You are here: Home > Medical Countermeasures Database > Lorazepam
Lorazepam - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Lorazepam
2. Chemical Defense therapeutic area(s)
— including key possible usesLorazepam has been shown to be effective as anticonvulsant against nerve agents such as sarin (GB), soman (GD), cyclosarin (GF), tabun (GA), VX, and organophosphorus pesticides.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
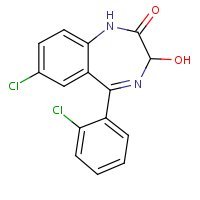
US NLM. ChemIDplus Lite. Lorazepam
Mechanism of action
Lorazepam interacts with the γ-aminobutyric acid (GABA)-benzodiazepine receptor complex, which is widespread in the brain of humans as well as other species. This interaction is presumed to be responsible for lorazepam's mechanism of action. Lorazepam exhibits relatively high and specific affinity for its recognition site but does not displace GABA. Attachment to the specific binding site enhances the affinity of GABA for its receptor site on the same receptor complex. The pharmacodynamic consequences of benzodiazepine agonist actions include antianxiety effects, sedation, and reduction of seizure activity. The intensity of action is directly related to the degree of benzodiazepine receptor occupancy.
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
Summary of clinical and non-clinical studies
Organophosphates (OPs) are commonly used as pesticides and as military nerve agents including sarin, soman, tabun, and VX. OPs irreversibly inhibit acetylcholinesterase (AChE), the enzyme that breaks down the neurotransmitter acetylcholine (ACh). As a result, ACh accumulates at synapses, perpetually stimulating the cholinergic receptors and inducing convulsions, behavioral impairments, and eventually death, if untreated. The benzodiazepine lorazepam is an anticonvulsant which interacts with γ-aminobutyric acid (GABA)-benzodiazepine receptors which are widespread in mammalian brains. Lorazepam is widely used for its antianxiety, sedative, and antiepileptic effects, and its clinically demonstrated safety and efficacy against extended seizures (status epilepticus) has made it the treatment of choice in the hospital setting (Silbergleit et al., 2012). In two randomized clinical trials in patients treated for seizure by paramedics, status epilepticus was terminated by the time of arrival at the hospital in 63.4% of patients (Silbergleit et al., 2012) and in 59.1% of patients (Alldredge et al., 2001) receiving intravenous 2 mg doses of lorazepam. In the latter study, lorazepam was significantly (p>0.001) superior to diazepam (status epilepticus terminated in 42.6% of patients receiving diazepam) and placebo (21.1%) (Alldredge et al., 2001). Some preclinical studies in animals indicate that lorazepam, as well as other benzodiazapines, may be effective in reducing or eliminating seizures occurring as a result of OP intoxication. Oxime-pretreated rats that were exposed to 180 μg/kg soman received 3.0 mg/kg lorazepam by intraperitoneal injection 5 minutes and 40 minutes after seizure onset (Shih et al., 1999). Seizures were terminated in 5 of 6 animals that received lorazepam 5 minutes after seizure onset, and in 1 of 5 animals in the 40-minute group. In another study, guinea pigs were pretreated with pyridostigmine, exposed to double the 50% lethal dose of soman, and 1 minute later were treated with atropine and an oxime (McDonough et al., 1999). Either 5 or 40 minutes after seizure onset, animals received intramuscular injections of lorazepam or another benzodiazepine. All benzodiazepines tested (lorazepam, avizafone, clonazepam, diazepam, loprazolam and midazolam) offered equal protection against the lethal effects of the nerve agent (11% mortality at 24 hours), and were effective in terminating seizures 5 minutes and 40 minutes after onset. Under these conditions, lorazepam lost very little effectiveness at 40 minutes, having a similar (slightly higher, but not statistically significant) 50% effective dose (ED50) at the later time point.
B. Link to clinical studies
Studies involving multiple populations
-
Early treatment of prolonged seizures with benzodiazepines given intravenously by paramedics in the prehospital setting has been shown to be associated with improved outcomes. However, an increasing number of Emergency Medical System (EMS) protocols use an intramuscular (IM) route because it is faster and consistently achievable. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial) is a double-blind randomized clinical trial to determine if the efficacy of IM midazolam is noninferior by a margin of 10% to that of intravenous (IV) lorazepam in patients treated by paramedics for status epilepticus (SE). Children and adults with >5 min of convulsions who are still seizing after paramedic arrival are administered study medication by IM autoinjector or IV infusion. The primary efficacy outcome is absence of seizures at emergency department (ED) arrival, without EMS rescue therapy. Safety outcomes include acute endotracheal intubation and recurrent seizures. Secondary outcomes include timing of treatment and initial seizure cessation. At the time of writing this communication, enrollment of all subjects is near completion and the study data will soon be analyzed (Class II).
Silbergleit R, Lowenstein D, Durkalski V, Conwit R; Neurological Emergency Treatment Trials (NETT) Investigators. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia. 2011 Oct;52 Suppl 8:45-7. [PubMed Citation]
-
Early termination of prolonged seizures with intravenous administration of benzodiazepines improves outcomes. For faster and more reliable administration, paramedics increasingly use an intramuscular route. This double-blind, randomized, noninferiority trial compared the efficacy of intramuscular midazolam with that of intravenous lorazepam for children and adults in status epilepticus treated by paramedics. Subjects whose convulsions had persisted for more than 5 minutes and who were still convulsing after paramedics arrived were given the study medication by either intramuscular autoinjector or intravenous infusion. The primary outcome was absence of seizures at the time of arrival in the emergency department without the need for rescue therapy. Secondary outcomes included endotracheal intubation, recurrent seizures, and timing of treatment relative to the cessation of convulsive seizures. This trial tested the hypothesis that intramuscular midazolam was noninferior to intravenous lorazepam by a margin of 10 percentage points. At the time of arrival in the emergency department, seizures were absent without rescue therapy in 329 of 448 subjects (73.4%) in the intramuscular-midazolam group and in 282 of 445 (63.4%) in the intravenous-lorazepam group (absolute difference, 10 percentage points; 95% confidence interval, 4.0 to 16.1; P<0.001 for both noninferiority and superiority). The two treatment groups were similar with respect to need for endotracheal intubation (14.1% of subjects with intramuscular midazolam and 14.4% with intravenous lorazepam) and recurrence of seizures (11.4% and 10.6%, respectively). Among subjects whose seizures ceased before arrival in the emergency department, the median times to active treatment were 1.2 minutes in the intramuscular-midazolam group and 4.8 minutes in the intravenous-lorazepam group, with corresponding median times from active treatment to cessation of convulsions of 3.3 minutes and 1.6 minutes. Adverse-event rates were similar in the two groups. For subjects in status epilepticus, intramuscular midazolam is at least as safe and effective as intravenous lorazepam for prehospital seizure cessation. (Funded by the National Institute of Neurological Disorders and Stroke and others; ClinicalTrials.gov number, ClinicalTrials.gov NCT00809146.)(Class II).
Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, Barsan W. NETT Investigators. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012 Feb 16;366(7):591-600. [PubMed Citation]
Adult
-
It is uncertain whether the administration of benzodiazepines by paramedics is an effective and safe treatment for out-of-hospital status epilepticus. We conducted a randomized, double-blind trial to evaluate intravenous benzodiazepines administered by paramedics for the treatment of out-of-hospital status epilepticus. Adults with prolonged (lasting five minutes or more) or repetitive generalized convulsive seizures received intravenous diazepam (5 mg), lorazepam (2 mg), or placebo. An identical second injection was given if needed. Of the 205 patients enrolled, 66 received lorazepam, 68 received diazepam, and 71 received placebo. Status epilepticus had been terminated on arrival at the emergency department in more patients treated with lorazepam (59.1 percent) or diazepam (42.6 percent) than patients given placebo (21.1 percent) (P=0.001). After adjustment for covariates, the odds ratio for termination of status epilepticus by the time of arrival in the lorazepam group as compared with the placebo group was 4.8 (95 percent confidence interval, 1.9 to 13.0). The odds ratio was 1.9 (95 percent confidence interval, 0.8 to 4.4) in the lorazepam group as compared with the diazepam group and 2.3 (95 percent confidence interval, 1.0 to 5.9) in the diazepam group as compared with the placebo group. The rates of respiratory or circulatory complications (indicated by bag valve-mask ventilation or an attempt at intubation, hypotension, or cardiac dysrhythmia) after the study treatment was administered were 10.6 percent for the lorazepam group, 10.3 percent for the diazepam group, and 22.5 percent for the placebo group (P=0.08). Benzodiazepines are safe and effective when administered by paramedics for out-of-hospital status epilepticus in adults. Lorazepam is likely to be a better therapy than diazepam (Class II).
Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, Gottwald MD, O'Neil N, Neuhaus JM, Segal MR, Lowenstein DH. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001 Aug 30;345(9):631-7. [PubMed Citation]
Pediatric studies
-
Pharmacokinetic data were evaluated in 10 term neonates with seizures after intravenous administration of lorazepam, 0.05 mg/kg or 0.1 mg/kg. All seizure activity ceased, with no adverse effects. Pharmacokinetic data revealed a decreased volume of distribution and clearance, and a prolonged half-life in comparison with data from older children and adults. These findings are consistent with physiologic differences in the neonate (Class III).
McDermott CA, Kowalczyk AL, Schnitzler ER, Mangurten HH, Rodvold KA, Metrick S. Pharmacokinetics Pharmacokinetics of lorazepam in critically ill neonates with seizures. J Pediatr. 1992 Mar;120(3):479-83. [PubMed Citation]
-
To report a case of lorazepam toxicity in a premature infant and discuss the importance of altered pharmacodynamics and pharmacokinetics in the neonatal population. A 2025-g, 33-weeks' gestation infant was born with respiratory distress syndrome that required mechanical ventilation. Lorazepam was used to establish sedation and prevent asynchronous breathing while the infant was on the ventilator. Shortly after the first dose of lorazepam, the infant experienced a seizure and was subsequently given a loading dose of phenobarbital. Lorazepam therapy was continued for sedation. The patient was transferred to our tertiary care center on day 2 of life for evaluation of possible cardiac disease. Upon arrival, the infant was extremely hypotonic and unresponsive; therefore, all sedative medications were discontinued. Two days after admission, the infant continued to exhibit very little spontaneous activity and a lorazepam serum concentration was obtained (63 h after the last dose). Analysis revealed a toxic lorazepam serum concentration of 4453 nmol/L. The patient eventually was weaned to room air and was transported back to the referring hospital. Lorazepam is commonly prescribed in the pediatric population for sedative, anticonvulsant, anxiolytic, antiemetic, and amnestic activity. Few data exist regarding the safety of long-term lorazepam therapy in the neonatal subpopulation. There have been some reports of neurologic toxicity secondary to lorazepam in preterm infants. Its metabolism depends on glucuronidation, an enzymatic process that is very depressed in the premature infant. Accumulation of the drug in the neonate accompanied by clinical toxicity is highly likely. The inability to establish a clear pharmacokinetic-pharmacodynamic relationship, along with the increased incidence of reported adverse events of lorazepam in neonates, is concerning. Clinicians should be aware of the altered metabolism and elimination of lorazepam in the premature infant (Class IV).
Reiter PD, Stiles AD. Lorazepam toxicity in a premature infant. Ann Pharmacother. 1993 Jun;27(6):727-9. [PubMed Citation]
Pregnancy, breastfeeding studies
-
In humans, blood levels obtained from umbilical cord blood indicate placental transfer of lorazepam and lorazepam glucuronide (Class IV).
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
Clinical reviews
-
Nerve agents (NA) are simple and cheap to produce but can produce casualties on a massive scale. They have already been employed by terrorist organizations and rogue states on civilians and armed forces alike. By inhibiting the enzyme acetylcholine esterase, NAs prevent the breakdown of the neurotransmitter acetylcholine. This results in over-stimulation of muscarinic and nicotinic receptors in the autonomic and central nervous systems and at the neuromuscular junction. Increased parasympathetic stimulation produces miosis, sialorrhea, bronchospasm and bronchorrhea. Effects at the neuromuscular junction cause weakness, fasciculations, and eventually paralysis. Central effects include altered behavior and mental status, loss of consciousness, seizures, or apnea. Most deaths are due to respiratory failure. Treatment with atropine competitively blocks the parasympathetic effects. Oximes like pralidoxime salvage acetylcholine esterase by "prying off" NA, provided the attachment has not "aged" to an irreversible bond. This reverses weakness. Benzodiazepines like diazepam are effective against NA induced seizures. Mortality has been surprisingly low. If victims can survive the first 15 to 20 min of a vapor attack, they will likely live. The low mortality rate to date underscores that attacks are survivable and research reveals even simple barriers such as clothing offer substantial protection. This article reviews the properties of NAs and how to recognize the clinical features of NA intoxication, employ the needed drugs properly, and screen out anxious patients who mistakenly believe they have been exposed (Class IV).
Cannard K. The acute treatment of nerve agent exposure. J Neurol Sci. 2006 Nov 1;249(1):86-94. [PubMed Citation]
-
The National Institutes of Health (NIH) supports research about and the development of better therapies for treating exposure to toxic chemicals that could be used in a terrorist attack or released during an industrial accident. A review of recent research published by NIH investigators working in this field indicates that scientific advances in this area also have implications for reducing the burden of other neurological diseases and disorders. Some key examples discussed include studies on the development of therapeutic drugs to treat seizures and the neuropathology caused by chemical nerve agents, which may help find better cures for epilepsy, stroke, and neurodegenerative diseases (Class IV).
Jett DA. Finding new cures for neurological disorders: A possible fringe benefit of biodefense research? Sci Transl Med. 2010 Mar
17;2(23):23ps12. [PubMed Citation]
-
Nerve agents (NAs) are the most lethal chemical weapons. We review the pathophysiology and management of NA poisoning of children. NAs cause cholinergic crisis. Children may manifest signs of cholinergic poisoning differently than adults. Children may be less likely to manifest miosis and glandular secretions. They may present with neurologic derangements alone. The goals of treatment should be to limit additional exposure, to provide respiratory support, and to prevent neurologic morbidity. Autoinjectors are optimal delivery vehicles for intramuscular antidotes and are likely to be used in civilian prehospital care. Antidotes include anticholinergics, oximes, and benzodiazepines. Several medications may be available within each class of antidotes. Clinicians will select an antidote based on the status of the individual victim, the accessibility of supportive care, and the availability of the drug. Atropine is well-tolerated and high doses may be required. The oxime pralidoxime chloride has a longer half-life in children. Currently, diazepam is the standard NA anticonvulsant. Midazolam may be the most effective intramuscular anticonvulsant after NA exposure, but, despite its efficacy, it is not an approved agent for seizures. Supportive care and long-term complications are summarized (Class IV).
Rotenberg JS, Newmark J. Nerve Agent Attacks on Children: Diagnosis and Management. Pediatrics. 2003 Sep;112(3 Pt 1):648-58. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
This study evaluated the ability of six benzodiazepines to stop seizures produced by exposure to the nerve agent soman. Guinea pigs, previously prepared with electrodes to record electroencephalographic (EEG) activity, were pretreated with pyridostigmine (0.026 mg/kg, i.m.) 30 min before challenge with soman (56 lg/kg, s.c.) and then treated 1 min after soman exposure with atropine (2.0 mg/kg, i.m.) and pralidoxime chloride (2-PAM Cl; 25 mg/kg, i.m.). All animals developed seizures following this treatment. Benzodiazepines (avizafone, clonazepam, diazepam, loprazolam, lorazepam, and midazolam) were given i.m. 5 or 40 min after seizure onset. All benzodiazepines were effective in stopping soman induced seizures, but there were marked differences between drugs in the rapidity of seizure control. The 50% effective dose (ED50) values and latencies for anticonvulsant effect for a given benzodiazepine were the same at the two times of treatment delay. Midazolam was the most potent and rapidly acting compound at both treatment times. Since rapid seizure control minimizes the chance of brain damage, use of midazolam as an anticonvulsant may lead to improved clinical outcome in the treatment of nerve agent seizures.
McDonough JH Jr, McMonagle J, Copeland T, Zoeffel D, Shih TM. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol. 1999 Nov;73(8-9):473-8. [PubMed Citation]
-
This report describes studies of anticonvulsants for the organophosphorus (OP) nerve agent soman: a basic research effort to understand how different pharmacological classes of compounds influence the expression of seizure produced by soman in rats, and a drug screening effort to determine whether clinically useful antiepileptics can modulate soman-induced seizures in rats. Electroencephalographic (EEG) recordings were used in these studies. Basic studies were conducted in rats pretreated with HI-6 and challenged with 1.6 x LDs0 soman. Antimuscarinic compounds were extremely effective in blocking (pretreatment) or terminating soman seizures when given 5 min after seizure onset. However, significantly higher doses were required when treatment was delayed for more than 10 min, and some antimuscarinic compounds lost anticonvulsant efficacy when treatment was delayed for more than 40 min. Diazepam blocked seizure onset, yet seizures could recur after an initial period of anticonvulsant effect at doses </=2.5 mg/kg. Diazepam could terminate ongoing seizures when given 5 min after seizure onset, but doses up to 20 mg/kg were ineffective when treatment was delayed for 40 min. The GABA uptake inhibitor, tiagabine, was ineffective in blocking or terminating soman motor convulsions or seizures. The glutamate receptor antagonists, NBQX, GYKI 52466, and memantine, had weak or minimal antiseizure activity, even at doses that virtually eliminated signs of motor convulsions. The antinicotinic, mecamylamine, was ineffective in blocking or stopping seizure activity. Pretreatment with a narrow range of doses of a2-adrenergic agonist, clonidine, produced variable protection (40- 60%) against seizure onset; treatment after seizure onset with clonidine was not effective. Screening studies in rats, using HI-6 pretreatment, showed that benzodiazepines (diazepam, midazolam and Iorazepam) were quite effective when given 5 min after seizure onset, but lost their efficacy when given 40 min after onset. The barbiturate, pentobarbital, was modestly effective in terminating seizures when given 5 or 40 min after seizure onset, while other clinically effective antiepileptic drugs, trimethadione and valproic acid, were only slightly effective when given 5 min after onset. In contrast, phenytoin, carbamazepine, ethosuximide, magnesium sulfate, lamotrigine, primidone, felbamate, acetazolamide, and ketamine were ineffective.
Shih T, McDonough JH Jr, Koplovitz I. Anticonvulsants for Soman-induced Seizure Activity. J Biomed Sci. 1999 Mar-Apr;6(2):86-96. [PubMed Citation]
Pregnant animal studies
-
An increased risk of congenital malformations associated with the use of minor tranquilizers (chlordiazepoxide, diazepam and meprobamate) during the first trimester of pregnancy has been suggested in several studies.
-
Reproductive studies in animals were performed in mice, rats, and two strains of rabbits. Occasional anomalies (reduction of tarsals, tibia, metatarsals, malrotated limbs, gastroschisis, malformed skull, and microphthalmia) were seen in drug-treated rabbits without relationship to dosage. Although all of these anomalies were not present in the concurrent control group, they have been reported to occur randomly in historical controls. At doses of 40 mg/kg orally or 4 mg/kg intravenously and higher, there was evidence of fetal resorption and increased fetal loss in rabbits which was not seen at lower doses.
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
A 4-mg intravenously (IV)-delivered dose provides an initial concentration of approximately 70 ng/mL. Following intramuscular (IM) administration, lorazepam is completely and rapidly absorbed reaching peak concentrations within 3 hours. A 4-mg dose provides a Cmax of approximately 48 ng/mL. Following administration of 1.5 to 5.0 mg of lorazepam IM, the amount of lorazepam delivered to the circulation is proportional to the dose administered. At clinically relevant concentrations, lorazepam is 91±2% bound to plasma proteins; its volume of distribution is approximately 1.3 L/kg. Unbound lorazepam penetrates the blood/brain barrier freely by passive diffusion, a fact confirmed by CSF sampling. Following parenteral administration, the terminal half-life and total clearance averaged 14±5 hours and 1.1±0.4 mL/min/kg, respectively. Lorazepam is extensively conjugated to the 3-O-phenolic glucuronide in the liver and is known to undergo enterohepatic recirculation. Lorazepam glucuronide is an inactive metabolite and is eliminated mainly by the kidneys. Following a single 2-mg oral dose of 14C-lorazepam to 8 healthy subjects, 88±4% of the administered dose was recovered in urine and 7±2% was recovered in feces. The percent of administered dose recovered in urine as lorazepam glucuronide was 74±4%. Only 0.3% of the dose was recovered as unchanged lorazepam, and the remainder of the radioactivity represented minor metabolites.
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
-
The effects of neomycin and cholestyramine on the disposition of lorazepam was examined in seven healthy drug-free men. Half-life as determined for the oral route was, in all subjects, 15% to 35% less than that determined for the intravenous route. Free oral clearance was slightly but not significantly less than free systemic clearance, but the ratio of the AUC of lorazepam glucuronide corrected for dose was twofold greater by the oral route. Urinary recoveries also differed (71.6% and 50.4%, oral versus intravenous). Neomycin and cholestyramine treatment resulted in a 19% to 26% reduction in half-life attendant on a 34% increase in free oral clearance and a 24% increase in free systemic clearance. This suggests that lorazepam undergoes significant enterohepatic recirculation in human beings and that there exists an extrahepatic pathway, at least for the intravenous route. Since pharmacokinetic measurements do not take these physiologic processes into account, the drug cannot properly be used as a marker of conjugative metabolism.
Herman RJ, Van Pham JD, Szakacs CB. Disposition of lorazepam in human beings: enterohepatic recirculation and first pass effect. Clin Pharmacol Ther. 1989 Jul;46(1):18-25. [PubMed Citation]
-
Six healthy volunteers received a single i.v. dose of 'low dose' lorazepam (0.0225 mg/kg), 'high dose' lorazepam (0.045 mg/kg) and placebo by 1-min infusion in a double-blind three-way crossover study. Plasma concentrations were measured 24 hr after dosage, and the EEG power spectrum was simultaneously computed by fast-Fourier transform to determine the percentage of total EEG amplitude occurring in the 13-30-Hz range. Low and high dose lorazepam did not differ significantly in distribution volume (1.89 versus 1.81 l/kg) or elimination half-life (11.5 versus 12.2 hr); clearance was slightly although significantly reduced at the higher dose (2.08 versus 1.88 ml/min/kg, P less than .005). EEG effects were of relatively slow onset, reaching their maximum change over baseline 30 min after infusion. The duration of action was prolonged, with the fraction of EEG activity in the 13-30-Hz range still significantly above baseline 8 hr after the 0.045 mg/kg dose. Five of these subjects received 0.15 mg/kg of i.v. diazepam in a companion study of identical design. EEG effects of diazepam were shorter than those of lorazepam, probably because of the more rapid and extensive decline in plasma diazepam concentrations in the postinfusion distribution phase. In addition, the onset of diazepam's effect was immediate. In male CD-1 mice that received i.v. diazepam (8.3 mg/kg) or lorazepam (3.3 mg/kg), the brain:plasma concentration ratio was maximal 2.5 min after dosage for diazepam, but equilibration was delayed at least 30 min after dosage for lorazepam. Thus the slow onset of action of lorazepam is probably attributable to slow entry into brain.
Greenblatt DJ, Ehrenberg BL, Gunderman J, Scavone JM, Tai NT, Harmatz JS, Shader RI. Kinetic and dynamic study of intravenous lorazepam; comparison with intravenous diazepam. J Pharmacol Exp Ther. 1989 Jul;250(1):134-40. [PubMed Citation]
-
It is well established that there is a wide intra- and interindividual variability in dose requirements for lorazepam and midazolam in intensive care patients. The objective of this study was to compare the population pharmacokinetics of lorazepam and midazolam after long-term continuous infusion in mechanically ventilated critically ill patients. Forty-nine critically ill patients randomly received either lorazepam (n = 28) or midazolam (n = 21) by continuous infusion for at least 24 h. Multiple blood samples were obtained for determination of the drug and metabolite concentrations by HPLC. Population pharmacokinetic models were developed using the Non-Linear Mixed Effect Modelling (NONMEM) program. The influence of selected covariates was investigated. The prospective performance of the models was evaluated on the basis of results in separate groups of patients for lorazepam (n = 31) and midazolam (n = 33). The pharmacokinetics of lorazepam were best described by a two-compartment model. Alcohol abuse, positive end expiratory pressure (PEEP) and age were identified as significant covariates. Total body clearance for patients without alcohol abuse was 4.13 - (PEEP - 5) x 0.42 l h-1, and 0.74 l h-1 for patients with alcohol abuse. The volume of distribution was 0.74 l, the steady state volume of distribution was 56 - (age - 58) x 2.1 l and the intercompartmental clearance was 10 l h-1. The proportional residual error was 15% and the median absolute prediction error was 13.6% with a bias of 1.5%. The pharmacokinetics of midazolam were best described by a two-compartment model with alcohol abuse, APACHE score and age as significant covariates. Total body clearance for patients without alcohol abuse was 11.3 - (age - 57) x 0.14 l h-1, and 7.27 - (age -57) x 0.14 l h-1 for patients with alcohol abuse. The volume of distribution was 7.15 l, the steady state volume of distribution was 431 l, and the intercompartmental clearance was 40.8 - (APACHE score - 26) x 2.75 l h-1. The proportional residual error was 31% with an additive residual error of 32 ng ml-1. The median absolute prediction error was 12.9% with a bias of 1.2%. The prospective performance in the lorazepam evaluation group was better with the covariate adjusted model, but in the midazolam evaluation group it was not better than with the simple model. In all models a tendency to overestimate the lower plasma concentrations was observed. The pharmacokinetics of both lorazepam and midazolam were well described by a two-compartment model. Inclusion of alcohol abuse and age as covariates improved both models. PEEP was identified as an additional covariate for lorazepam, and the APACHE score for midazolam. For both drugs there is a large interindividual variability in their pharmacokinetics when used for long-term sedation in critically ill patients. However, the intra-individual variability is much lower for lorazepam.
Swart EL, Zuideveld KP, de Jongh J, Danhof M, Thijs LG, Strack van Schijndel RM. Comparative population pharmacokinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients. Br J Clin Pharmacol. 2004 Feb;57(2):135-45. [PubMed Citation]
Children
-
NEONATES (BIRTH TO 1 MONTH): Following a single 0.05 mg/kg (n=4) or 0.1 mg/kg (n=6) intravenous dose of lorazepam, mean total clearance normalized to body weight was reduced by 80% compared to normal adults, terminal half-life was prolonged 3-fold, and volume of distribution was decreased by 40% in neonates with asphyxia neonatorum compared to normal adults. All neonates were of ≥37 weeks of gestational age.
-
INFANTS (1 MONTH UP TO 2 YEARS): There is no information on the pharmacokinetic profile of lorazepam in infants in the age range of 1 month to 2 years.
-
CHILDREN (2 YEARS TO 12 YEARS): Total (bound and unbound) lorazepam had a 50% higher mean volume of distribution (normalized to body-weight) and a 30% longer mean half-life in children with acute lymphocytic leukemia in complete remission (2 to 12 years, n=37) compared to normal adults (n=10). Unbound lorazepam clearance normalized to body-weight was comparable in children and adults.
-
ADOLESCENTS (12 YEARS TO 18 YEARS): Total (bound and unbound) lorazepam had a 50% higher mean volume of distribution (normalized to body-weight) and a mean half-life that was two fold greater in adolescents with acute lymphocytic leukemia in complete remission (12 to 18 years, n=13) compared to normal adults (n=10). Unbound lorazepam clearance normalized to body-weight was comparable in adolescents and adults.
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
-
An investigation has been made of the excretion of alpha-methyldopa, alpha-methyldopa sulphate, lorazepam and lorazepam glucuronide in the urine of neonates. The rate of elimination of both the drugs in the newborn is slow compared with the adult rate, and apparent half-life being 3 to 4 times longer than the reported adult values. The newborn appear able to readily metabolise alpha-methyldopa to alpha-methyldopa sulphate and to slowly conjugate lorazepam with glucuronic acid. alpha-Methyldopa tends to be conjugated to a greater extent and lorazepam to about the same or slightly lesser extent in the newborn than in adults. It is postulated that elimination in the neonate is mainly controlled by the rate of renal excretion in the case of alpha-methyldopa and by the rate of conjugation in the case of lorazepam.
Cummings AJ, Whitelaw AGL. A study of conjugation and drug elimination in the human neonate. Br J Clin Pharmacol. 1981 Oct;12(4):511-5. [PubMed Citation]
-
Pharmacokinetic data were evaluated in 10 term neonates with seizures after intravenous administration of lorazepam, 0.05 mg/kg or 0.1 mg/kg. All seizure activity ceased, with no adverse effects. Pharmacokinetic data revealed a decreased volume of distribution and clearance, and a prolonged half-life in comparison with data from older children and adults. These findings are consistent with physiologic differences in the neonate.
McDermott CA, Kowalczyk AL, Schnitzler ER, Mangurten HH, Rodvold KA, Metrick S. Pharmacokinetics Pharmacokinetics of lorazepam in critically ill neonates with seizures. J Pediatr. 1992 Mar;120(3):479-83. [PubMed Citation]
-
We evaluated the effects of low doses of lorazepam on episodic versus long-term memory, attention, and somatic and affective symptoms, as well as its pharmacokinetics, in a group of 16 children aged 2.8 to 14.2 years. Psychologic assessments of each child were performed twice before intravenous administration of lorazepam (0.03 mg/kg), and 1 1/2 hours and 24 hours after lorazepam; there were no significant changes in long-term memory, attention, or somatic symptoms. There was a significant decrease in affective symptoms at 1 1/2 hours (p = 0.011), with a trend toward decreased anxiety at 1 1/2 and 24 hours after lorazepam (p = 0.026 and 0.028, respectively). There was also a selective anterograde amnestic effect in 5 of 16 children after lorazepam (p = 0.06). Mean (+/- SD) lorazepam systemic clearance was 1.3 +/- 0.4 ml/min/kg, with a terminal half-life of 10.5 +/- 2.9 hours and an unbound clearance of 15.9 +/- 5.2 ml/min/kg. A group of healthy adult volunteers who were given lorazepam had a mean systemic clearance of 1.0 +/- 0.4 ml/min/kg, somewhat less than that of the children (p = 0.069). There were no significant differences in any lorazepam pharmacokinetic parameter between those children who did versus those who did not have changes in affective symptoms or amnesia. These data should be helpful in establishing the dose of lorazepam in children, as the drug becomes more widely used as an antiemetic, premedicant, and anticonvulsant agent.
Relling MV, Mulhern RK, Dodge RK, Johnson D, Pieper JA, Rivera GK, Evans WE. Lorazepam pharmacodynamics and pharmacokinetics in children. J Pediatr. 1989 Apr;114(4 Pt 1):641-6. [PubMed Citation]
Geriatric
-
Following single intravenous doses of 1.5 to 3 mg of ATIVAN Injection, mean total body clearance of lorazepam decreased by 20% in 15 elderly subjects of 60 to 84 years of age compared to that in 15 younger subjects of 19 to 38 years of age. Consequently, no dosage adjustment appears to be necessary in elderly subjects based solely on their age.
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
Renal Impairment
-
Because the kidney is the primary route of elimination of lorazepam glucuronide, renal impairment would be expected to compromise its clearance. This should have no direct effect on the glucuronidation (and inactivation) of lorazepam. There is a possibility that the enterohepatic circulation of lorazepam glucuronide leads to a reduced efficiency of the net clearance of lorazepam in this population.
-
Six normal subjects, six patients with renal impairment (Clcr of 22±9 mL/min), and four patients on chronic maintenance hemodialysis were given single 1.5 to 3.0 mg intravenous doses of lorazepam. Mean volume of distribution and terminal half-life values of lorazepam were 40% and 25% higher, respectively, in renally impaired patients than in normal subjects. Both parameters were 75% higher in patients undergoing hemodialysis than in normal subjects. Overall, though, in this group of subjects the mean total clearance of lorazepam did not change. About 8% of the administered intravenous dose was removed as intact lorazepam during the 6-hour dialysis session.
-
The kinetics of lorazepam glucuronide were markedly affected by renal dysfunction. The mean terminal half-life was prolonged by 55% and 125% in renally impaired patients and patients under hemodialysis, respectively, as compared to normal subjects. The mean metabolic clearance decreased by 75% and 90% in renally impaired patients and patients under hemodialysis, respectively, as compared with normal subjects.
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
Hepatic Impairment
-
Oxazepam and lorazepam are 3-hydroxy benzodiazepine derivatives used as sedatives and anxiolytics. The major metabolic pathway for both compounds involves conjugation to glucuronic acid at the 3-position, followed by urinary excretion of the inactive glucuronide metabolite. Oxazepam has been administered to humans by the oral route only. Usual ranges for kinetic parameters are: elimination half-life, 5 to 15 hours; volume of distribution, 0.6 to 2.0 L/kg; clearance, 0.9 to 2.0 ml/min/kg. Age and liver disease have a minimal influence on oxazepam kinetics, but renal disease is associated with a prolonged half-life and increased volume of distribution. Typical kinetic values for lorazepam are: elimination half-life, 8 to 25 hours; volume of distribution, 1.0 to 1.3 L/kg; clearance, 0.7 to 1.2 ml/min/kg. Lorazepam clearance is somewhat reduced in old age, but liver disease has a minimal effect on clearance. Oral and intramuscular lorazepam are rapidly absorbed, with systemic availability averaging 90% or more. Both oxazepam and lorazepam are extensively bound to plasma protein, but the free fraction for lorazepam (8 to 12%) is greater than that for oxazepam (2 to 4%).
Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981 Mar-Apr;6(2):89-105. [PubMed Citation]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
Status epilepticus: usual dose 4 mg given slowly (2 mg/min) IV (preffered), or IM. May repeat in 10 to 15 minutes if seizures continue or recur. Experience with further doses of lorazepam is very limited.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1516-7
Anxiety: Ativan (lorazepam) is administered orally. For optimal results, dose, frequency of administration, and duration of therapy should be individualized according to patient response. To facilitate this, 0.5 mg, 1 mg, and 2 mg tablets are available.The usual range is 2 to 6 mg/day given in divided doses, the largest dose being taken before bedtime, but the daily dosage may vary from 1 to 10 mg/day. For anxiety, most patients require an initial dose of 2 to 3 mg/day given b.i.d. or t.i.d.
Insomnia due to anxiety or transient situational stress: a single daily dose of 2 to 4 mg may be given, usually at bedtime.
The dosage of Ativan (lorazepam) should be increased gradually when needed to help avoid adverse effects. When higher dosage is indicated, the evening dose should be increased before the daytime doses.
Product label: ATIVAN (lorazepam) tablet [Cardinal Health] Last revised: December 2010 [DailyMed]
Pregnancy (FDA)
Pregnancy Category D
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
Nursing Mothers (FDA)
Lorazepam has been detected in human breast milk. Therefore, lorazepam should not be administered to nursing mothers because, like other benzodiazepines, the possibility exists that lorazepam may sedate or otherwise adversely affect the infant.
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
Geriatric (FDA)
Anxiety: for elderly or debilitated patients, an initial dosage of 1 to 2 mg/day in divided doses is recommended, to be adjusted as needed and tolerated.
Product label: ATIVAN (lorazepam) tablet [Cardinal Health] Last revised: December 2010 [DailyMed]
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Lorazepam has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Formulation
Oral For solution concentrate 2 mg/mL
Tablets 0.5 mg, 1 mg, 2 mg
Parenteral Injection 2 mg/mL, 4 mg/mL
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2625-2627
Storage
Lorazepam oral concentrate solution and lorazepam injection should be stored at 2-8°C and protected from light; freezing of the injection should be avoided. Lorazepam tablets should be stored in well-closed containers at 20-25°C.
The manufacturer states that lorazepam injection should be diluted prior to IV administration with an equal volume of compatible diluent, including 0.9% sodium chloride injection or 5% dextrose injection. Solutions of lorazepam should not be used if they are discolored or contain a precipitate.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2625-2627
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
To compare the utility of intramuscular lorazepam (LZ) with the combination of intramuscular haloperidol (HDL) and LZ to control acutely agitated behavior. Randomized double-blind comparison. Psychiatric emergency service of a large, university-affiliated, municipal hospital. Twenty subjects treated on the psychiatric emergency service. Patients received an injection of either LZ 2 mg (11 patients) or HDL 5 mg plus LZ 2 mg (9 patients). The Overt Aggression Scale (OAS), visual analog scales reflecting agitation and hostility, and the Clinical Global Impressions (CGI) severity scale were administered at baseline and 30, 60, 120, and 180 minutes after the injection. Planned data comparisons included categoric assignment of patients as improved, as defined by decreases in outcome measures 60 minutes after the injection, as well as continuous variables up to 180 minutes after the injection. A significantly greater percentage of subjects receiving combined treatment improved on the specific measures 60 minutes after dosing (p<0.05). Kaplan-Meier survival analyses showed significant between-group differences in survival curves plotted for the entire study period (p<0.05). Repeated measures analyses of variance studying group differences showed that both groups improved over time, but between-group differences were not significant. The powers of these analyses were low due to the small sample. No serious adverse effects occurred in either treatment group. Our results suggest superior efficacy for HDL-LZ over LZ alone. Categoric tests of improvement at 60 minutes provided the strongest evidence of group differences.
Bieniek SA, Ownby RL, Penalver A, Dominguez RA. A double-blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacotherapy. 1998 Jan-Feb;18(1):57-62. [PubMed Citation]
-
Rapid tranquilization is a routinely practiced method of calming agitated psychotic patients by use of neuroleptics, benzodiazepines, or both in combination. Although several studies have examined the efficacy of the three approaches, none have compared these treatments in a prospective, randomized, double-blind, multicenter trial. Ninety-eight psychotic, agitated, and aggressive patients (73 men and 25 women) were prospectively enrolled during an 18-month period in emergency departments in five university or general hospitals. Patients were randomly assigned to receive intramuscular injections of lorazepam (2 mg), haloperidol (5 mg), or both in combination. Patients in each treatment group received 1 to 6 injections of the same study drug within 12 hours, based on clinical need. They were evaluated hourly after the first injection until at least 12 hours after the last. Efficacy was assessed on the Agitated Behavior Scale (ABS), a modified Brief Psychiatric Rating Scale (MBPRS), Clinical Global impressions (CGI) scale, and an Alertness Scale. Effective symptom reduction was achieved in each treatment group with significant (P < .01) mean decreases from baseline at every hourly ABS evaluation. Significant (P < .05) mean differences on the ABS (hour 1) and MBPRS (hours 2 and 3) suggest that tranquilization was most rapid in patients receiving the combination treatment. Study event incidence (side effects) did not differ significantly between treatment groups, although patients receiving haloperidol alone tended to have more extrapyramidal system symptoms. The superior results produced by the combination treatment support the use of lorazepam plus haloperidol as the treatment of choice for acute psychotic agitation.
Battaglia J, Moss S, Rush J, Kang J, Mendoza R, Leedom L, Dubin W, McGlynn C, Goodman L. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997 Jul;15(4):335-40. [PubMed Citation]
-
The suitability of lorazepam as a sedative for use in intensive care has been critically evaluated in 36 severely ill patients. The efficacy of lorazepam has been evaluated by regular measurements of both cardiovascular and neurological status as well as by the nursing staff directly involved with the care of the patients. A variety of patients have received lorazepam for sedation both while on intermittent positive pressure ventilation and when breathing spontaneously. The benefits of the predictable and even sedation produced by a long-acting drug of this nature are discussed. Particular attention has been paid to the possibility of accumulation of lorazepam in a similar way to that of diazepam, and the relative advantages of the two drugs for use in the intensive care situation have been compared.
Simpson PJ, Eltringham RJ. Lorazepam in intensive care. Clin Ther. 1981;4(3):150-63. [PubMed Citation]
-
Alcohol abuse is one of the most common causes of seizures in adults. In a randomized, double-blind study, we compared lorazepam with placebo for the prevention of recurrent seizures related to alcohol. Over a 21-month period, we studied consecutive patients with chronic alcohol abuse who were at least 21 years of age and who presented to the emergency departments of two hospitals in Boston after a witnessed, generalized seizure. The patients were randomly assigned to receive either 2 mg of lorazepam in 2 ml of normal saline or 4 ml of normal saline intravenously and then observed for six hours. The primary end point was the occurrence of a second seizure during the observation period. Of the 229 patients who were initially evaluated, 186 met the entry criteria. In the lorazepam group, 3 of 100 patients (3 percent) had a second seizure, as compared with 21 of 86 patients (24 percent) in the placebo group (odds ratio for seizure with the use of placebo, 10.4; 95 percent confidence interval, 3.6 to 30.2; P<0.001). Forty-two percent of the placebo group were admitted to the hospital, as compared with 29 percent of the lorazepam group (odds ratio for admission, 2.1; 95 percent confidence interval, 1.1 to 4.0; P=0.02). Seven patients in the placebo group and one in the lorazepam group were transported to an emergency department in Boston with a second seizure within 48 hours after hospital discharge. Treatment with intravenous lorazepam is associated with a significant reduction in the risk of recurrent seizures related to alcohol.
D'Onofrio G, Rathlev NK, Ulrich AS, Fish SS, Freedland ES. Lorazepam for the prevention of recurrent seizures related to alcohol. N Engl J Med. 1999 Mar 25;340(12):915-9. [PubMed Citation]
-
Lorazepam was studied in a double-blind, placebo-controlled, crossover trial in eight patients with frequent partial complex seizures refractory to therapy with a combination of standard anticonvulsant drugs. Concomitant antiepileptic drugs were maintained at therapeutic serum levels throughout the study, and concentrations of lorazepam were monitored. Following an 8-week baseline observation, patients were randomly assigned to placebo or lorazepam (1 mg BID). The dose was increased biweekly until seizures stopped or unacceptable side effects occurred. Eight weeks later, patients were crossed over, and the same escalating dose paradigm was followed. When seizure frequency during the last 2 weeks of each treatment was compared, seven of eight patients had fewer seizures on lorazepam, and the eighth had decreased seizure duration (a significant difference: p less than 0.01, two-tailed sign test). Blood level data suggest a narrow therapeutic window, with seizure improvement occurring at concentrations of 20-30 ng/ml and side effects at greater than 33 ng/ml. Lorazepam appears to be a useful adjunct in refractory partial complex seizure therapy. It should not be stopped abruptly, as an increase in seizure frequency may result.
Walker JE, Homan RW, Crawford IL. Lorazepam: a controlled trial in patients with intractable partial complex seizures. Epilepsia. 1984 Aug;25(4):464-6. [PubMed Citation]
-
We report our experience with 300 consecutive parenteral doses of lorazepam (LOR) for status epilepticus (SE) or serial seizures in 77 children and young adults. The median dose for SE in children less than 12 years old was 0.10 mg/kg. LOR stopped the SE in 79% and diminished the intensity of SE in an additional 4%. Prior acute or chronic anticonvulsant use (excepting chronic benzodiazepines) did not alter effectiveness or increase side effects. Duration of freedom from seizures following acute therapy was independent of LOR dosage. In patients requiring sequential doses, LOR becomes progressively less effective. Side effects were few and, when present, always associated with a single or first dose in a series. LOR is a safe and effective acute anticonvulsant agent for in-hospital control of SE in the pediatric age group. Tachyphylaxis of anticonvulsant action occurs when serial doses are used.
Crawford TO, Mitchell WG, Snodgrass S. Lorazepam in childhood status epilepticus and serial seizures: effectiveness and tachyphylaxis. Neurology. 1987 Feb;37(2):190-5. [PubMed Citation]
Children
-
Anxiety: usual oral dose 0.05 mg/kg/dose every 4 to 8 hours, or IV 0.05 mg/kg every 4 to 8 hours. Maximum dose - 2 mg/dose.
-
Status epilepticus: neonates and children younger than 18 years of age - usual dosage 0.05 to 0.1 mg/kg given IV over 2 to 5 minutes. If needed, a dosage of 0.05 mg/kg may be repeated in 10 to 15 minutes. Maximum dose - 4 mg/dose.
-
Lorazepam injection may contain benzyl alcohol and propylene glycol, which may be toxic to newborns at high dose.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1516-7
-
Sublingual lorazepam was successful in controlling serial seizures in ten children. There was both intrasubject and intersubject variability in the effective dose, which ranged from about 0.05 mg/kg to 0.15 mg/kg. Side effects were minimal and consisted of drowsiness, unsteadiness, nausea, and hyperactivity. Sublingual lorazepam is an easy and effective way to treat serial seizures at home.
Yager JY, Seshia SS. Sublingual lorazepam in childhood serial seizures. Am J Dis Child. 1988 Sep;142(9):931-2. [PubMed Citation]
-
Seven neonatal patients with severe seizures unresponsive to conventional anticonvulsant therapy were treated with lorazepam. Immediate cessation of seizure activity occurred in all patients within five minutes. Although seizures recurred in two infants eight hours later, frequency and severity diminished. There were no apparent significant side effects attributed to the medication.
Deshmukh A, Wittert W, Schnitzler E, Mangurten HH. Lorazepam in the treatment of refractory neonatal seizures: a pilot study. Am J Dis Child. 1986 Oct;140(10):1042-4. [PubMed Citation]
-
Seizure is a recognized complication of high-dose busulfan (BU) therapy and phenytoin (DPH) is widely used as prophylaxis. A number of adverse effects have been associated with DPH and it may also interfere with BU metabolism. We used lorazepam (median dose 0.022 mg/kg) i.v. or p.o. before each dose and for 24 h after the last dose of BU as seizure prophylaxis to 29 children undergoing hematopoietic stem cell transplantation. The regimen was well tolerated and drowsiness was the only significant side-effect. Twelve patients were able to receive the entire prophylaxis by mouth. No seizure developed during and within 48 h of BU. Concomitant pharmacokinetic studies showed no alternation of the absorption and clearance of BU during lorazepam administration. Lorazepam can be used as an alternative for seizure prophylaxis during high-dose BU treatment.
Chan KW, Mullen CA, Worth LL, Choroszy M, Koontz S, Tran H, Slopis J. Lorazepam for seizure prophylaxis during high-dose busulfan administration. Bone Marrow Transplant. 2002 Jun;29(12):963-5. [PubMed Citation]
8. Route of Administration/Monitoring
-
Lorazepam is administered orally or by IM or IV injection. The drug should not be administered by intra-arterial injection since arteriospam can occur which may cause gangrene and possibly require an amputation. IM injections of lorazepam, administered as undiluted solutions, should be made deeply into a large muscle mass. Prior to IV injection lorazepam must be diluted with an equal volume of compatible diluents.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2625-2627
9. Adverse effects
-
Diarrhea occurred in an infant given large enteral doses of benzodiazepine solutions that contained the carriers propylene glycol and polyethylene glycol. This adverse drug effect may be avoided by prescribing the tablet formulations appropriately prepared for enteral administration.
Marshall JD, Farrar HC, Kearns GL. Diarrhea associated with enteral benzodiazepine solutions. J Pediatr. 1995 Apr;126(4):657-9. [PubMed Citation]
-
To report a case of lorazepam toxicity in a premature infant and discuss the importance of altered pharmacodynamics and pharmacokinetics in the neonatal population. A 2025-g, 33-weeks' gestation infant was born with respiratory distress syndrome that required mechanical ventilation. Lorazepam was used to establish sedation and prevent asynchronous breathing while the infant was on the ventilator. Shortly after the first dose of lorazepam, the infant experienced a seizure and was subsequently given a loading dose of phenobarbital. Lorazepam therapy was continued for sedation. The patient was transferred to our tertiary care center on day 2 of life for evaluation of possible cardiac disease. Upon arrival, the infant was extremely hypotonic and unresponsive; therefore, all sedative medications were discontinued. Two days after admission, the infant continued to exhibit very little spontaneous activity and a lorazepam serum concentration was obtained (63 h after the last dose). Analysis revealed a toxic lorazepam serum concentration of 4453 nmol/L. The patient eventually was weaned to room air and was transported back to the referring hospital. Lorazepam is commonly prescribed in the pediatric population for sedative, anticonvulsant, anxiolytic, antiemetic, and amnestic activity. Few data exist regarding the safety of long-term lorazepam therapy in the neonatal subpopulation. There have been some reports of neurologic toxicity secondary to lorazepam in preterm infants. Its metabolism depends on glucuronidation, an enzymatic process that is very depressed in the premature infant. Accumulation of the drug in the neonate accompanied by clinical toxicity is highly likely. The inability to establish a clear pharmacokinetic-pharmacodynamic relationship, along with the increased incidence of reported adverse events of lorazepam in neonates, is concerning. Clinicians should be aware of the altered metabolism and elimination of lorazepam in the premature infant.
Reiter PD, Stiles AD. Lorazepam toxicity in a premature infant. Ann Pharmacother. 1993 Jun;27(6):727-9. [PubMed Citation]
-
ATIVAN MAY CAUSE FETAL DAMAGE WHEN ADMINISTERED TO PREGNANT WOMEN. Ordinarily, ATIVAN Injection should not be used during pregnancy except in serious or life-threatening conditions where safer drugs cannot be used or are ineffective. Status epilepticus may represent such a serious and life-threatening condition.
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
-
To report a patient with a probable acute tubular necrosis (ATN) induced by chronic exposure to polyethylene glycol (PEG)-400 via long-term, massive dosage of intravenous lorazepam. A 57-year-old man with a history of alcohol abuse was admitted to the intensive care unit for acute respiratory failure. Lorazepam therapy was initiated in anticipation of alcohol withdrawal. Dosages up to 18 mg/h were required to provide adequate sedation and optimize ventilation. On day 43, the patient developed oliguric ATN of unknown etiology. The cumulative intravenous lorazepam dose was 4089 mg, equivalent to approximately 220 mL of PEG-400. Blood urea nitrogen concentrations followed a pattern that paralleled lorazepam dosage increases and decreases. Protein and granular casts were evident in urinalyses performed on days 12 and 29. The patient eventually experienced complete recovery. ATN associated with intravenous PEG was last reported in 1959 in 6 of 32 patients receiving a cumulative PEG-300 dose of 120-200 mL over 3-5 days via an intravenous nitrofurantoin preparation. Two of the 6 patients died. Chronic administration of intravenous PEG to rabbits over a 5-week period has caused cloudy swelling of the renal tubular epithelium, increased blood urea concentrations, and death in some animals. ATN probably resulted from chronic PEG exposure via massive doses of lorazepam injection, possibly enhanced by concurrent administration of vancomycin.
Laine GA, Hossain SM, Solis RT, Adams SC. Polyethlene glycol nephrotoxicity secondary to prolonged high dose intravenous lorazepam. Ann Pharmacother. 1995 Nov;29(11):1110-4. [PubMed Citation]
10. Contraindication(s)
Lorazepam is contraindicated in:
-
Patients with a known sensitivity to benzodiazepines or its vehicle (polyethylene glycol, propylene glycol, and benzyl alcohol).
-
Patients with acute narrow-angle glaucoma.
-
Patients with sleep apnea syndrome.
-
Patients with severe respiratory insufficiency, except in those patients requiring relief of anxiety and/or diminished recall of events while being mechanically ventilated.
-
Intra-arterial injection is contraindicated because, as with other injectable benzodiazepines, inadvertent intra-arterial injection may produce arteriospasm resulting in gangrene which may require amputation.
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
-
Drugs that affect the CNS (e.g., phenothiazines, opiate agonists or partial agonists, barbiturates, antidepressants, alcohol, scopolamine, monoamine oxidase inhibitors) may have additive CNS effects when used concomitantly with, or during the period of recovery from, lorazepam. Such combinations, or IV lorazepam used alone in higher than recommended doses, can produce excessive sedation which may result in partial airway obstruction. The manufacturer warns that scopolamine does not provide additional benefit when used concomitantly with lorazepam, but may increase sedation, hallucinations, and irrational behavior.
-
Benzodiazepines should not be administered IV to patients in whom the hypnotic or hypotensive effects may be prolonged or intensified such as those with shock or coma, to patients with depressed respiration, or to those who have recently received other respiratory depressant drugs.
-
Benzodiazepines should not be used in patients with depressive neuroses or psychotic reactions in which anxiety is not prominent. Benzodiazepines are in patients with acute alcohol intoxication with depressed vital signs and in patients with known hypersensitivity to the drugs. Because the frequency of suicide appears to be increased in untreated patients with panic disorder, and because panic disorder may be associated with primary and secondary major depressive disorders, the usual precautions of psychotropic therapy in depressed patients or those at risk for concealed suicidal ideation should be exercised during benzodiazepine therapy for panic disorder.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2625-2627
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesTitle: Efficacy and Safety Study Comparing Lorazepam and Diazepam for Children in the Emergency Department With Seizures (Status 2)
Condition: Status Epilepticus
Intervention: Drug: lorazepam or diazepam
Title: Acute Effect of Lorazepam on Brain Activity Measured by Magnetoencephalograpy (MEG) and Electroencephalography (EEG)
Condition: Healthy Subjects
Interventions: Drug: Lorazepam; Drug: Placebo
ClinicalTrials.gov. Lorazepam
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations- No data available at this time.
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations- No data available at this time.
15. Study-related ethical concerns
— including review panel recommendations- No data available at this time.
16. Global regulatory status
U.S.
Ativan (lorazepam) is indicated for the management of anxiety disorders or for the short-term relief of the symptoms of anxiety or anxiety associated with depressive symptoms. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The effectiveness of Ativan (lorazepam) in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
Product label: ATIVAN (lorazepam) tablet [Cardinal Health] Last revised: December 2010 [DailyMed]
17. Other potentially useful information
-
Lorazepam has been shown to be effective against both Convulsant (e.g., picrotoxin, hydrazine, strychnine, tetramethylenedisulfotetramine) and Cholinergic (e.g., sarin (GB), soman (GD), cyclosarin (GF), tabun (GA), VX, and organophosphorus pesticides) Toxidromes to enhance the inhibitory effect of gamma-aminobutyric acid at GABA(a) receptors and counter excess central nervous system excitation and seizures.
Department of Homeland Security (DHS). Chemical Security Analysis Center (CSAC). 2011. Chemical Segregation by Toxidrome for the Chemical Terrorism Risk Assessment. PowerPoint available in Report on the Toxic Chemical Syndrome Definition and Nomenclature Workshop (May, 2012)
-
Log P (octanol-water) = 2.39 (exp)
US NLM. ChemIDplus Lite. Lorazepam
18. Publications
Alldredge BK, Gelb AM, Isaacs SM, Corry MD, Allen F, Ulrich S, Gottwald MD, O'Neil N, Neuhaus JM, Segal MR, Lowenstein DH. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med. 2001 Aug 30;345(9):631-7. [PubMed Citation]
Bieniek SA, Ownby RL, Penalver A, Dominguez RA. A double-blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacotherapy. 1998 Jan-Feb;18(1):57-62. [PubMed Citation]
Battaglia J, Moss S, Rush J, Kang J, Mendoza R, Leedom L, Dubin W, McGlynn C, Goodman L. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double-blind, emergency department study. Am J Emerg Med. 1997 Jul;15(4):335-40. [PubMed Citation]
Cannard K. The acute treatment of nerve agent exposure. J Neurol Sci. 2006 Nov 1;249(1):86-94. [PubMed Citation]
Chan KW, Mullen CA, Worth LL, Choroszy M, Koontz S, Tran H, Slopis J. Lorazepam for seizure prophylaxis during high-dose busulfan administration. Bone Marrow Transplant. 2002 Jun;29(12):963-5. [PubMed Citation]
ClinicalTrials.gov. Lorazepam
Crawford TO, Mitchell WG, Snodgrass S. Lorazepam in childhood status epilepticus and serial seizures: effectiveness and tachyphylaxis. Neurology. 1987 Feb;37(2):190-5. [PubMed Citation]
Cummings AJ, Whitelaw AGL. A study of conjugation and drug elimination in the human neonate. Br J Clin Pharmacol. 1981 Oct;12(4):511-5. [PubMed Citation]
Deshmukh A, Wittert W, Schnitzler E, Mangurten HH. Lorazepam in the treatment of refractory neonatal seizures: a pilot study. Am J Dis Child. 1986 Oct;140(10):1042-4. [PubMed Citation]
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
D'Onofrio G, Rathlev NK, Ulrich AS, Fish SS, Freedland ES. Lorazepam for the prevention of recurrent seizures related to alcohol. N Engl J Med. 1999 Mar 25;340(12):915-9. [PubMed Citation]
Greenblatt DJ. Clinical pharmacokinetics of oxazepam and lorazepam. Clin Pharmacokinet. 1981 Mar-Apr;6(2):89-105. [PubMed Citation]
Greenblatt DJ, Ehrenberg BL, Gunderman J, Scavone JM, Tai NT, Harmatz JS, Shader RI. Kinetic and dynamic study of intravenous lorazepam; comparison with intravenous diazepam. J Pharmacol Exp Ther. 1989 Jul;250(1):134-40. [PubMed Citation]
Herman RJ, Van Pham JD, Szakacs CB. Disposition of lorazepam in human beings: enterohepatic recirculation and first pass effect. Clin Pharmacol Ther. 1989 Jul;46(1):18-25. [PubMed Citation]
Jett DA. Finding new cures for neurological disorders: A possible fringe benefit of biodefense research? Sci Transl Med. 2010 Mar 17;2(23):23ps12. [PubMed Citation]
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1516-7
Laine GA, Hossain SM, Solis RT, Adams SC. Polyethlene glycol nephrotoxicity secondary to prolonged high dose intravenous lorazepam. Ann Pharmacother. 1995 Nov;29(11):1110-4. [PubMed Citation]
Marshall JD, Farrar HC, Kearns GL. Diarrhea associated with enteral benzodiazepine solutions. J Pediatr. 1995 Apr;126(4):657-9. [PubMed Citation]
McDermott CA, Kowalczyk AL, Schnitzler ER, Mangurten HH, Rodvold KA, Metrick S. Pharmacokinetics Pharmacokinetics of lorazepam in critically ill neonates with seizures. J Pediatr. 1992 Mar;120(3):479-83. [PubMed Citation]
McDonough JH Jr, McMonagle J, Copeland T, Zoeffel D, Shih TM. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol. 1999 Nov;73(8-9):473-8. [PubMed Citation]
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2625-2627
Product label:
ATIVAN (lorazeapm) injection
[West-ward Pharmaceutical Corp.] Last revised: November 2011
[DailyMed]
Product label: ATIVAN (lorazepam) tablet [Cardinal Health] Last revised: December 2010 [DailyMed]
Reiter PD, Stiles AD. Lorazepam toxicity in a premature infant. Ann Pharmacother. 1993 Jun;27(6):727-9. [PubMed Citation]
Relling MV, Mulhern RK, Dodge RK, Johnson D, Pieper JA, Rivera GK, Evans WE. Lorazepam pharmacodynamics and pharmacokinetics in children. J Pediatr. 1989 Apr;114(4 Pt 1):641-6. [PubMed Citation]
Rotenberg JS, Newmark J. Nerve Agent Attacks on Children: Diagnosis and Management. Pediatrics. 2003 Sep;112(3 Pt 1):648-58. [PubMed Citation]
Shih T, McDonough JH Jr, Koplovitz I. Anticonvulsants for Soman-induced Seizure Activity. J Biomed Sci. 1999 Mar-Apr;6(2):86-96. [PubMed Citation]
Silbergleit R, Lowenstein D, Durkalski V, Conwit R; Neurological Emergency Treatment Trials (NETT) Investigators. RAMPART (Rapid Anticonvulsant Medication Prior to Arrival Trial): a double-blind randomized clinical trial of the efficacy of intramuscular midazolam versus intravenous lorazepam in the prehospital treatment of status epilepticus by paramedics. Epilepsia. 2011 Oct;52 Suppl 8:45-7. [PubMed Citation]
Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, Barsan W. NETT Investigators. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012 Feb 16;366(7):591-600. [PubMed Citation]
Simpson PJ, Eltringham RJ. Lorazepam in intensive care. Clin Ther. 1981;4(3):150-63. [PubMed Citation]
Swart EL, Zuideveld KP, de Jongh J, Danhof M, Thijs LG, Strack van Schijndel RM. Comparative population pharmacokinetics of lorazepam and midazolam during long-term continuous infusion in critically ill patients. Br J Clin Pharmacol. 2004 Feb;57(2):135-45. [PubMed Citation]
US NLM. ChemIDplus Lite. Lorazepam
Walker JE, Homan RW, Crawford IL. Lorazepam: a controlled trial in patients with intractable partial complex seizures. Epilepsia. 1984 Aug;25(4):464-6. [PubMed Citation]
Department of Homeland Security (DHS). Chemical Security Analysis Center (CSAC). 2011. Chemical Segregation by Toxidrome for the Chemical Terrorism Risk Assessment. PowerPoint available in Report on the Toxic Chemical Syndrome Definition and Nomenclature Workshop (May, 2012)
Yager JY, Seshia SS. Sublingual lorazepam in childhood serial seizures. Am J Dis Child. 1988 Sep;142(9):931-2. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
