted with 200 mL of diluent (not provided with Cyanokit) using the supplied sterile transfer spike. The recommended diluent is 0.9% Sodium Chloride injection (0.9% NaCl). Lactated Ringers injection and 5% Dextrose injection (D5W) have also been found to be compatible with hydroxocobalamin and may be used if 0.9% NaCl is not readily available. The line on the vial label represents 200 mL volume of diluent. Following the addition of diluent to the lyophilized powder, the vial should be repeatedly inverted or rocked, not shaken, for at least 60 seconds prior to infusion.
Hydroxocobalamin solutions should be visually inspected for particulate matter and color prior to administration. If the reconstituted solution is not dark red or if particulate matter is seen after the solution has been appropriately mixed, the solution should be discarded
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
-
STUDY OBJECTIVE: Management of chemical weapon casualties includes the timely administration of antidotes without contamination of rescuers. Personal protective equipment makes intravenous access difficult but does not prevent intraosseous drug administration. We therefore measured the systemic bioavailability of antidotes for organophosphorus nerve agent and cyanide poisoning when administered by the intraosseous, intravenous, and intramuscular routes in a small study of Göttingen minipigs. METHODS: Animals were randomly allocated to sequentially receive atropine (0.12 mg/kg by rapid injection), pralidoxime (25 mg/kg by injection during 2 minutes), and hydroxocobalamin (75 mg/kg during 10 minutes) by the intravenous or intraosseous route, or atropine and pralidoxime by the intramuscular route. Plasma concentrations were measured for 6 hours to characterize the antidote concentration-time profiles for each route. RESULTS: Maximum plasma concentrations of atropine and pralidoxime occurred within 2 minutes when administered by the intraosseous route compared with 8 minutes by the intramuscular route. Maximum plasma hydroxocobalamin concentration occurred at the end of the infusion when administered by the intraosseous route. The mean area under the concentration-time curve by the intraosseous route was similar to the intravenous route for all 3 drugs and similar to the intramuscular route for atropine and pralidoxime. CONCLUSION: This study showed rapid and substantial antidote bioavailability after intraosseous administration that appeared similar to that of the intravenous route. The intraosseous route of antidote administration should be considered when intravenous access is difficult.
Murray DB, Eddleston M, Thomas S, Jefferson RD, Thompson A, Dunn M, Vidler DS, Clutton RE, Blain PG. Rapid and Complete Bioavailability of Antidotes for Organophosphorus Nerve Agent and Cyanide Poisoning in Minipigs After Intraosseous Administration. Ann Emerg Med. 2012 Jun 25. [Epub ahead of print] [PubMed Citation]
9. Adverse effects
-
Allergic Reaction: Use caution in the management of patients with known anaphylactic reactions to hydroxocobalamin or cyanocobalamin. Consideration should be given to use of alternative therapies, if available. Allergic reactions may include: anaphylaxis, chest tightness, edema, urticaria, pruritus, dyspnea, and rash. Allergic reactions including angioneurotic edema have also been reported in postmarketing experience.
-
Blood Pressure Increase: Many patients with cyanide poisoning will be hypotensive; however, elevations in blood pressure have also been observed in known or suspected cyanide poisoning victims.
Elevations in blood pressure (≥180 mmHg systolic or ≥110 mmHg diastolic) were observed in approximately 18% of healthy subjects (not exposed to cyanide) receiving hydroxocobalamin 5 g and 28% of subjects receiving 10 g. Increases in blood pressure were noted shortly after the infusions were started; the maximal increase in blood pressure was observed toward the end of the infusion. These elevations were generally transient and returned to baseline levels within 4 hours of dosing.
-
Photosensitivity: Hydroxocobalamin absorbs visible light in the UV spectrum. It therefore has potential to cause photosensitivity. While it is not known if the skin redness predisposes to photosensitivity, patients should be advised to avoid direct sun while their skin remains discolored
-
Erythema and Chromaturia: Patients should be advised that skin redness may last up to 2 weeks and urine coloration may last for up to 5 weeks after administration of Cyanokit. While it is not known if the skin redness predisposes to photosensitivity, patients should be advised to avoid direct sun while their skin remains discolored.
-
Rash: In some patients an acneiform rash may appear anywhere from 7 to 28 days following hydroxocobalamin treatment. This rash will usually resolve without treatment within a few weeks.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
-
Known hypersensitivity to hydroxocobalamin or vitamin B12 must be taken into benefit-risk consideration before administration of Cyanokit, since hypersensitive reactions may occur in patients receiving hydroxocobalamin.
European Public Assessment Report (EPAR): Product Information (Cyanokit). Last updated May 2011 (EMA)
-
A total of 347 subjects were exposed to hydroxocobalamin in clinical studies. Of these 347 subjects, 245 patients had suspected exposure to cyanide at the time of hydroxocobalamin administration. The remaining 102 subjects were healthy volunteers who had not been exposed to cyanide at the time of hydroxocobalamin administration.
Most patients will experience a reversible red colouration of the skin and mucous membranes that may last up to 15 days after administration of Cyanokit. All patients will show a dark red colouration of the urine quite marked during the three days following administration. Urine colouration may last up to 35 days after administration of Cyanokit.
List of adverse reactions: The following adverse reactions have been reported in association with Cyanokit use. However, because of the limitations of the available data, it is not possible to apply frequency estimations:
-
Blood and lymphatic system disorders - Decrease in the percentage of lymphocytes.
-
Immune system disorders - Allergic reactions including angioneurotic oedema, skin eruption, urticaria and pruritus.
-
Psychiatric disorders - Restlessness.
-
Nervous system disorders - Memory impairment; dizziness.
-
Eye disorders - Swelling, irritation, redness.
-
Cardiac disorders - Ventricular extrasystoles. An increase in heart rate was observed in cyanide-poisoned patients.
-
Vascular disorders - Transient increase in blood pressure, usually resolving within several hours; hot flush. A decrease in blood pressure was observed in cyanide-poisoned patients.
-
Respiratory, thoracic and mediastinal disorders - Pleural effusion, dyspnoea, throat tightness, dry throat, chest discomfort.
-
Gastrointestinal disorders - Abdominal discomfort, dyspepsia, diarrhoea, vomiting, nausea, dysphagia.
-
Skin and subcutaneous tissue disorders - Reversible red colouration of the skin and mucous membranes (see above). Pustular rashes, which may last for several weeks, affecting mainly the face and the neck.
-
Renal and urinary disorders - Chromaturia
-
General disorders and administration site conditions - Headache; injection site reaction; peripheral oedema.
-
European Public Assessment Report (EPAR): Product Information (Cyanokit). Last updated May 2011 (EMA)
10. Contraindication(s)
None.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues-
Title: Hydroxocobalamin and Rural Emergency Medical Services Cyanide Exposure Patients: A Cost Analysis
Condition: Cyanide Poisoning
Intervention: Drug: Cyanide antidote administration
ClinicalTrials.gov; Hydroxocobalamin and Rural Emergency Medical Services Cyanide Exposure Patients: A Cost Analysis (NIH)
-
Title: Development of a field-deployable device to rapidly measure blood cyanide levels
The objective of this proposal is to assess the feasibility of a fully integrated device for the rapid and early diagnosis of cyanide poisoning in whole blood using the spectral shift of the vitamin B12 precursor cobinamide upon binding with cyanide as an indicator. Cyanide is an extremely potent and rapid acting poison with as little as 50 mg fatal to humans. Currently there are no portable rapid tests for the detection of cyanide in whole blood available. The primary goal of this proposal is to demonstrate feasibility of the cobinamide-cyanide chemistry in a rapid test using a whole blood sample from a finger-stick. The total assay time from the sample collection to a valid result will be less than 5 minutes. The innovation in this proposal is to incorporate the cobinamide chemistry with blood separation technology, fluid path designs, and a detection area, all integrated in a compact, disposable device which can be interpreted with a handheld visualization system. Feasibility work of this novel rapid assay for detecting blood cyanide through cobinamide will initially focus on several technical approaches for the device design. Parallel paths to pursue a wet chemistry design, a design where the reagents are dried in the device, and a combination thereof will be considered, compared and evaluated. In all of the proposed methods the whole blood sample will be added to the device, followed by an integrated whole blood processing step to yield a plasma sample. Either prior to or immediately after the whole blood separation and lysis, the sample will be mixed with the on-board reagent, dried or wet, and possibly buffers. The processed sample-reagent mixture then moves through the device through various fluid paths to a detection area. To allow for sufficient mixing time between the sample and the reagents, different fluid paths, mixing chambers, polymeric time-gates, and dissolvable films will be evaluated with the goal of maximizing the interaction time. The spectral shift of cobinamide upon binding of cyanide to cobinamide will be measured in the detection area using a handheld or small, portable reader instrument. Upon successful completion of this Phase 1 project we intend to further develop our concept/prototype assay, complete the device design and integrate the finished test with a reader instrument through a Phase 2 proposal. Specific aims for a Phase 2 of this project include the validation of the cobinamide-based device for measuring cyanide in blood. Results will be compared to two established methods for measuring cyanide in biological samples. The sensitivity, specificity, accuracy, precision, and reproducibility of the devices will be determined. Additional studies will be designed to obtain regulatory approval and subsequently we will commercialize the test. If successful, this test will provide the medical community and first responders with a fast, reliable and economic alternative to determine cyanide poisoning. PUBLIC HEALTH RELEVANCE: Cyanide poisoning has been recognized as a threat from smoke inhalation and potentially through weapons of mass destruction, but there are currently no methods available to rapidly detect cyanide in blood from at-risk patients. The objective of the proposed work is to assess the feasibility of a fully integrated method for the rapid and early detection and diagnosis of cyanide poisoning in whole blood using the spectral shift of the vitamin B12 precursor cobinamide upon binding with cyanide as an indicator. Successful accomplishment of the goals of this project will be the basis for the development of a test to detect and measure cyanide blood poisoning which will allow healthcare providers to treat patients quickly and effectively.
RePORTER. NIH. Development of a field-deployable device to rapidly measure blood cyanide levels.
-
Title: Countermeasures against chemical threats: countermeasures against cyanide
Sources for potential exposure to cyanide of both civilians and military personnel include combustion of nitrogenous materials, commercial accidents and the deliberate release of a cyanogenic chemical. The relative ease in obtaining and releasing cyanide means that the risk of cyanide use in a terrorist attack resulting in a mass casualty situation should not be ignored. Rapid intervention is critical for effective medical intervention in cases of cyanide exposure: treatments require a "three minute solution."
The availability of a rapid, IM injectable antidote should meet this requirement. However, there are no such available treatments - the current cyanide antidotes are administered intravenously. Because intravenous administration is time consuming and requires well trained medical personnel mass exposure to cyanide would likely leave many victims untreated. Because IM administration can be performed via an autoinjector and can therefore be done rapidly by minimally trained personnel there is a critical need for a rapid-acting, IM-injectable antidote for the treatment of mass cyanide casualties. In order to address this need /the authors/ are advancing /a/preclinical lead, sulfanegen to clinical development. /They/ have previously demonstrated efficacy of sulfanegen in murine, swine and rabbit models of cyanide toxicity. In these models sulfanegen is effective in reversal of cyanide toxicity by IM injection and therefore should meet the three minute solution. /The authors/ have recently held a pre-IND meeting with the FDA and /their/ goal is to advance this cyanide antagonist to the clinic by validation of animal models, demonstrate efficacy of sulfanegen in these animal models and perform the required GLP pharmacokinetic and safety evaluation required for clinical trials. /The authors/ will then commence a Phase 1 human safety study while simultaneously performing the required GLP studies for NDA submission under the animal rule. Successful completion of the aims of this project should lead to a clinical countermeasure for cyanide toxicity that will be applicable in all cases of cyanide exposure from individuals to mass casualty settings. PUBLIC HEALTH RELEVANCE: /The authors/ have discovered a novel cyanide antidote that /they/ have named sulfanegen and demonstrated that it is more effective than existing antidotes in models of cyanide toxicity. /They/will perform the necessary studies to translate this antidote from the bench to the bedside. Successful completion of the goals of this project will therefore result in a clinical antidote that could be useful in the case of a terrorist attack involving cyanide.
RePORTER. NIH. Countermeasures against chemical threats: countermeasures against cyanide.
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations-
Identify appropriate pediatric dose.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Pediatric studies of hydroxocobalamin.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Route of administration/monitoring:
-
Need more sensitive method for measuring cyanide in blood
-
New technology may be available soon
-
Need to explore more routes of administration for example, IM
-
Need to explore safety of different concentrations
-
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Studies of hydroxocobalamin in lactating women.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
Obtain more pediatric data, for example, by contacting appropriate agents in France, where hydroxocobalamin is being given regularly to children.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Studies in the use of hydroxocobalamin in patients receiving nitroprusside.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Studies of pediatric fire victims
-
Hydroxocobalamin versus cyanide antidote kit
-
Need more sensitive measure of blood cyanide
-
Consider three-arm trial (one group receives all)
-
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Studies of hydroxocobalamin without sodium thiosulfate
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
A better understanding of the mechanism of action of cyanide at the molecular level is needed.
-
The use of antidotes to cyanide poisoning in patients exposed to other toxic hazards such as smoke or industrial chemicals needs further evaluation.
-
Models should be developed to characterize the long-term effects of acute exposure to cyanide and identify how cyanide-containing compounds are metabolized.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
15. Study-related ethical concerns
— including review panel recommendations-
May require input from EMS community.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
16. Global regulatory status
U.S.
Cyanokit is indicated for the treatment of known or suspected cyanide poisoning.
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
-
Pernicious anemia, both uncomplicated and accompanied by nervous system involvement.
-
Dietary deficiency of Vitamin B12, occurring in strict vegetarians and in their breast-fed infants. (Isolated vitamin B12 deficiency is very rare).
-
Malabsorption of vitamin B12, resulting from structural or functional damage to the stomach, where intrinsic factor is secreted or to the ileum, where intrinsic factor facilitates vitamin B12 absorption. These conditions include tropical sprue, and nontropical sprue (idiopathic steatorrhea, gluten-induced enteropathy). Folate deficiency in these patients is usually more severe than vitamin B12 deficiency.
-
Inadequate secretion of intrinsic factor, resulting from lesions that destroy the gastric mucosa (ingestion of corrosives, extensive neoplasia), and a number of conditions associated with a variable degree of gastric atrophy (such as multiple sclerosis, certain endocrine disorders, iron deficiency, and subtotal gastrectomy). Total gastrectomy always produces vitamin B12 deficiency. Structural lesions leading to vitamin B12 deficiency include regional ileitis, ileal resections, malignancies, etc.
-
Competition for Vitamin B12 by intestinal parasites or bacteria. The fish tapeworm (Diphyilobothrium latum) absorbs huge quantities of vitamin B12 and infested patients often have associated gastric atrophy. The blind-loop syndrome may produce deficiency of Vitamin B12 or folate.
-
Inadequate utilization of vitamin B12. This may occur if antimetabolites for the vitamin are employed in the treatment of neoplasia.
-
For the Schilling Test.
Product label: HYDROXOCOBALAMIN injection, solution [Watson Laboratories, Inc.] Last revised August 2011 [DailyMed]
E.U.
Hydroxocobalamin is used in the treatment of known or suspected cyanide poisoning in all age ranges.
European Public Assessment Report (EPAR): Product Information (Cyanokit). Last updated May 2011 (EMA)
Adult Dosage:
The initial dose of Cyanokit is 5 g (200 ml, complete volume of reconstituted solution).
Depending upon the severity of the poisoning and the clinical response, a second dose may be administered.
The subsequent dose of Cyanokit is 5 g (200 ml, complete volume of reconstituted solution).
The maximum total recommended dose is 10 g.
Children Dosage:
In infants to adolescents (0 to 18 years old), the initial dose of Cyanokit is 70 mg/kg body weight not exceeding 5 g.
| Body weight in kg | 5 | 10 | 20 | 30 | 40 | 50 | 60 |
|---|---|---|---|---|---|---|---|
| Initial dose | |||||||
| in g | 0.35 | 0.70 | 1.40 | 2.10 | 2.80 | 3.50 | 4.20 |
| in ml | 14 | 28 | 56 | 84 | 112 | 140 | 168 |
Depending upon the severity of the poisoning and the clinical response (see section 4.4), a second dose may be administered.
In infants to adolescents (0 to 18 years old), the subsequent dose of Cyanokit is 70 mg/kg body weight not exceeding 5 g.
Pregnancy Dosage:
There are no adequate data from the use of hydroxocobalamin in pregnant women and the potential risk for humans is unknown.
However, taken into account:
-
that no more than two injections of hydroxocobalamin are to be administered,
-
the potentially life threatening condition,
-
the lack of alternative treatment,
hydroxocobalamin may be given to a pregnant woman.
In case of known pregnancy at the time of treatment with Cyanokit or in case that pregnancy becomes known after treatment with Cyanokit, health care professionals are requested to promptly report the exposure during pregnancy to the Marketing Authorisation Holder and to carefully follow-up on the pregnancy and its outcome.
Nursing Mothers Dosage:
Because hydroxocobalamin will be administered in potentially life-threatening situations, breastfeeding is not a contraindication to its use. In the absence of data in breast-fed infants, breast-feeding discontinuation is recommended after receiving Cyanokit.
Geriatric Dosage:
Approximately 50 known or suspected cyanide victims aged 65 or older received hydroxocobalamin in clinical studies. In general, the effectiveness of hydroxocobalamin in these patients was similar to that of younger patients.
Renal and Hepatic Impairment Dosage:
Although the safety and efficacy of hydroxocobalamin have not been studied in renal and hepatic impairments, Cyanokit is administered as emergency therapy in an acute, life-threatening situation only and no dose adjustment is required in these patients.
European Public Assessment Report (EPAR): Product Information (Cyanokit). Last updated May 2011 (EMA)
Other
Cyanokit has marketing authorisations in France (1996), Hong Kong (1999) and USA (2006). Cyanokit has been distributed, based on the French marketing authorisation, in 37 other countries (18 EU member states and 19 countries outside Europe).
European Public Assessment Report (EPAR): Scientific Discussion (Cyanokit). Last updated May 2011 (EMA)
17. Other potentially useful information
-
Sources of cyanide exposure are many, including combustion of plastic and vinyl, such as in a house fire, laboratory or industrial exposures including exposure in the electroplating industry both of printed circuit boards and in jewelry work. Rapid and definitive diagnosis of cyanide poisoning is unavailable in the emergency department setting. It is desirable to make a definitive diagnosis in order to prevent potential complications of empiric treatment of presumptive cyanide poisoning from the cyanide antidote kit currently approved by the US Food and Drug Administration (FDA). We investigated a technique to detect cyanide currently utilized by water treatment facilities to determine if it can be applied to rapidly detect concentrations of cyanide in the clinically important range. Varying standardized dilutions of KCN ranging from 0.25 microg/mL to 30 microg/mL were acidified with a drop of sulphuric acid in a closed system under a ventilation hood. Cyantesmo test strips were placed into the test tubes above the fluid level where liberated HCN gas interacted with the test strip to effect a color change. Color changes were compared to negative controls and to each other. The test strips demonstrated an incrementally increasing deep blue color change over a progressively longer portion of the test strip in less than 5 minutes for each concentration of KCN including 1, 3, 10, and 30 microg/mL. The concentrations of 0.25, 0.5, and 0.75 required more than 2 hours to begin demonstration of any color change. The Cyantesmo test strips accurately and rapidly detected, in a semi-quantifiable manner, concentrations of CN greater than 1 microg/mL contained in each test sample. Future work to validate this test in blood and in clinical specimens is planned.
Rella J, Marcus S, Wagner BJ. Rapid cyanide detection using the Cyantesmo kit. J Toxicol Clin Toxicol. 2004; 42(6):897-900. [PubMed Citation]
-
Exposure to cyanide can occur in a variety of ways, including exposure to smoke from cigarettes or fires, accidental exposure during industrial processes, and exposure from the use of cyanide as a poison or chemical warfare agent. Confirmation of cyanide exposure is difficult because, in vivo, cyanide quickly breaks down by a number of pathways, including the formation of both free and protein-bound thiocyanate. A simple method was developed to confirm cyanide exposure by extraction of protein-bound thiocyanate moieties from cyanide-exposed plasma proteins. Thiocyanate was successfully extracted and subsequently derivatized with pentafluorobenzyl bromide for GC-MS analysis. Thiocyanate levels as low as 2.5 ng mL(-1) and cyanide exposure levels as low as 175 μg kg(-1) were detected. Samples analyzed from smokers and non-smokers using this method showed significantly different levels of protein-bound thiocyanate (p<0.01). These results demonstrate the potential of this method to positively confirm chronic cyanide exposure through the analysis of protein-bound cyanide in human plasma.
Youso SL, Rockwood GA, Lee JP, Logue BA. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2010 Sep 10;677(1):24-8. [PubMed Citation]
-
Diaquacobinamide (H(2)O)(2)Cbi(2+) or its conjugate base hydroxyaquacobinamide (OH(H(2)O)Cbi(+))) can bind up to two cyanide ions, making dicyanocobinamide. This transition is accompanied by a significant change in color, previously exploited for cyanide determination. The reagent OH(H(2)O)Cbi(+) is used in excess; when trace amounts of cyanide are added, CN(H(2)O)Cbi(+) should be formed. But the spectral absorption of CN(H(2)O)Cbi(+) is virtually the same as that of OH(H(2)O)Cbi(+). It has been inexplicable how trace amounts of cyanide are sensitively measured by this reaction. It is shown here that even with excess OH(H(2)O)Cbi(+), (CN)(2)Cbi is formed first due to kinetic reasons; this only slowly forms CN(H(2)O)Cbi(+). This understanding implies that CN(H(2)O)Cbi(+) will itself be a better reagent. We describe a single valve merging zone flow analyzer that allows both sample and reagent economy. With a 50cm liquid core waveguide (LCW) flow cell and an inexpensive fiber optic - charge coupled device array spectrometer, a S/N=3 limit of detection of 8nM, a linear dynamic range to 6μM, and excellent precision (RSD 0.49% and 1.07% at 50 and 100nM, respectively, n=5 each) are formed. At 1% carryover, sample throughput is 40h(-1). The setup is readily used to measure thiocyanate with different reagents. We demonstrate applicability to real samples by analyzing human saliva samples and hydrolyzed extracts of apple seeds, peach pits, and almonds.
Ma J, Dasgupta PK, Zelder FH, Boss GR. Cobinamide chemistries for photometric cyanide determination. A merging zone liquid core waveguide cyanide analyzer using cyanoaquacobinamide. Anal Chim Acta. 2012 Jul 29;736:78-84. [PubMed Citation]
-
When cyanide is introduced into the body, it quickly transforms through a variety of chemical reactions, normally involving sulfur donors, to form more stable chemical species. Depending on the nature of the sulfur donor, cyanide may be transformed into free thiocyanate, the major metabolite of cyanide transformation, 2-amino-2-thiazoline-4-carboxylic acid or protein-bound thiocyanate (PB-SCN) adducts. Because protein adducts are generally stable in biological systems, it has been suggested that PB-SCN may have distinct advantages as a marker of cyanide exposure. In this study, plasma was analyzed from 25 smokers (chronic low-level cyanide exposure group) and 25 non-smokers for PB-SCN. The amount of PB-SCN found in the plasma of smokers, 1.35 μM, was significantly elevated (p < 0.0001) when compared to non-smokers, 0.66 μM. Differences in sub-groups of smokers and non-smokers were also evaluated. The results of this study indicate the effectiveness of analyzing PB-SCN in determining instances of chronic cyanide exposure with possible extension to confirmation of acute cyanide exposure.
Youso SL, Rockwood GA, Logue BA. The analysis of protein-bound thiocyanate in plasma of smokers and non-smokers as a marker of cyanide exposure. J Anal Toxicol 2012 May;36(4):265-9. [PubMed Citation]
-
The duration of action of the cyanide antidotes sodium thiosulphate and hydroxocobalamin was investigated in guinea-pigs after prophylactic administration before a long-term infusion of NaCN. The parameter for the diminution of the antidote action was the point of time at which the concentration of HCN in the exhaled air of the animals exceeded 100 nmol/kg per min. The time taken to reach this threshold level in the control animals was 12 min. While the threshold level could be extended only to 35 min with hydroxocobalamin (300 mg/kg i.v.) the protective action of sodium thiosulphate (100, 500 and 1000 mg/kg i.v.) persisted dose dependently for about 1, 2 and 4 h, respectively. Additionally we found a plasma half-life of sodium thiosulphate in guinea-pigs of 26 min. This value corresponds approximately to the plasma half-life of sodium thiosulphate in humans given in the literature. Because of the large injection volume necessary, sodium thiosulphate is not suitable for prophylactic use in man.
Mengel, K., Krämer W, Isert B, Friedberg KD. Thiosulphate and hydroxocobalamin prophylaxis in progressive cyanide poisoning in guinea-pigs. Toxicology. 1989 Mar;54(3):335-42. [PubMed Citation]
-
BACKGROUND: Antidotal doses of hydroxocobalamin are associated with transient increases in blood pressure in some animals and humans. These studies in anesthetized rabbits were undertaken to explore the possible mechanisms underlying the hemodynamic effects of hydroxocobalamin by investigating 1) possible hemodynamic effects of cyanocobalamin, which is formed on a molar-to-molar basis when hydroxocobalamin binds cyanide, and 2) the interference of hydroxocobalamin with the endothelial nitric oxide system. METHODS: Study 1 investigated the hemodynamic effects of cyanocobalamin. This study included two treatment arms: 1) cyanocobalamin (75 mg/kg, IV) followed by saline (n = 7) and 2) saline followed by cyanocobalamin (n = 7). Study 2 assessed the hemodynamic effects of hydroxocobalamin (75 mg/kg, IV) in the presence and absence of the nitric oxide synthase inhibitor L-Nomega-nitro-L-arginine methyl ester (L-NAME; 30 mg/kg, IV). Nitric oxide synthase inhibition itself increases blood pressure. Thus, as part of Study 2, the hemodynamic effects of hydroxocobalamin were also investigated in the presence of an equipressor dose of angiotensin II (ANGII; 0.05 microg/kg/min, IV) in order to determine whether elevated blood pressure per se could interfere with hydroxocobalamin's hemodynamic effects. This study included six treatment arms (designated as first treatment + second treatment): saline + saline (n = 5), L-NAME + saline (n = 7), saline + hydroxocobalamin (n = 7), L-NAME + hydroxocobalamin (n = 7), ANGII + hydroxocobalamin (n = 7), and ANGII + saline (n = 7). RESULTS: In Study 1, the effects of cyanocobalamin on hemodynamic parameters were indistinguishable from those of saline. In Study 2, hydroxocobalamin infusion was associated with moderate hemodynamic effects, including an increase in systemic vascular resistance, an increase in blood pressure, and a decrease in cardiac output. Administration of L-NAME abolished the effects of hydroxocobalamin on all hemodynamic parameters. ANGII at a dose producing a pressor response comparable to that of L-NAME did not influence the hydroxocobalamin-associated hemodynamic changes. CONCLUSION: These studies in anesthetized rabbits demonstrate that the moderate pressor effect of hydroxocobalamin is not related to the formation of cyanocobalamin but is very likely related to the scavenging of nitric oxide by hydroxocobalamin.
Gerth K, Ehring T, Braendle M, Schelling P. Nitric oxide scavenging by hydroxocobalamin may account for its hemodynamic profile. Clin Toxicol (Phila). 2006;44 Suppl 1:29-36. [PubMed Citation]
-
BACKGROUND: Hydroxocobalamin (OHCbl) and cyanocobalamin (CNCbl) are dark-red colored analogs of vitamin B-12. OHCbl is used as an alternate antidote for cyanide poisoning. Due to the strong red color, if uncorrected, these cobalamins interfere with hemoglobin measurements and can introduce errors in spectrophotometric assay on co-oximeters. The impact of cobalamins on commonly used co-oximetry systems was compared to evaluate the accuracy of hemoglobin measurements and to further assess the ability of the instruments to detect and flag cobalamin interference. METHODS: Blood samples spiked with varying concentrations of hydroxocobalamin or cyanocobalamin (0-2 g/l), were measured on 3 GEM Premier 4000 s, 3 IL 682 CO-Oximeter, 1 Radiometer ABL 735 and 1 Siemens Rapidpoint 405 analyzer. The effect of OHCbl or CNCbl interference on total hemoglobin and hemoglobin derivatives is evaluated using the measured difference between the control sample and the spiked sample. RESULTS: Spectral measurements, blood measurements and delta spectral data confirmed that hemoglobin measurement accuracy is affected by the presence of OHCbl or CNCbl and that the source of interference is from errors in measurement algorithms, and not due to cobalamin induced hemoglobin changes. CONCLUSIONS: Among the 4 co-oximeters tested in this evaluation, GEM Premier 4000 and Rapidpoint 405 analyzer showed minimal impact for hydroxocobalamin concentrations around 0.5 g/l. Cyanocobalamin displayed similar interference effect on co-oximetry measurements as OHCbl. The error detection system in the GEM Premier 4000 appropriately detected and flagged interferences on sample measurements.
Pamidi PV, DeAbreu M, Kim D, Mansouri S. Hydroxocobalamin and cyanocobalamin interference on co-oximetry based hemoglobin measurements. Clin Chim Acta. 2009 Mar;401(1-2):63-7. [PubMed Citation]
-
BACKGROUND: Hydroxocobalamin has been shown to be a rapid and powerful antidote in acute cyanide poisoning and to prevent cyanide poisoning during sodium nitroprusside administration. This cobalt-containing compound has been shown to be devoid of significant immediate side effects during acute administration. However, its potential delayed toxicity related to cobalt accumulation in tissue remains unknown. Therefore, we evaluated the toxicity of hydroxocobalamin as compared with that of cobalt salts on rat cardiac and diaphragmatic muscles. METHODS: For a 21-day period, rats were treated intraperitoneally with either hydroxocobalamin (70 mg kg-1 per day, n = 14), cobalt chloride hexahydrate (12 mg kg-1 per day, n = 14) or saline (n = 10). Hydroxocobalamin and cobalt chloride groups received equimolar doses of cobalt. We studied: (1) the mechanical properties of isolated left ventricular papillary muscles and diaphragmatic strips, (2) the cardiac and diaphragmatic cobalt tissue concentrations, and (3) the myocardial histological aspect. RESULTS: During the study period, no significant increase in body weight was noted in the cobalt-treated group (-4 +/- 1%), which was in contrast to the hydroxocobalamin-treated group (+21 +/- 2%) and the saline-treated group (22 +/- 2%). Compared with controls, the mechanical properties of cardiac and diaphragmatic muscles were unchanged after either hydroxocobalamin or cobalt salt treatments, and myocardial histological characteristics were similar in all groups. Conversely, large amounts of cobalt deposit were observed in the cobalt-treated group in both the diaphragm (41.90 +/- 16.30 vs 0.70 +/- 0.40 mumol mug-1 in the control group, P < 0.001) and the myocardium (16.90 +/- 6.40 vs 0.14 +/- 0.01 mumol mug-1 in the control group, P < 0.001). After hydroxocobalamin administration, cobalt concentrations were significantly lower in the diaphragm (25.10 +/- 16.50 mu mol mug-1, P < 0.001 vs cobalt-treated group) and the myocardium (4.50 +/- 1.20 mumol mug, P < 0.001 vs cobalt-treated group). CONCLUSION: These results indicate that repeated administration of hydroxocobalamin was devoid of significant diaphragmatic and cardiac muscle toxicity and therefore remains a safe antidote for acute cyanide poisoning.
Pery-Man N, Houeto P, Coirault C, Suard I, Perennec J, Riou B, Lecarpentier Y. Hydroxocobalamin vs cobalt toxicity on rat cardiac and diaphragmatic muscles. Intensive Care Med. 1996 Feb;22(2):108-15. [PubMed Citation]
-
On October 27, 2011, Mederidian Medical Technologies announced the launch of a new single-vial dose of cyanokit. The new presentation of cyanokit will have the whole starting dose of 5 g in a single vial replacing the previous kit consisting of two 2.5 g vials. According to the press release, in addition to the full starting dose of cyanokit contained in one vial, the new packaging is more compact than the previous presentation. The new dose also features a 20% increase in shelf life (36 months from the date of formulation).
Merdian Medical Technologies (For Immediate Release), CYANOKIT Treatment for Known or Suspected cyanide Poisoning Now Available in 5 g Single-Vial Dose, Replacing Two, 2.5 g Vial Dose October 27, 2011
-
Sodium nitroprusside (SNP) and hydroxocobalamin (HC) - in molar ratios of 1:4, 1:5, and 1:8, respectively - were infused simultaneously during 4 h into two veins of separate ears of conscious rabbits. Controls received HC only. Sodium thiosulphate (ST) was infused with SNP at molar ratios of 1:4, 1:5, and 1:10. The observation period was 48 h. With the lowest dose of HC (1:4), SNP produced a severe metabolic acidosis; three of ten animals died during the infusion, an additional six within 24 h. When the 1:5 ratio was administered, the acidosis was less marked, but still three of seven animals succumbed within 24 h. The highest dose (1:8) prevented acidosis, however three of eight animals died. All doses of HC caused histological changes in the liver, the myocardium, and the kidney, independently if given alone or with SNP. In contrast to this, ST had a complete antidote effect, if administered in a 1:5 ratio; no acidosis was demonstrable and death did not occur. In neither dosage ST could prevent histological changes in the liver, but the kidney and the heart were not affected. In contrast to HC ST alone did not cause histological alterations. Consequently, ST is the preferable antidote and is superior to HC for preventing or treating intoxications with SNP.
Höbel M, Engeser P, Nemeth L, Pill J. The antidote effect of thiosulphate and hydroxocobalamin in formation of nitroprusside intoxication of rabbits. Arch Toxicol. 1980 Dec;46(3-4):207-13. [PubMed Citation]
-
The US government considers cyanide to be among the most likely agents of chemical terrorism. Cyanide differs from many other biological or chemical agents for which little or no defense is available because its individual and public health effects are largely remediable through appropriate preparedness and response. Because the toxicity of the cyanide antidote currently available in the United States renders it ill-suited for use in terrorist incidents and other situations requiring rapid out-of-hospital treatment, hydroxocobalamin--an effective and safe cyanide antidote being used in other countries--has been introduced in the United States. Unlike the other available cyanide antidote, hydroxocobalamin can be administered at the scene of a cyanide disaster, and it need not be reserved for cases of confirmed cyanide poisoning but can be administered in cases of suspected poisoning. Both of these attributes facilitate the rapid intervention necessary for saving lives. To realize the potential benefits of hydroxocobalamin, progress also needs to be realized in other aspects of readiness, including but not limited to developing plans for ensuring local and regional availability of antidote, educating emergency responders and health care professionals in the recognition and management of cyanide poisoning, and raising public awareness of the potential for a chemical weapons attack and of how to respond.
Eckstein M. Enhancing public health preparedness for a terrorist attack involving cyanide. J Emerg Med. 2008 Jul;35(1):59-65. [PubMed Citation]
-
Hydroxocobalamin will lower blood cyanide concentrations. While determination of blood cyanide concentration is not required and must not delay treatment with hydroxocobalamin, it may be useful for documenting cyanide poisoning. If a cyanide blood level determination is planned, it is recommended to draw the blood sample before initiation of treatment with Cyanokit.
European Public Assessment Report (EPAR): Product Information (Cyanokit). Last updated May 2011 (EMA)
-
Because of its deep red color, hydroxocobalamin may cause haemodialysis machines to shut down due to an erroneous detection of a 'blood leak'. This should be considered before haemodialysis is initiated in patients treated with hydroxocobalamin.
European Public Assessment Report (EPAR): Product Information (Cyanokit). Last updated May 2011 (EMA)
-
Cyanokit was approved by the Food and Drug Administration on December 15, 2006 for the treatment of known or suspected cyanide poisoning. Cyanokit received a priority review and was approved under the Animal Efficacy Rule, which allows use of animal data for evidence of a drug's effectiveness for certain conditions when the drug cannot be ethically or feasibly tested in humans.
FDA News Release: FDA Approves Drug to Treat Cyanide Poisoning. December 15, 2006 (FDA)
-
STUDY HYPOTHESIS: Concentrated aqueous solutions of hydroxocobalamin (OHCob) are given intravenously for the treatment of cyanide poisoning. Because OHCob solutions are intensely red and have peak light absorptions at 352 nm and 525 nm, we investigated whether the presence of OHCob in serum would interfere with various automated, colorimetric chemistry measurements. DESIGN: Selected serum chemistry colorimetric measurements were compared in seven patients, using their own serum as control, with serum containing OHCob at the following concentrations: 100 mg/L, 500 mg/L, and 1,000 mg/L. These concentrations are in the range achieved with therapeutic doses of OHCob when given for cyanide poisoning. MEASUREMENTS AND MAIN RESULTS: Statistically significant alterations in serum values for aspartate aminotransferase, total bilirubin, creatinine, magnesium, and iron were seen in the presence of OHCob. CONCLUSION: The presence of OHCob in serum interferes with several chemistry methodologies, and such interference should be anticipated when this antidote is used.
Curry SC, Connor DA, Raschke RA. Effect of the cyanide antidote hydroxocobalamin on commonly ordered serum chemistry studies. Ann Emerg Med. 1994 Jul;24(1):65-7. [PubMed Citation]
-
Octanol/Water Partition Coefficient: log Kow = 5.28 (est)
HSDB. Hydroxocobalamin
18. Publications
Akyildiz BN, Kurtoğlu S, Kondolot M, Tunç A. Cyanide poisoning caused by ingestion of apricot seeds. Ann Trop Paediatr. 2010;30(1):39-43. [PubMed Citation]
Astier A, Baud FJ. Complexation of intracellular cyanide by hydroxocobalamin using a human cellular model. Hum Exp Toxicol. 1996 Jan;15(1):19-25. [PubMed Citation]
Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier S, Bush A. Hydroxocobalamin and Sodium Thiosulfate Versus Sodium Nitrite and Sodium Thiosulfate in the Treatment of Acute Cyanide Toxicity in a Swine (Sus scrofa) Model. Ann Emerg Med. 2010 Apr;55:345-351. [PubMed Citation]
Bebarta VS, Pitotti RL, Dixon PS, Valtier S, Esquivel L, Bush A, Little CM. Hydroxocobalamin and Epinephrine Both Improve Survival in a Swine Model of Cyanide-Induced Cardiac Arrest. Ann Emerg Med. 2012 Mar 14. [Epub ahead of print] [PubMed Citation]
Bebarta VS, Pitotti RL, Dixon P, Lairet JR, Bush A, Tanen DA. Hydroxocobalamin versus sodium thiosulfate for the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. Ann Emerg Med. 2012 Jun;59(6):532-9. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Borron SW, Srtonerook M, Reid F. Efficacy of hydroxocobalamin for the treatment of acute cyanide poisoning in adult beagle dogs; Clin Toxicol (Phil). 2006; 44 Suppl 1: 5-15. [PubMed Citation]
Borron SW, Baud FJ, Megarbane B, Bismuth C. Hydroxocobalamin for Severe Acute Cyanide Poisoning by Ingestion or Inhalation; Am J Emerg Med. 2007 Jun;25(5):551-8. [PubMed Citation]
Borron SW, Baud FJ, Barriot P, Imbert M, Bismuth C. Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med. 2007 Jun;49(6):794-801. [PubMed Citation]
Borron SW, Baud FJ. Antidotes for acute cyanide poisoning. Curr Pharm Biotechnol. 2012 Aug 1;13(10):1940-8. [PubMed Citation]
Cescon DW, Juurlink DN. Discoloration of skin and urine after treatment with hydroxocobalamin for cyanide poisoning. CMAJ. 2009 Jan 20;180(2):251. [PubMed Citation]
Cigolini D, Ricci G, Zannoni M, Codogni R, De Luca M, Perfetti P, Rocca G. Hydroxocobalamin treatment of acute cyanide poisoning from apricot kernels. Emerg Med J. 2011 Sep;28(9):804-5. [PubMed Citation]
ClinicalTrials.gov; Hydroxocobalamin and Rural Emergency Medical Services Cyanide Exposure Patients: A Cost Analysis (NIH)
Coentrão L, Moura D. Acute cyanide poisoning among jewelry and textile industry workers. Am J Emerg Med. 2011 Jan;29(1):78-81. [PubMed Citation]
Curry SC, Connor DA, Raschke RA. Effect of the cyanide antidote hydroxocobalamin on commonly ordered serum chemistry studies. Ann Emerg Med. 1994 Jul;24(1):65-7. [PubMed Citation]
Dart RC. Hydroxocobalamin for acute cyanide poisoning: new data from preclinical and clinical studies; new results from the prehospital emergency setting. Clin Toxicol (Phila). 2006;44 Suppl 1:1-3. (Abstract not available)
de La Coussaye JE, Houeto P, Sandouk P, Levillain P, Sassine A, Riou B. Pharmacokinetics of hydroxocobalamin in dogs. J Neurosurg Anesthesiol. 1994 Apr;6(2):111-5. [PubMed Citation]
DesLauriers CA, Burda AM, Wahl M. Hydroxocobalamin as a cyanide antidote. Am J Ther. 2006 Mar-Apr;13(2):161-5. (Abstract not available)
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
Eckstein M. Enhancing public health preparedness for a terrorist attack involving cyanide. J Emerg Med. 2008 Jul;35(1):59-65. [PubMed Citation]
European Public Assessment Report (EPAR): Product Information (Cyanokit). Last updated May 2011 (EMA)
European Public Assessment Report (EPAR): Scientific Discussion (Cyanokit). Last updated May 2011 (EMA)
FDA News Release: FDA Approves Drug to Treat Cyanide Poisoning. December 15, 2006 (FDA)
Forsyth JC, Mueller PD, Becker CE, Osterloh J, Benowitz NL, Rumack BH, Hall AH. Hydroxocobalamin as a cyanide antidote: safety, efficacy and pharmacokinetics in heavily smoking normal volunteers J Toxicol Clin 1993; Toxicol 31: 277-94. [PubMed Citation]
Fortin JL, Giocanti JP, Ruttimann M, Kowalski JJ. Prehospital administration of hydroxocobalamin for smoke inhalation - associated cyanide poisoning: 8 years of experience in the Paris Fire Brigade; Clin Toxicol. 2006; 44 (Suppl 1): 37-44. [PubMed Citation]
Fortin JL, Desmettre T, Manzon C, Judic-Peureux V, Peugeot-Mortier C, Giocanti JP, Hachelaf M, Grangeon M, Hostalek U, Crouzet J, Capellier G. Cyanide poisoning and cardiac disorders: 161 cases. J Emerg Med. 2010 May;38(4):467-76. [PubMed Citation]
Fortin JL, Waroux S, Giocanti JP, Capellier G, Ruttimann M, Kowalski JJ. Hydroxocobalamin for poisoning caused by ingestion of potassium cyanide: a case study. J Emerg Med 2010 Sep;39(3):320-4. [PubMed Citation]
Fortin JL, Baud FJ, Astier A, Barriot P, Lecarpentier Y. Hydrogen cyanide poisoning in a prison environment: a case report. J Correct Health Care. 2011 Jan;17(1):29-33. [PubMed Citation]
Fujita Y, Fujino Y, Onodera M, Kikuchi S, Kikkawa T, Inoue Y, Niitsu H, Takahashi K, Endo S. A fatal case of acute hydrogen sulfide poisoning caused by hydrogen sulfide: hydroxocobalamin therapy for acute hydrogen sulfide poisoning. J Anal Toxicol. 2011 Mar;35(2):119-23. [PubMed Citation]
Geller RJ, Barthold C, Saiers JA, Hall AH. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006 Nov; 118 (5): 2146-58. [PubMed Citation]
Gerth K, Ehring T, Braendle M, Schelling P. Nitric oxide scavenging by hydroxocobalamin may account for its hemodynamic profile. Clin Toxicol (Phila). 2006;44 Suppl 1:29-36. [PubMed Citation]
Guidotti T. Acute cyanide poisoning in prehospital care: new challenges, new tools for intervention. Prehosp Disaster Med. 2006 Mar-Apr;21(2):s40-8. [PubMed Citation]
Hall AH, Dart R, Bogdan G. Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning? Ann Emerg Med. 2007 Jun;49(6):806-13 [PubMed Citation]
Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol 2009;39(7):541-52. [PubMed Citation]
Hamel J; A Review of acute cyanide poisoning with a treatment update. Critical Care Nurse. 2011 Feb;31(1):72-82. [PubMed Citation]
Höbel M, Engeser P, Nemeth L, Pill J. The antidote effect of thiosulphate and hydroxocobalamin in formation of nitroprusside intoxication of rabbits. Arch Toxicol. 1980 Dec;46(3-4):207-13. [PubMed Citation]
Houeto P, Borron SW, Sandouk P, Imbert M, Levillain P, Baud FJ. Pharmacokinetics of hydroxocobalamin in smoke inhalation victims. J Toxicol Clin Toxicol. 1996;34(4):397-404. [PubMed Citation]
HSDB. Hydroxocobalamin
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.29-32
Kaya A, Okur M, Üstyol L, Temel H, Çaksen H. Acute cyanide poisoning after eating apricot pits: A case report. Turk Pediatri Arsivi. 2012 47(2):141-2.
Keim ME. Terrorism involving cyanide: the prospect of improving preparedness in the prehospital setting. Prehosp Disaster Med. 2006 Mar-Apr;21(2):s56-60. [PubMed Citation]
Kovalevsky P. Bengler C.
Cyanide intoxication after ingesting bitter almond. Case
report and review.
Reanimation Soins Intensifs Medecine d'Urgence. 1996
12(3):149-153.
Ma J, Dasgupta PK, Zelder FH, Boss GR. Cobinamide chemistries for photometric cyanide determination. A merging zone liquid core waveguide cyanide analyzer using cyanoaquacobinamide. Anal Chim Acta. 2012 Jul 29;736:78-84. [PubMed Citation]
Mannaioni G, Vannacci A, Marzocca C, Zorn AM, Peruzzi S, Moroni F. Acute cyanide intoxication treated with a combination of hydroxycobalamin, sodium nitrite, and sodium thiosulfate. J Toxicol Clin Toxicol. 2002;40(2):181-3. [PubMed Citation]
Mégarbane B, Delahaye A, Goldgran-Tolédano D, Baud FJ. Antidotal treatment of cyanide poisoning. J Chin Med Assoc. 2003 Apr;66(4): 193-203. [PubMed Citation]
Mengel, K., Krämer W, Isert B, Friedberg KD. Thiosulphate and hydroxocobalamin prophylaxis in progressive cyanide poisoning in guinea-pigs. Toxicology. 1989 Mar;54(3):335-42. [PubMed Citation]
Merdian Medical Technologies (For Immediate Release), CYANOKIT Treatment for Known or Suspected cyanide Poisoning Now Available in 5 g Single-Vial Dose, Replacing Two, 2.5 g Vial Dose October 27, 2011
Murray DB, Eddleston M, Thomas S, Jefferson RD, Thompson A, Dunn M, Vidler DS, Clutton RE, Blain PG. Rapid and Complete Bioavailability of Antidotes for Organophosphorus Nerve Agent and Cyanide Poisoning in Minipigs After Intraosseous Administration. Ann Emerg Med. 2012 Jun 25. [Epub ahead of print] [PubMed Citation]
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1695-1697
Pamidi PV, DeAbreu M, Kim D, Mansouri S. Hydroxocobalamin and cyanocobalamin interference on co-oximetry based hemoglobin measurements. Clin Chim Acta. 2009 Mar;401(1-2):63-7. [PubMed Citation]
Pery-Man N, Houeto P, Coirault C, Suard I, Perennec J, Riou B, Lecarpentier Y. Hydroxocobalamin vs cobalt toxicity on rat cardiac and diaphragmatic muscles. Intensive Care Med. 1996 Feb;22(2):108-15. [PubMed Citation]
Posner MA et al; Hydroxocobalamin Therapy of Cyanide Intoxication in Guinea Pigs. Anesthesiology. 1976; 44 (2): 157-60. [PubMed Citation]
Product label: CYANOKIT (hydroxocobalamin) injection, powder, lyophilized, for solution [Meridian Medical Technologies, Inc.] Last revised: April 2011 [DailyMed]
Product label: HYDROXOCOBALAMIN injection, solution [Watson Laboratories, Inc.] Last revised August 2011 [DailyMed]
Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC; Australian Resuscitation Council. Review article: management of cyanide poisoning. Emerg Med Australas. 2012 Jun;24(3):225-38. [PubMed Citation]
Rella J, Marcus S, Wagner BJ. Rapid cyanide detection using the Cyantesmo kit. J Toxicol Clin Toxicol. 2004; 42(6):897-900. [PubMed Citation]
RePORTER. NIH. Countermeasures against chemical threats: countermeasures against cyanide.
RePORTER. NIH. Development of a field-deployable device to rapidly measure blood cyanide levels.
Riou B, Baud FJ, Astier A, Barriot P, Lecarpentier Y. In vitro demonstration of the antidotal efficacy of hydroxocobalamin in cyanide poisoning. J Neurosurg Anesthesiol. 1990 Dec; 2(4): 296-304. [PubMed Citation]
Riou B, Gérard JL, La Rochelle CD, Bourdon R, Berdeaux A, Giudicelli JF. Hemodynamic effects of hydroxocobalamin in conscious dogs. Anesthesiology. 1991 Mar;74(3):552-8. [PubMed Citation]
Roderique EJ, Gebre-Giorgis AA, Stewart DH, Feldman MJ, Pozez Al. Smoke Inhalation Injury in a Pregnant Patient: A Literature Review of the Evidence and Current Best Practices in the Setting of a Classic Case; J Burn Care Res 2012 Jan 30 [Epub ahead of print] [PubMed Citation]
Sauer SW, Keim ME. Hydroxocobalamin: improved public health readiness for cyanide disasters. Ann Emerg Med. 2001 Jun;37(6):635-41. [PubMed Citation]
Shepherd G, Velez LI. Role of hydroxocobalamin in acute cyanide poisoning. Ann Pharmacother. 2008 May;42(5):661-9 [PubMed Citation]
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
Sutter M, Tereshchenko N, Rafii R, Daubert GP. Hemodialysis complications of hydroxocobalamin: a case report; J Med Toxicol 2010 Jun; 6 (2):165-7 [PubMed Citation]
Uhl W, Nolting A, Golor G, Rost KL, Kovar A. Safety of hydroxocobalamin in healthy volunteers in a randomized, placebo-controlled study; ClinToxicol 2006 (Phil) 44 Suppl 1: 17-28. [PubMed Citation]
Uhl W, Nolting A, Gallemann D, Hecht S, Kovar A. Changes in blood pressure after administration of hydroxocobalamin: relationship to changes in plasma cobalamins-(III) concentrations in healthy volunteers. Clin Toxicol (Phila). 2008 Jul;46(6):551-9. [PubMed Citation]
Van den Berg MP, Merkus P, Romeijn SG, Verhoef JC, Merkus FW. Hydroxocobalamin uptake into the cerebrospinal fluid after nasal and intravenous delivery in rats and humans. J Drug Target. 2003 Jul;11(6):325-31. [PubMed Citation]
Youso SL, Rockwood GA, Lee JP, Logue BA. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2010 Sep 10;677(1):24-8. [PubMed Citation]
Youso SL, Rockwood GA, Logue BA. The analysis of protein-bound thiocyanate in plasma of smokers and non-smokers as a marker of cyanide exposure. J Anal Toxicol 2012 May;36(4):265-9. [PubMed Citation]
Zerbe NF, Wagner BK. Use of vitamin B12 in the treatment and prevention of nitroprusside-induced cyanide toxicity. Crit Care Med. 1993 Mar;21(3):465-7. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Meridian Medical Technologies website addressing safety information regarding Cyanokit, ordering information, links to package labeling and links to various resources. http://www.cyanokit.com/
Record last updated 1/2/2013
