You are here: Home > Medical Countermeasures Database > Midazolam
Midazolam - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Midazolam
2. Chemical Defense therapeutic area(s)
— including key possible usesThe anticonvulsant midazolam has been shown to be effective against both convulsant (e.g., picrotoxin, hydrazine, strychnine, tetramethylenedisulfotetramine) and cholinergic (e.g., sarin (GB), soman (GD), cyclosarin (GF), tabun (GA), VX, and organophosphorus pesticides) agents by enhancing the inhibitory effect of gamma-aminobutyric acid at GABA(a) receptors and limiting excess central nervous system excitation and seizures.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
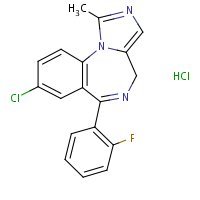
US NLM. ChemIDplus Lite Midazolam Hydrochloride
Mechanism of action
-
Midazolam, like other benzodiazepines, is presumed to interact with the gamma-aminobutyric acid (GABA)-benzodiazepine receptor complex, which is widespread in the brain of humans as well as other species. The pharmacodynamic consequences of benzodiazepine agonist actions include antianxiety effects, sedation, and reduction of seizure activity. Midazolam binds to the GABA receptor but does not displace GABA; rather, it enhances the affinity of GABA for its receptor site on the same receptor complex. The pharmacodynamic consequences of benzodiazepine agonist actions include antianxiety effects, sedation, and reduction of seizure activity. The intensity of action is directly related to the degree of benzodiazepine receptor occupancy.
Brunton, L.L, et al. (eds.). (2006). (Goodman & Gilman's) The Pharmacological Basis of Therapeutics (11th Ed.). McGraw-Hill-Medical Publishing Division, New York, NY. p.402-407.
-
Exposure to high doses of organophosphorus nerve agents such as soman, even with carbamate pretreatment, produces a variety of toxic cholinergic signs, including secretions, convulsions and death. Evidence suggests that soman-induced convulsions may be associated with postexposure brain neuropathology. The purpose of this study was to investigate the pharmacologic mechanism of action of soman-induced convulsions and of anticonvulsant drugs. Various classes of compounds were evaluated for their efficacy in preventing soman-induced convulsions in rats pretreated with the oxime HI-6 to increase survival time, along with various doses of the test compounds (IM) either in the absence or presence of atropine sulfate (16 mg/kg, IM) 30 minutes prior to a soman challenge dose (180 micrograms/kg, SC; equivalent to 1.6 x LD50) that produced 100% convulsions. Without atropine sulfate, only tertiary anticholinergics (scopolamine, trihexyphenidyl, biperiden, benactyzine, benztropine, azaprophen and aprophen), caramiphen, carbetapentane and MK-801 were effective anticonvulsants. In the presence of atropine sulfate, the benzodiazepines (diazepam, midazolam, clonazepam, loprazolam and alprazolam), mecamylamine, flunarizine, diphenylhydantoin, clonidine, CGS 19755 and Organon 6370 studied were effective. We have examined the possibility that diazepam may exert some of its anticonvulsant effects through cholinergic mechanisms and found that a reduced release of ACh into synapses after diazepam and atropine treatment may account for diazepam's anticonvulsant activity against soman. We also found that at anticonvulsant doses biperiden and trihexyphenidyl each significantly reversed the effects of soman on striatal levels of DOPAC and HVA, the metabolites of dopamine, and have concluded that in addition to actions on muscarinic receptors, the anticonvulsant effects of these anticholinergics in soman poisoning may be partially related to their actions on the striatal dopaminergic system. These findings allow us to postulate that central muscarinic cholinergic mechanisms are primarily involved in eliciting the convulsions following exposure to soman and that subsequent recruitment of other excitatory neurotransmitter systems and loss of inhibitory control may be responsible for sustaining the convulsions and for producing the subsequent brain damage. Future studies to confirm these neuropharmacological mechanisms are proposed.
Shih TM, Koviak TA, Capacio BR; Anticonvulsants Anticonvulsants for poisoning by the organophosphorus compound soman: pharmacological mechanisms. Neurosci Biobehav Rev. 1991 Fall; 15 (3): 349-62.
Summary of clinical and non-clinical studies
Organophosphates (OPs) are commonly used as pesticides and as military nerve agents including sarin, soman, tabun, and VX. OPs irreversibly inhibit the enzyme acetylcholinesterase (AChE), which breaks down the neurotransmitter acetylcholine (ACh) (Zilker, 2005). As a result, ACh accumulates and overstimulates glands, producing profuse sweating, rhinorrhea, salivation, lacrimation, convulsions, coma, respiratory distress, and, if untreated, death (Zilker, 2005). Nerve agents have been used in the Iran-Iraq War and in terrorist attacks in Japan and pose significant threats to both military and civilian populations. Therapy for acute OP intoxication involves pre-treatment with pyridostigmine bromide (when possible), and post-exposure treatment with atropine, an oxime, and an anticonvulsant. The benzodiazepine midazolam is used clinically as a sedative and anxiolytic, and, like other benzodiazepines, has anticonvulsant properties. There are no controlled clinical studies demonstrating the effectiveness of midazolam in humans against OP poisoning, but individual case studies indicate that midazolam can successfully treat the condition. A 5-year old girl was exposed to an OP insecticide via tainted food; four hours later, she experienced respiratory failure and unconsciousness, and fell into a coma 16 hours after eating (Sumi et al., 2008). Intravenous midazolam, atropine, and pralidoxime were administered, and the patient gradually recovered, was extubated on Day 8, and was discharged without sequelae on Day 25. In a randomized clinical trial in patients treated for seizures by paramedics, status epilepticus was terminated by the time of hospital arrival in 73.4% of patients receiving an intramuscular midazolam injection, a significantly greater benefit compared to patients receiving intravenous lorazepam (seizures terminated in 63.4%, p<0.001) (Silbergleit et al., 2012). Midazolam also had a comparable safety profile; combined with the greater ease of intramuscular injection compared to intravenous infusion, midazolam may become the preferred treatment for seizures outside of the hospital setting.
Animal studies indicate that midazolam is effective in terminating seizures specifically caused by OP nerve agent exposure. In rats treated with the oxime HI-6 30 minutes before soman exposure, midazolam was one of the most effective compounds in terminating seizures when administered 5 minutes after seizure onset (9 of 11 rats); its effectiveness was reduced when administered 40 minutes after onset (1 of 7 rats) (Shih et al., 1999). In another study, guinea pigs were pretreated with pyridostigmine, exposed to double the 50% lethal dose (2×LD50) of soman, and 1 minute later were treated with atropine and an oxime (McDonough et al., 1999). At 5 minutes or 40 minutes after seizure onset, animals received intramuscular injections of lorazepam or another benzodiazepine. All benzodiazepines tested (lorazepam, avizafone, clonazepam, diazepam, loprazolam and midazolam) offered equal protection against the lethal effects of the nerve agent (11% mortality at 24 hours), and in terms of ending seizures, midazolam was the most potent and the fastest-acting at both time points (5 min and 40 min). When this experimental design was expanded to a range of nerve agents (tabun, sarin, soman, cyclosarin, VX, Russian VX), midazolam was again superior to other benzodiazepines (in potency and rapidity of action) against all tested nerve agents (Shih et al., 2003). A comparison of different routes of midazolam delivery (intramuscular, intranasal, sublingual) in similarly-treated guinea pigs (pyridostigmine, followed by 2×LD50 soman, followed by atropine/oxime; plus midazolam immediately after or 40 minutes after seizure onset) indicated that intranasal administration had a potency of anticonvulsant effect comparable to intramuscular injection (McDonough et al., 2009). Furthermore, although the intramuscular route resulted in a significantly lower ED50 than the intranasal route at 5 minutes, the speed of anticonvulsant effect was equivalent for the two routes. At 40-minutes, there were no differences between the intramuscular and intranasal routes in either ED50 or speed of anticonvulsant effect.
B. Link to clinical studies
Studies involving multiple populations
-
BACKGROUND: Early termination of prolonged seizures with intravenous administration of benzodiazepines improves outcomes. For faster and more reliable administration, paramedics increasingly use an intramuscular route. METHODS: This double-blind, randomized, noninferiority trial compared the efficacy of intramuscular midazolam with that of intravenous lorazepam for children and adults in status epilepticus treated by paramedics. Subjects whose convulsions had persisted for more than 5 minutes and who were still convulsing after paramedics arrived were given the study medication by either intramuscular autoinjector or intravenous infusion. The primary outcome was absence of seizures at the time of arrival in the emergency department without the need for rescue therapy. Secondary outcomes included endotracheal intubation, recurrent seizures, and timing of treatment relative to the cessation of convulsive seizures. This trial tested the hypothesis that intramuscular midazolam was noninferior to intravenous lorazepam by a margin of 10 percentage points. RESULTS: At the time of arrival in the emergency department, seizures were absent without rescue therapy in 329 of 448 subjects (73.4%) in the intramuscular-midazolam group and in 282 of 445 (63.4%) in the intravenous-lorazepam group (absolute difference, 10 percentage points; 95% confidence interval, 4.0 to 16.1; P<0.001 for both noninferiority and superiority). The two treatment groups were similar with respect to need for endotracheal intubation (14.1% of subjects with intramuscular midazolam and 14.4% with intravenous lorazepam) and recurrence of seizures (11.4% and 10.6%, respectively). Among subjects whose seizures ceased before arrival in the emergency department, the median times to active treatment were 1.2 minutes in the intramuscular-midazolam group and 4.8 minutes in the intravenous-lorazepam group, with corresponding median times from active treatment to cessation of convulsions of 3.3 minutes and 1.6 minutes. Adverse-event rates were similar in the two groups. CONCLUSIONS: For subjects in status epilepticus, intramuscular midazolam is at least as safe and effective as intravenous lorazepam for prehospital seizure cessation. (Funded by the National Institute of Neurological Disorders and Stroke and others; ClinicalTrials.gov number, ClinicalTrials.gov NCT00809146.) (Class II).
Silbergleit R, Durkalski V, Lowenstein D et al; Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med, 2012; 366(7):591-600. [PubMed Citation]
-
Midazolam (Dormicum) is a benzodiazepine used for the premedication and induction of anaesthesia. Its diazepine nucleus is fused with a nucleus of the imidazol group having a basic azote in position 2, which allows the formation of water soluble salts. Its antiepileptic properties have been most recently studied on interictal spikes' and on status epilepticus. Fourteen patients, nine females and five males, from 19 to 72 years old, suffering from subsequent repeated epileptic seizures were treated with 0-2 mg/kg intramuscular midazolam. We performed 18 interventions: three for complex partial seizures, nine for tonic-clonic seizures, two for myoclonic, one for tonic, one for atonic seizures, and two for partial motor prolonged seizures. The clinical status (heart and respiratory rate, blood pressure, waking state and clinical evolution of seizures) was followed during one hour after the injection. In nine cases, classical EEG was recorded for the whole of this period. A slight decrease of blood pressure (5%) was noted in only three cases with a slower heart rate (10%) in one of them. The disappearance of the epileptic seizures was observed in all cases, 5 to 10 minutes after the injection. This response was complete in 15 interventions and partial in three. On the nine recorded EEGs, the eileptiform activities had disappeared in 10 min in five cases, but reappeared at the end of the recording in one of them. They diminished significantly in three cases and remained unchanged in one case. The EEG changes were concomitant with the clinical improvement. The response to this treatment was stable in all but four cases, who presented with a relapse within the first 24 hours after the injection (two after 3 hours, one after 4 hours and one after 18 hours), two of which were then treated by a second injection of midazolam and responded very well. Our results are comparable with those of Egli and Albani' and Jawad et al,2 reporting respectively 12 complete clinical responses on 15 cases of tonic-clonic and complex partial seizures, and 21 complete responses on 26 cases. In our study, the mean of complete clinical responses reached 82%. Compared with other benzodiazepines used in emergency for iterative epileptic seizures, midazolam offers two advantages. First, its action is rapid and effective by the intramuscular route. Jawad et al3 have compared intramuscular midazolam with intramuscular and intravenous diazepam on the ability to reduce interictal spikes. It appears that 10 or 15 mg intramuscular midazolam is as effective as 20 mg intravenous diazepam, while 10 or 15 mg intramuscular midazolam is more effective than 10 mg diazepam administered, intramuscularly or intravenously. The second advantage of midazolam is its short half life (1 to 2 hours), as compared with diazepam, lorazepam and clonazepam which have values from 15 to 31 hours. This short half life allows us to exclude from the number of relapses the case who presented with a seizure after 18 hours, as we consider this seizure as a new event. In conclusion, these results indicate that midazolam is an effective treatment of repeated epileptic seizures. In addition, this drug may be easily and safely administrated by the intramuscular route (Class IV).
Ghilain S, Rijckevorsel-Harmant K, Harmant J, de Barsy TH; Midazolam in the treatment of epileptic seizures. J Neurol Neurosurg Psych. 1988; 51 (5): 732. [PubMed Citation]
Adult
-
Midazolam in an autoinjector was evaluated in an open-label dose escalation study involving 39 healthy participants. Safety and pharmacokinetic parameters were determined for doses ranging from 5 to 30 mg. No serious adverse events were noted during the study. Two participants (30 mg) experienced changes in their electrocardiogram (trigeminy and prolongation of QRS complex) that met the criteria for dose-limiting adverse events. No significant respiratory depression was noted during the study. The midazolam doses studied exhibited a median t(max) of 0.5 hours with a geometric mean terminal elimination half-life value of 4.1 hours (range, 2.9-4.5 hours). The extent of systemic exposure, assessed by area under the curve (AUC) and maximum concentration (C(max)), tended to increase proportionally with increasing doses from 5 to 30 mg; however, for the male 30-mg group, there was evidence of a larger than proportional increase in AUC (Class III).
Reichard DW, Atkinson AJ, Hong SP, Burback BL, Corwin MJ, Johnson JD; Human safety and pharmacokinetic study of intramuscular midazolam administered by autoinjector. J Clin Pharmacol. 2010 Oct; 50 (10): 1128-35. [PubMed Citation]
Pediatric studies
-
Objective: To compare intranasal midazolam, using a Mucosal Atomization Device (IN-MMAD), with rectal diazepam (RD) for the home treatment of seizures in children with epilepsy. Design: Prospective randomized study. Setting: Patients' homes and a freestanding children's hospital that serves as a referral center for 5 states. Patients: A total of 358 pediatric patients who visited a pediatric neurology clinic from July 2006 through September 2008 and were prescribed a home rescue medication for their next seizure. Intervention: Caretakers were randomized to use either 0.2 mg/kg of IN-MMAD (maximum, 10 mg) or 0.3 to 0.5 mg/kg of RD (maximum, 20 mg) at home for their child's next seizure if it lasted more than 5 minutes. Outcome Measures: The primary outcome measure was total seizure time after medication administration. Our secondary outcomemeasures were total seizure time, time to medication administration, respiratory complications, emergency medical service support, emergency department visits, hospitalizations, and caretakers' ease of administration and satisfaction with the medication. Results: A total of 92 caretakers gave the study medication during a child's seizure (50 IN-MMAD, 42 RD).The median time from medication administration to seizure cessation for IN-MMAD was 1.3 minutes less than for RD (95% confidence interval, 0.0-3.5 minutes; P=.09). The median time to medication administration was 5.0 minutes for each group. No differences in complications were found between treatment groups. Caretakers were more satisfied with IN-MMAD and report that it was easier to give than RD. Conclusions: There was no detectable difference in efficacy between IN-MMAD and RD as a rescue medication for terminating seizures at home in pediatric patients with epilepsy. Ease of administration and overall satisfaction was higher with IN-MMAD compared with RD (Class II).
Holsti M, Dudley N, Schunk J, Adelgais K, Greenberg R, Olsen C, Healy A, Firth S, Filloux F; Intranasal Midazolam vs. Rectal Diazepam for the Home Treatment of Acute Seizures in Pediatric Patients With Epilepsy. Arch Pediatr Adolesc Med. 2010; 164 (8): 747-753.
-
A study was done to examine the efficacy of buccal midazolam in controlling convulsion in children by comparing it with intravenous diazepam, a standard mode of treating convulsions. One hundred and twenty cases presenting with convulsions to emergency were treated randomly with either buccal midazolam (in a dose of 0.2 mg/kg) or intravenous diazepam (in a dose of 0.3 mg/kg). Partial seizures, generalized tonic, clonic and tonic-clonic convulsions were included irrespective of duration or cause. One episode per child only was included. The frequency of overall control of convulsive episodes within 5 min were 85% and 93.3% in buccal midazolam and intravenous diazepam groups, respectively; the difference was, however, not statistically significant (p = 0.142). The mean time needed for controlling the convulsive episodes after administration of the drugs was significantly less with intravenous diazepam (p = <0.001). The mean time for initiation of treatment was significantly less with buccal midazolam (p = <0.001). The mean time for controlling the convulsive episodes after noticing these first were significantly less with buccal midazolam than with intravenous diazepam (p = 0.004) that is likely to be due to longer time needed for initiating treatment with intravenous diazepam in preparing the injection and establishing an IV line. There was no significant side effect in both the groups. The findings suggest that buccal midazolam can be used as an alternative to intravenous diazepam especially when getting an IV line becomes difficult. In situations where establishing an IV line is a problem, buccal midazolam may be the first choice (Class IV).
Talukdar B, Chakrabarty B; Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: A randomized controlled trial. Brain & Development 2009; 31 (10): 744-749. [PubMed Citation]
-
An outbreak of food poisoning that affected at least ten people in various regions of Japan was traced to exposure to Chinese dumplings contaminated with the organophosphate insecticide Methamidophos. We experienced the most serious case, a five years old girl, who suffered coma. She presented with features of cholinergic overactivity and her serum cholinesterase activity was 9 U/l. We started intravenous treatment with pralidoxime iodide, atropine sulfate, and midazolam. Her symptoms improved gradually and she was discharged on day 25 without any sequelae. Though poisoning attributed to organophosphate insecticides has become less common in recent years, it is even more important to diagnose the problem rapidly based on the characteristic symptoms and to start specific treatment at the earliest possible stage after poisoning (Class IV).
Sumi Y, Oode Y, Tanaka H; Chinese Dumpling Scare Hits Japan--A Case of Methamidophos Poisoning. J Toxicol Sci, 2008; 33(4):485-6. [PubMed Citation]
Pregnancy, breastfeeding studies
-
Midazolam crosses the human placenta, but this transfer, at least after oral and IM use, appears to be slower than that experienced with other benzodiazepines, such as diazepam, oxazepam, or lorazepam (2). In 13 patients given 15 mg of midazolam orally, a mean of 11.4 hours (range 1 0.5-12.4 hours) before cesarean section, only 1 had measurable levels of the drug at the time of surgery. Maternal venous level was 12 ng/mL and cord venous level was 7 ng/mL. No patient had detectable levels of the drug in the amniotic fluid. A second group of patients (N = 11) was administered 15 mg of midazolam orally at a mean of 34.3 minutes (15-60 minutes) before cesarean section. The mean serum concentrations in maternal venous, umbilical venous, and umbilical arterial blood were 12.7, 8.4, and 5.7 ng/mL, respectively. The cord venous:maternal venous and cord arterial:maternal venous ratios were 0.74 and 0.45, respectively. Six patients in a third group were administered midazolam (0.05 mg/kg) IM 18-45 minutes (mean 30.5 minutes) before cesarean section. Drug levels from the same sampling sites and ratios obtained in the second group were measured in this group, with results of 40.0, 21.7, and 12.8 ng/mL, respectively, and 0.56 and 0.32, respectively. None of the 1- and 5-minute Apgar scores of the 30 infants was less than 7, and no adverse effects attributable to midazolam were observed in the newborns (Class III).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.960-961
-
A 1989 study compared the effects of midazolam (0.3 mg/kg; N = 20) with thiopental (4 mg/kg; N = 20) in mothers undergoing induction of anesthesia before cesarean section. The only difference found between the groups was a significantly higher (mean 7.9 mm Hg) diastolic blood pressure in the midazolam group during, but not after, induction. Characteristics of the newborns from the midazolam and thiopental groups (both N = 19) were compared in the second part of this study. Oxygen via face mask was required in five midazolam exposed newborns compared with three in the thiopental group. Respiratory depression on day 1 and hypoglycemia and jaundice on day 3 were also observed in the midazolam group. Moreover, three statistically significant (p <0.05) neurobehavioral adverse effects, from the 19 tested, consisting of body temperature, general body tone, and arm recoil, were noted after midazolam exposure during the first 2 hours after birth. Other parameters that were inferior in the midazolam groups but that did not reach statistical significance, were palmar grasp, resistance against pull, and startle reaction. Although these differences do not meet all of the criteria for "floppy infant syndrome", they do indicate that the use of midazolam before cesarean section has a depressant effect on the newborn that is greater than that observed with thiopental (Class III).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.960-961
-
Midazolam is excreted into breast milk. In a study published in 1990, 12 women in the immediate postpartum period took 15 mg orally at night for 5 nights. No measurable concentrations of midazolam or the metabolite, hydroxymidazolam, were detected (<10 nmol/L) in milk samples collected a mean 7 hours (range 6-8 hours) after drug intake during the 5-day period. One mother, however, who was accidentally given a second dose (total dose 30 mg) did have a milk concentration of 30 nmol/L (milk:plasma ratio 0.20) at 7 hours. The authors estimated that the exposure of the infant would be nil in early breast milk if nursing was held for 4 hours after a 15-mg dose. Two women also were studied at 2-3 months postpartum. In six paired milk and serum samples collected up to 6 hours after a 1 5-mg dose, the mean milk:plasma ratio was 0.15. Based on an average milk concentration of 10 nmol/L, a nursing infant would ingest an estimated 0.33 mcg of midazolam and 0.34 mcg of metabolite per 100 mL of milk if nursed within 4-6 hours of the maternal dose (Class III).
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.960-961
-
BACKGROUND AND OBJECTIVES: Lactating women undergoing operations requiring general anesthesia are advised to pump and discard their milk for 24 hours after the procedure. Data on anesthetic drug transfer into breast milk are limited. This study determined thepharmacokinetics of midazolam, propofol, and fentanyl transfer into milk to provide caregivers with data regarding the safety of breast milk after administration of these drugs. METHODS: Five lactating women participated in this study after providing institutionally approved written informed consent. Patients underwent premedication with midazolam before induction of anesthesia with propofol and fentanyl. Anesthesia was maintained with a potent volatile anesthetic. Milk and blood were collected before drug administration. Milk was collected 5, 7, 9, 11, and 24 hours after drug administration. Venous blood was collected at intervals up to 7 hours. Plasma and milk midazolam, propofol, and fentanyl concentrations were measured by HPLC with tandem mass spectrometric or fluorescence detection. The pharmacokinetics of drug transfer into milk was modeled with plasmapharmacokinetics. RESULTS: Plasma midazolam, propofol, and fentanyl pharmacokinetics were consistent with reports of others. In 24 hours of milk collection, averages of 0.005% (range, 0.002%-0.013%) of the maternal midazolam dose, 0.027% (0.004%-0.082%) of the propofol dose, and 0.033% (0.006%-0.073%) of the fentanyl dose were collected in milk, representing averages of 0.009%, 0.025%, and 0.039% of the respective elimination clearances. CONCLUSION: The amount of midazolam, propofol, and fentanyl excreted into milk within 24 hours of induction of anesthesia provides insufficient justification for interrupting breast-feeding (Class III).
Nitsun M, Szokol JW, Saleh HJ, Murphy GS, Vender JS, Luong L, Raikoff K, Avram MJ; Pharmacokinetics of midazolam, propofol, and fentanyl transfer to human breast milk. Clin Pharmacol Ther. 2006 Jun; 79 (6): 549-57. [PubMed Citation]
Clinical reviews
-
Although intravenous (i.v.) administration of antiepileptic drugs is the preferred route of therapy in status epilepticus, intramuscular (i.m.) delivery may provide a valuable alternative when there are obstacles to venous access. Compared to other treatment options such as rectal drug administration, which is as challenging as the i.v. route in a convulsing patient, the i.m. route is easier and less invasive. The two most commonly used first-line anticonvulsants, diazepam and lorazepam, may be administered i.m., but are absorbed from the i.m. site more slowly than midazolam. Midazolam, a fairly new benzodiazepine, is a potent anticonvulsant with a fast onset of effect. Because of its water solubility, midazolam is rapidly absorbed from the injection site and has excellent local tolerability. The pharmacodynamic effects of midazolam can be seen within seconds of its administration, and seizure arrest is usually attained within 5 to 10 min. Case reports and a recent randomized trial that demonstrate the successful use of i.m. midazolam in the termination of epileptic seizures are reviewed (Class IV).
Towne AR, DeLorenzo RJ; Use of Intramuscular Midazolam for Status Epilepticus. The Journal of Emergency Medicine, 1999; 17 (2): 323-328. [PubMed Citation]
-
Successful management of incidents with chemical warfare agents strongly depends on the speed of medical help and the ability of helpers to react properly. Though the general principles of clinical toxicology, such as decontamination, stabilization, patient evaluation and symptomatic treatment are similar for many toxicants, chemical warfare agents deserve special attention because of their very high inhalative and cutaneous toxicity, rapid onset of the disease and multiple organ failures. This article describes the medical management of mass casualties with blister agents, nerve agents and blood agents from the viewpoint of a clinical toxicologist. Characteristic diagnostic signs, decontamination procedures and therapeutic schemes for these agents are described. Treatment options are discussed. The importance of planning (e.g. antidote availability) and preparedness is emphasized (Class IV).
Zilker T; Medical management of incidents with chemical warfare agents. Toxicology 2005 Oct; 214 (3): 221-31. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
Control of seizure activity is critical to survival and neuroprotection following nerve agent exposure. Extensive research has shown that three classes of drugs, muscarinic antagonists, benzodiazepines, and N-methyl-D-aspartate antagonists, are capable of moderating these seizures. This study began to map the neural areas in rat brain that respond to these three drug classes resulting in anticonvulsant effects. Drugs of each class (scopolamine, midazolam, MK-801) were evaluated for their ability to prevent sarin-induced seizures when injected into specific brain areas (lateral ventricle, anterior piriform cortex, basolateral amygdala, area tempestas). Animals were pretreated by microinjection with saline or a dose of drug from one of the three classes 30 min prior to receiving 150 microg/kg sarin, subcutaneously, followed by 2.0 mg/kg atropine methylnitrate, intramuscularly. Animals were then returned to their cages, where electroencephalographic activity was monitored for seizures. Anticonvulsant effective doses (ED(50)) were determined using an up-down dosing procedure over successive animals. Scopolamine provided anticonvulsant effects in each area tested, while midazolam was effective in each area except the lateral ventricle. MK-801 was only effective at preventing seizures when injected into the basolateral amygdala or area tempestas. The results show a unique neuroanatomical and pharmacological specificity for control of nerve agent-induced seizures.
Skovira JW, McDonough JH, Shih TM; Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. J Mol Neurosci. 2010 Jan; 40 (1-2): 56-62. [PubMed Citation]
-
This study evaluated the anticonvulsant effectiveness of midazolam to stop seizures elicited by the nerve agent soman when midazolam was administered by different routes (intramuscular, intranasal or sublingual) at one of two different times after the onset of seizure activity. Guinea pigs previously prepared with cortical electrodes to record brain electroencephalographic activity were pre-treated with pyridostigmine (0.026 mg/kg, intramuscularly) 30 min. before challenge with a seizure-inducing dose of the nerve agent soman (56 microg/kg, subcutaneously), and 1 min. later, they were administered 2.0 mg/kg atropine sulfate admixed with 25.0 mg/kg 2-PAM Cl (intramuscularly). Groups of animals were administered differing doses of midazolam by the intramuscular, intranasal or sublingual route at either the onset of seizure activity or 40 min. after the onset of seizure activity that was detected in the electroencephalographic record. When given immediately after seizure onset, the anticonvulsant ED50 of intramuscular midazolam was significantly lower than that of intranasal midazolam, which in turn was significantly lower than sublingual midazolam at that time. At the 40-min. treatment delay, the anticonvulsant ED50s of intramuscular or intranasal midazolam did not differ and both were significantly lower than the sublingual route. Higher doses of midazolam were required to stop seizures at the 40-min. treatment delay time compared to immediate treatment. The speed of seizure control for intramuscular or intranasal midazolam was the same while sublingual midazolam acted significantly slower. Midazolam was effective in treating soman-induced seizures when given by all three routes, but with differences in potency and speed of action.
McDonough JH, Van Shura KE, LaMont JC, et al; Comparison of the intramuscular, intranasal or sublingual routes of midazolam administration for the control of soman-induced seizures. Basic Clin Pharmacol Toxicol, 2009 Jan; 104(1):27-34. [PubMed Citation]
-
Two guinea pig models were used to study the anticonvulsant potency of diazepam, midazolam, and scopolamine against seizures induced by the nerve agents tabun, sarin, soman, cyclosarin, O-ethyl S-(2-(diisopropylamino)ethyl)methylphosphonothioate (VX), and O-isobutyl S-(2-diethylamino)ethyl)-methyl phosphonothioate (VR). Animals instrumented for electroencephalogram recording were pretreated with pyridostigmine bromide (0.026 mg/kg i.m.) 30 min before challenge with 2 x LD50 (s.c.) of a nerve agent. In model A, atropine sulfate (2.0 mg/kg i.m.) and pyridine-2-aldoxime methylchloride (2-PAM; 25.0 mg/kg i.m.) were given 1 min after nerve agent challenge, and the tested anticonvulsant was given (i.m.) 5 min after seizure onset. In model B, a lower dose of atropine sulfate (0.1 mg/kg i.m.) was given along with 2-PAM 1 min after nerve agent challenge, and the anticonvulsant was given at seizure onset. With the lower dose of atropine, seizure occurrence increased to virtually 100% for all agents; the time to seizure onset decreased for sarin, cyclosarin, and VX; the signs of nerve agent intoxication were more severe; and coma resulted frequently with cyclosarin. The anticonvulsant ED50 doses for scopolamine or diazepam were, in general, not different between the two models, whereas the anticonvulsant ED50 values of midazolam increased 3- to 17-fold with the lower atropine dose. Seizure termination times were not systematically effected by the different doses of atropine. The order of anticonvulsant effectiveness within each model was scopolamine > or = midazolam > diazepam. The findings indicate that the dose of atropine given as antidotal therapy can significantly influence measures of nerve agent toxicity and responsiveness to anticonvulsant therapy.
Shih TM, Rowland TC, McDonough JH; Anticonvulsants for nerve agent-induced seizures: The influence of the therapeutic dose of atropine. J Pharmacol Exp Ther. 2007 Jan; 320 (1): 154-61. [PubMed Citation]
-
Centrally mediated seizures and convulsions are common consequences of exposure to organophosphates (OPs). These seizures rapidly progress to status epilepticus (SE) and contribute to profound brain injury. Effective management of these seizures is critical for minimization of brain damage. Nasal application of midazolam (1.5 mg/kg) after 5 min of sarin-induced electrographic seizure activity (EGSA) ameliorated EGSA and convulsive behavior (238 +/- 90 s). Identical treatment after 30 min was not sufficient to ameliorate ECoG paradoxical activity and convulsive behavior. Nasalmidazolam (1.5 mg/kg), together with scopolamine (1 mg/kg, im) after 5 min of EGSA, exerted a powerful and rapid anticonvulsant effect (53 +/- 10 s). Delaying the same treatment to 30 min of EGSA leads to attenuation of paroxysmal ECoG activity in all cases but total cessation of paroxysmal activity was not observed in most animals tested. Cognitive tests utilizing the Morris Water Maze demonstrated that nasal midazolam alone or together with scopolamine (im), administered after 5 min of convulsions, abolished the effect of sarin on learning. Both these treatments, when given after 30 min of convulsions, only decreased the sarin-induced learning impairments. Whereas rats which were not subject to the anticonvulsant agents did not show any memory for the platform location, both treatments (at 5 min as well as at 30 min) completely abolished the memory deficits. Both treatments equally blocked the impairment of reversal learning when given at 5 min. However, when administered after 30 min, midazolam alone reversed the impairments in reversal learning, while midazolam with scopolamine did not. Rats exposed to sarin and treated with the therapeutic regimen with the exclusion of midazolam exhibited severe brain lesions that encountered the hippocampus, pyriform cortex, and thalamus. Nasalmidazolam at 5 min prevented brain damage, while delaying the midazolam treatment to 30 min of EGSA resulted in brain damage. The addition of scopolamine to midazolam did not alter the above observation. In summary, nasal midazolam treatment briefly after initiation of OP-induced seizure leads to cessation of EGSA and prevented brain lesions and behavioral deficiencies in the rat model.
Gilat E, Kadar T, Levy A, Rabinovitz I, Cohen G, Kapon Y, Sahar R, Brandeis R; Anticonvulsant treatment of sarin-induced seizures with nasal midazolam: an electrographic, behavioral, and histological study in freely moving rats. Toxicol Appl Pharmacol. 2005 Nov 15; 209 (1): 74-85. [PubMed Citation]
-
Benzodiazepine drugs are effective anticonvulsant treatments for terminating nerve agent-induced seizures. However, only two benzodiazepines, diazepam and lorazepam, are approved for use in the acute treatment of seizures. Previous tests with diazepam and lorazepam have shown that they control nerve agent seizures very slowly when administered intramuscularly (IM) in contrast to the benzodiazepine midazolam. The present studies addressed whether the speed of seizure control by a benzodiazepine could be enhanced by two manipulations, either increasing the administered dose or by combining the benzodiazepine with an anticholinergic drug in a standardized guinea pig seizure model. Animals were implanted with recording electrodes to monitor cortical electroencephalographic (EEG) activity one week before experiments. On the day of exposure the animals were pretreated with pyridostigmine Br (0.026 mg/kg, IM) 30 min prior to challenge with 56 ug/kg, SC, (2 x LD50) soman. One min after soman challenge the animals were treated (IM) with 2 mg/kg atropine SO4 admixed with 25 mg/kg 2-PAM Cl, and the EEG was observed for seizure onset. Typically, 4-6 doses of drug were tested with 4-7 animals/group. When treatment was given 5 min after seizure onset, all three benzodiazepines controlled seizures in virtually 100% of the cases at the higher doses. Time to terminate seizures tended to decrease at the higher doses of diazepam, although there were still a number of outliers at all doses tested. Seizure termination times were short (15-20 min) with midazolam and did not substantially decrease at higher doses, again with a number of outliers. Seizure termination times remained long (67-92 min) across all tested doses of lorazepam. When scopolamine was combined with diazepam or midazolam there was some evidence of drug potentiation in the combinations when treatment was delayed for 40 min after seizure onset. For example, diazepam (4.8 mg/kg) adminis-tered 40 min after seizure onset terminated seizures in 67% (4 of 6) of the animals tested; scopolamine (0.1 mg/kg) administered under the same conditions failed to terminate seizures (0 of 6). When these doses were combined, seizures were controlled in 92% (11 of 12) of the animals tested. This potentiative effect of scopolamine was similar with midazolam. A dose of 2.0 mg/kg midazolam terminated seizures in 60% (3 of 5) animals tested, but when combined with the 0.1 mg/kg dose of scopolamine seizures were terminated in 92% (11 of 12) of the animals tested. There was no appreciable enhancement of seizure termination speed. The results show that increasing the treatment dose of benzodiazepine can produce a successful anticon-vulsant effect in all animals and can possibly enhance the speed of anticonvulsant effect, especially with diazepam. The results also show that administration of a strong centrally active anticholinergic drug such as scopolamine in combination with either diazepam or midazolam can modestly enhance the anticonvulsant action of low doses of a benzodiazepine, but does not increase the speed of seizure control.
McDonough JH, McMonagle J, Gonzales M, Rowland R, Shih TM; Studies on anticonvulsants against nerve agent-induced seizures. Pharmacology Division U.S. Army Medical Research Institute of Chemical Defense Aberdeen Proving Ground, MD 21010-5400 USA 2003
-
Seizures and status epilepticus, which may contribute to brain injury, are common consequences of exposure to organophosphorus (OP) cholinesterase inhibitors. Effective management of these seizures is critical. To investigate the efficacy of nasal midazolam as an anticonvulsive treatment for OP exposure, as compared to intramuscular midazolam, guinea pigs were connected to a recording swivel for electrocorticograph (ECoG) monitoring and clinical observation. The experimental paradigm consisted of pyridostigmine pretreatment (0.1 mg/kg i.m.) 20 min prior to sarin exposure (1.2x LD(50) 56 microg/kg i.m.). One minute post-exposure, atropine (3 mg/kg i.m.) and TMB-4 (1 mg/kg im) were administered. Within 3-8 min after sarin exposure all animals developed electrographic seizure activity (EGSA), with convulsive behavior. Treatment with midazolam (1 mg/kg i.m.) 10 min after the onset of EGSA abolished EGSA within 389+/-181 s. The same dose was not effective, in most cases, when given 30 min after onset. However, a higher dose (2 mg/kg) was found efficacious after 30 min (949+/-466 s). In contrast, nasal application of midazolam (1 mg/kg) was found most effective, with significant advantages, in amelioration of EGSA and convulsive behavior, when given 10 min (216+/-185 s) or 30 min (308+/-122 s) following the onset of EGSA ( P<0.001). Thus, nasal midazolam could be used as a novel, rapid and convenient route of application against seizure activity induced by nerve agent poisoning.
Gilat E, Goldman M, Lahat E, Levy A, Rabinovitz I, Cohen G, Brandeis R, Amitai G, Alkalai D, Eshel G; Nasal midazolam as a novel anticonvulsive treatment against organophosphate-induced seizure activity in the guinea pig. Arch Toxicol. 2003 Mar; 77 (3): 167-72. [PubMed Citation]
-
This study evaluated the potency and rapidity of some anticholinergics (atropine, biperiden, and trihexyphenidyl) and benzodiazepines (diazepam and midazolam) as an anticonvulsant treatment against seizures induced by six nerve agents (tabun, sarin, soman, cyclosarin, VR, and VX) and summarized the relationship between anticonvulsant activity and nerve agent-induced lethality and neuropathology. Guinea pigs, previously implanted with cortical electrodes for EEG recording, were pretreated with pyridostigmine bromide (0.026 mg/kg im) 30 min prior to challenge with 2xLD50 dose (sc) of a given nerve agent; in a separate experiment, animals were challenged with 5x LD50 sc of soman. One minute after agent challenge the animals were treated im with 2 mg/kg atropine SO4 admixed with 25 mg/kg 2-PAM Cl. Five minutes after the start of EEG seizures, animals were treated im with different doses of anticholinergics or benzodiazepines and observed for seizure termination. The time to seizure onset, the time to seizure termination, and 24-h lethality were recorded. The anticonvulsant ED50 of each drug for termination of seizures induced by each agent was calculated and compared. Brain tissue from animals that survived 24 h was examined for pathology. All drugs were capable of terminating seizure activity, with midazolam and trihexyphenidyl being significantly more potent than the other drugs, and midazolam being more rapid in controlling seizure than atropine, trihexyphenidyl, or diazepam against each agent. Seizures induced by sarin or VX required lower doses of all the test anticonvulsants. The dose of a given drug that was an effective anticonvulsant against a 2xLD50 challenge of soman was equally effective against seizures induced by a 5xLD50 challenge. All nerve agents were capable of producing neuropathology. Seizure control was strongly associated with protection against acute lethality and brain pathology.
Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003 Apr 15;188(2):69-80. [PubMed Citation]
-
These studies investigated the effectiveness of combination treatment with a benzodiazepine and an anticholinergic drug against soman-induced seizures. The anticholinergic drugs considered were biperiden, scopolamine, trihexaphenidyl, and procyclidine; the benzodiazepines were diazepam and midazolam. Male guinea pigs were implanted surgically with cortical screw electrodes. Electrocorticograms were displayed continually and recorded on a computerized electroencephalographic system. Pyridostigmine (0.026 mg x kg(-1), i.m.) was injected as a pretreatment to inhibit red blood cell acetylcholinesterase by 30-40%. Thirty minutes after pyridostigmine, 2 x LD50 (56 microg x kg(-1)) of soman was injected s.c., followed 1 min later by i.m. treatment with atropine (2 mg x kg(-1)) + 2-PAM (25 mg x kg(-1)). Electrographic seizures occurred in all animals. Anticonvulsant treatment combinations were administered i.m. at 5 or 40 min after seizure onset. Treatment consisted of diazepam or midazolam plus one of the above-mentioned anticholinergic drugs. All doses of the treatment compounds exhibited little or no antiseizure efficacy when given individually. The combination of a benzodiazepine and an anticholinergic drug was effective in terminating soman-induced seizure, whether given 5 or 40 min after seizure onset. The results suggest a strong synergistic effect of combining benzodiazepines with centrally active anticholinergic drugs and support the concept of using an adjunct to supplement diazepam for the treatment of nerve-agent-induced seizures.
Koplovitz I, Schulz S, Shutz M, Railer R, Macalalag R, Schons M, McDonough J; Combination anticonvulsant treatment of soman-induced seizures. J Appl Toxicol. 2001 Dec; 21 Suppl 1:S53-5. [PubMed Citation]
-
This study evaluated the ability of six benzodiazepines to stop seizures produced by exposure to the nerve agent soman. Guinea pigs, previously prepared with electrodes to record electroencephalographic (EEG) activity, were pretreated with pyridostigmine (0.026 mg/kg, i.m.) 30 min before challenge with soman (56 microg/kg, s.c.) and then treated 1 min after soman exposure with atropine (2.0 mg/kg, i.m.) and pralidoxime chloride (2-PAM Cl; 25 mg/kg, i.m.). All animals developed seizures following this treatment. Benzodiazepines (avizafone, clonazepam, diazepam, loprazolam, lorazepam, and midazolam) were given i.m. 5 or 40 min after seizure onset. All benzodiazepines were effective in stopping soman-induced seizures, but there were marked differences between drugs in the rapidity of seizure control. The 50% effective dose (ED50) values and latencies for anticonvulsant effect for a given benzodiazepine were the same at the two times of treatment delay. Midazolam was the most potent and rapidly acting compound at both treatment times. Since rapid seizure control minimizes the chance of brain damage, use of midazolam as an anticonvulsant may lead to improved clinical outcome in the treatment of nerve agent seizures.
McDonough JH Jr, McMonagle J, Copeland T, Zoeffel D, Shih TM. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol. 1999 Nov;73(8-9):473-8. [PubMed Citation]
-
This report describes studies of anticonvulsants for the organophosphorus (OP) nerve agent soman: a basic research effort to understand how different pharmacological classes of compounds influence the expression of seizure produced by soman in rats, and a drug screening effort to determine whether clinically useful antiepileptics can modulate soman-induced seizures in rats. Electroencephalographic (EEG) recordings were used in these studies. Basic studies were conducted in rats pretreated with HI-6 and challenged with 1.6 x LD50 soman. Antimuscarinic compounds were extremely effective in blocking (pretreatment) or terminating soman seizures when given 5 min after seizure onset. However, significantly higher doses were required when treatment was delayed for more than 10 min, and some antimuscarinic compounds lost anticonvulsant efficacy when treatment was delayed for more than 40 min. Diazepam blocked seizure onset, yet seizures could recur after an initial period of anticonvulsant effect at doses </=2.5 mg/kg. Diazepam could terminate ongoing seizures when given 5 min after seizure onset, but doses up to 20 mg/kg were ineffective when treatment was delayed for 40 min. The GABA uptake inhibitor, tiagabine, was ineffective in blocking or terminating soman motor convulsions or seizures. The glutamate receptor antagonists, NBQX, GYKI 52466, and memantine, had weak or minimal antiseizure activity, even at doses that virtually eliminated signs of motor convulsions. The antinicotinic, mecamylamine, was ineffective in blocking or stopping seizure activity. Pretreatment with a narrow range of doses of alpha2-adrenergic agonist, clonidine, produced variable protection (40-60%) against seizure onset; treatment after seizure onset with clonidine was not effective. Screening studies in rats, using HI-6 pretreatment, showed that benzodiazepines (diazepam,midazolam and lorazepam) were quite effective when given 5 min after seizure onset, but lost their efficacy when given 40 min after onset. The barbiturate, pentobarbital, was modestly effective in terminating seizures when given 5 or 40 min after seizure onset, while other clinically effective antiepileptic drugs, trimethadione and valproic acid, were only slightly effective when given 5 min after onset. In contrast, phenytoin, carbamazepine, ethosuximide, magnesium sulfate, lamotrigine, primidone, felbamate, acetazolamide, and ketamine were ineffective.
Shih T-M, McDonough JH, McMonagle J, Koplovitz I.; Anticonvulsants for soman-induced seizure activity. J Biomed Sci, 1999 Mar-Apr; 6(2):86-96. [PubMed Citation]
-
The effects of midazolam (MDZ), diazepam (DZ) and scopolamine (SCP) therapies on soman-induced electrocorticogram (ECoG) and biceps femoris electromyogram (EMG) activities and brain lesions were assessed in male rats. Animals received pyridostigmine (26 micrograms/kg, im) 30 min before soman (87.1 micrograms/kg, im) followed by therapy consisting of atropine (1.5 mg/kg) admixed with 2-PAM (25 mg/kg, im) 1 min later; MDZ (0.5 mg/kg), DZ (1.77 mg/kg) or SCP (0.43 mg/kg) was administered im at 1 min after the onset of convulsions (CVs). Typically, within 5 min after soman the ECoG profile changed to a full-blown, spike-and-dome epileptiform (SDE) pattern followed by CVs and increased amplitude of EMG activity. Treatment with SCP restored ECoG and EMG profiles by 30 min. At 2 hr after exposure only 1 animal demonstrated a slight abnormality in ECoG activity which was normal at 24 hr. Similarly, DZ and MDZ restored EcoG and EMG profiles by 30 min; however, in contrast to SCP, 83% of the animals demonstrated reappearance of SDE 2 hrs after soman. SCP therapy also enabled rats to move about in their cages by 30 min post treatment. In contrast, DZ- and MDZ-treated rats remained incapacitated as late as 2 hr post-exposure. Animals were euthanized at 24 hr, and the extent of soman-induced brain lesions was determined by light microscopic analysis. When present, brain lesions were minimal in SCP-treated rats. The mean brain lesion scores across all experimental conditions ranked as follows: soman control > MDZ > DZ > or = SCP = saline control. These observations suggest that SCP may be highly effective in severe soman intoxication.
Anderson DR, Harris LW, Chang FC, Baze WB, Capacio BR, Byers SL, Lennox WJ; Antagonism of soman-induced convulsions by midazolam, diazepam and scopolamine. Drug Chem Toxicol. 1997 Aug; 20 (3): 115-31. [PubMed Citation]
-
The aim of this study was to compare the anticonvulsive and protective effects of diazepam and midazolam in rats poisoned by chemical warfare agents. In rats treated with soman, sarin or VX, the anticonvulsive effects of midazolam and diazepam were of similar magnitude. Atropine and oxime HI-6 decreased the toxicity of soman, sarin and VX 1.65, 2.06 and 18.3 times, respectively. The introduction of diazepam and midazolam in the therapy of rats poisoned by VX and sarin led to further improvement of protective indices. Midazolam was even more effective than diazepam. A reliable protective effect was obtained with the lowest dose of both benzodiazepines used (0.5 mg/kg). The specific benzodiazepine antagonist flumazenil abolished, almost completely, the protective effect of both benzodiazepines. These data confirmed a significant role of the gabaergic system in poisoning with organophosphorus compounds, especially during the initial stage of intoxication.
Bokonjić D, Rosić N; Anticonvulsive and protective effects of diazepam and midazolam in rats poisoned by highly toxic organophosphorus compounds. Arh Hig Rada Toksikol. 1991 Dec; 42 (4): 359-65. [PubMed Citation]
-
Two benzodiazepine compounds, midazolam and diazepam, were administered as adjunctive treatment to soman-exposed rhesus monkeys to evaluate their effects on acute soman intoxication. Monkeys were pretreated orally with pyridostigmine, exposed to soman, and treated i.m. with atropine, pralidoxime chloride (2-PAM), and with midazolam, diazepam or sterile water (control). All monkeys that received the benzodiazepines recovered sooner and exhibited no convulsions. Neuronal degenerative and necrotic lesions were decreased or eliminated in the entorhinal cortex, caudate nucleus, and hippocampus of those animals that received benzodiazepine therapy. These findings support the continued evaluation of drugs with anticonvulsant activity as standard adjunct therapy for soman intoxication.
Hayward IJ, Wall HG, Jaax NK, Wade JV, Marlow DD, Nold JB; Decreased brain pathology in organophosphate-exposed rhesus monkeys following benzodiazepine therapy. J Neurol Sci. 1990 Aug; 98 (1): 99-106. [PubMed Citation]
Other non-clinical studies
Animal in vitro studies-
Soman, a potent irreversible acetylcholinesterase (AChE) inhibitor, induces delayed neuronal injury by reactive oxygen species (ROS). Midazolam is used in patients with pathologic effects of oxidative stresses such as infection, hemodynamic instability and hypoxia. We investigated whether midazolam protects the Central Nervous System (CNS) from soman intoxication. The present study was performed to determine whether midazolam protects B35 cells from ROS stress for the purpose of exploring an application of midazolam to soman intoxication. Glucose oxidase (GOX) induced ROS stress was used in a B35 neuroblastoma cell model of ROS induced neuronal injury. To investigate the effect of midazolam on cell viability, LDH assays and fluorescence activated cell sorting (FACS) analysis was performed. Western blotting was used for evaluating whether Akt-phosphorylation is involved in cell-protective effects of midazolam. GOX derived ROS injury decreased cell viability about 1.6-2 times compared to control; midazolam treatment (5 and 10 μg/ml) dose-dependently increased cell viability during ROS injury. On western blots, Akt-phosphorylation was induced during pretreatment with midazolam; it was diminished during co-treatment with LY-294002, an inhibitor of Akt-phosphorylation. FACS analysis confirmed that the cell protective effect ofmidazolam is mediated by an anti-apoptotic effect. GOX-induced apoptosis was inhibited by midazolam and the finding was diminished by LY-294002. Midazolam protects neuronal cells from GOX-induced ROS injury; this effect is mediated by an anti-apoptotic effect through Akt-phosphorylation. This shows that midazolam may be useful in soman intoxication.
Chong WS, Hyun CL, Park MK, Park JM, Song HO, Park T, Lim YS, Cho CK, Kang PS, Kwon HU; Midazolam protects B35 neuroblastoma cells through Akt-phosphorylation in reactive oxygen species derived cellular injury. Korean J Anesthesiol. 2012 Feb; 62 (2): 166-71.
Non-clinical reviews
-
A review of the literature was conducted to provide an overview of organophosphorus (OP)-induced morphological changes in the non-human primate. Most studies have evaluated effects of the OP nerve agent soman (pinacolyl methylphosphonofluoridate), an irreversible inhibitor of acetylcholinesterase. Soman-induced acute and chronic morphological changes have been examined. The effects of nerve agent therapy (i.e. pyridostigmine, praloxidime chloride and atropine), with and without an anticonvulsant (i.e. diazepam, midazolam), on soman-induced lesions have also been studied. Acute changes in the central nervous system of rhesus and cynomolgus monkeys exposed to soman alone or soman and therapy, without an anticonvulsant, were characterized by neuronal degeneration and necrosis and neuropil edema. The lesions were usually present in the frontal cortex, entorhinal cortex, amygdaloid complex, caudate nucleus, thalamus and hippocampus. Morphologically, these lesions resemble lesions produced by hypoxic-ischemic injury or by seizures and are similar to soman-induced changes in other laboratory animals. Nerve agent therapy supplemented with an anticonvulsant reduced or prevented soman-induced acute neural lesions. Acute changes in non-neural tissues were limited to the heart (e.g. hemorrhage, myofiber necrosis, myocarditis) and skeletal muscle (e.g. myofiber necrosis). Heart lesions in the non-human primate are similar to OP-induced heart lesions in man. The pathogenesis of the acute lesions in both the central nervous system and heart is discussed. Consistent soman-induced chronic morphological changes have not been produced in the rhesus monkey or baboon.
Baze WB; Soman-induced morphological changes: an overview in the non-human primate. J Appl Toxicol. 1993 May-Jun; 13 (3): 173-7. [PubMed Citation]
-
The drug midazolam has been recommended to replace diazepam as the immediate anticonvulsant treatment for nerve agent-induced seizures. This recommendation marks the latest decision in an ongoing program to improve medical countermeasures to treat nerve agent poisoning. Extensive rodent screening studies first identified midazolam as the most promising compound to focus on for advanced testing. Midazolam was then evaluated directly with diazepam for the ability to terminate nerve agent seizures in a nonhuman primate model. In all animal tests midazolam was twice as potent and more rapidly acting than diazepam, thus minimizing the possibility of seizure-induced brain damage.
McDonough, JH; Pharmacology Division, U.S. Army Medical Research Institute of Chemical Defense. Midazolam: An Improved Anticonvulsant Treatment For Nerve Agent-Induced Seizures. Proceedings of the 2001 ECBC Scientific Conference on Chemical and Biological Defense Research, 6-8 March , Marriott's Hunt Valley Inn, Hunt Valley, MD.
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdults
-
The absolute bioavailability of the intramuscular route was greater than 90% in a crossover study in which healthy subjects (n=17) were administered a 7.5 mg IV or IM dose. The mean peak concentration (Cmax) and time to peak (Tmax) following the IM dose was 90 ng/mL (20% CV) and 0.5 hr (50% CV). Cmax for the 1-hydroxy metabolite following the IM dose was 8 ng/mL (Tmax = 1 hour). Following IM administration, Cmax for midazolam and its 1-hydroxy metabolite were approximately one-half of those achieved after intravenous injection.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
The volume of distribution (Vd) determined from six single-dose pharmacokinetic studies involving healthy adults ranged from 1 to 3.1 L/kg. Female gender, old age, and obesity are associated with increased values of midazolam Vd. In humans, midazolam has been shown to cross the placenta and enter into fetal circulation and has been detected in human milk and CSF. In adults and pediatric patients older than 1 year, midazolam is approximately 97% bound to plasma protein, principally albumin.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
Six single-dose pharmacokinetic studies involving healthy adults yield pharmacokinetic parameters for midazolam in the following ranges: volume of distribution (Vd), 1.0 to 3.1 L/kg; elimination half-life, 1.8 to 6.4 hours (mean approximately 3 hours); total clearance (Cl), 0.25 to 0.54 L/hr/kg. In a parallel group study, there was no difference in the clearance, in subjects administered 0.15 mg/kg (n=4) and 0.30 mg/kg (n=4) IV doses indicating linear kinetics. The clearance was successively reduced by approximately 30% at doses of 0.45 mg/kg (n=4) and 0.6 mg/kg (n=5) indicating non-linear kinetics in this dose range.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
The principal urinary excretion product is 1-hydroxy-midazolam in the form of a glucuronide conjugate; smaller amounts of the glucuronide conjugates of 4-hydroxy- and dihydroxy-midazolam are detected as well. The amount of midazolam excreted unchanged in the urine after a single IV dose is less than 0.5% (n=5). Following a single IV infusion in 5 healthy volunteers, 45% to 57% of the dose was excreted in the urine as 1-hydroxymethyl midazolam conjugate. Studies of the intravenous administration of 1-hydroxy-midazolam in humans suggest that 1-hydroxy-midazolam is at least as potent as the parent compound and may contribute to the net pharmacologic activity of midazolam. In vitro studies have demonstrated that the affinities of 1- and 4-hydroxy-midazolam for the benzodiazepine receptor are approximately 20% and 7%, respectively, relative to midazolam.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
Midazolam in an autoinjector was evaluated in an open-label dose escalation study involving 39 healthy participants. Safety and pharmacokinetic parameters were determined for doses ranging from 5 to 30 mg. No serious adverse events were noted during the study. Two participants (30 mg) experienced changes in their electrocardiogram (trigeminy and prolongation of QRS complex) that met the criteria for dose-limiting adverse events. No significant respiratory depression was noted during the study. The midazolam doses studied exhibited a median t(max) of 0.5 hours with a geometric mean terminal elimination half-life value of 4.1 hours (range, 2.9-4.5 hours). The extent of systemic exposure, assessed by area under the curve (AUC) and maximum concentration (C(max)), tended to increase proportionally with increasing doses from 5 to 30 mg; however, for the male 30-mg group, there was evidence of a larger than proportional increase in AUC.
Reichard DW, Atkinson AJ, Hong SP, Burback BL, Corwin MJ, Johnson JD; Human safety and pharmacokinetic study of intramuscular midazolam administered by autoinjector. J Clin Pharmacol. 2010 Oct; 50(10):1128-35. [PubMed Citation]
-
There have been conflicting reports on the pharmacokinetics of midazolam, administered i.m. The aims of this study were to determine the pharmacokinetic data of midazolam following different doses and to test whether a correlation exists between its plasma level and sedative effect. METHODS: Fifteen patients between the ages of 18 and 50 were divided into three groups for i.m. administration of midazolam 0.05 mg/kg (group 1), 0.1 mg/kg (group 2), or 0.15 mg/kg (group 3) i.m. Venous blood was drawn 6, 12, 18, 24, 30, 36, 42, 48, 54, 60 min, and 2, 3, 4, 6, 8 h after the injection. After the same times the sedative effect was estimated by the anesthetist (awake, sleeping but easy to wake, sleeping and difficult to wake, unconscious). The plasma midazolam levels were determined by gas chromatography. The following pharmacokinetic parameters were ascertained: Cmax (peak concentration), tmax (time to attain peak concentration), clearance, elimination half-life. RESULTS: The peak concentration is directly proportional to the dosage of midazolam and the relation between the two is linear. The median Cmax values were 35.3 ng/ml (group 1), 103 ng/ml (group 2) and 123.5 ng/ml (group 3). The duration of tmax was between 12 and 36 min (means = 27 min). There was no significant difference between the groups in clearance, tmax, or elimination half-life. A significant correlation was found between the plasma midazolam levels and the degree of sedation. However, we observed a considerable variability in the effect. CONCLUSION: A 95% confidence interval for the prediction of the peak concentration of midazolam after i.m. injection is stated. Midazolam should be administered at a dose of 0.05 mg/kg at the most, if unconsciousness after premedication is to be avoided.
Behne M, Janshon G, Asskali F, Förster H; The pharmacokinetics of midazolam following intramuscular administration. Anaesthesist. 1989 Jun; 38 (6): 278-84. [PubMed Citation]
Children
-
The pharmacokinetics of midazolam and its major metabolite, α-hydroxymidazolam, and the absolute bioavailability of midazolam HCl syrup were studied in pediatric patients of different ages (6 months to <16 years old) over a 0.25 to 1.0 mg/kg dose range. The mean Tmax values across dose groups (0.25, 0.5, and 1.0 mg/kg) range from 0.17 to 2.65 hours. Midazolam exhibits linear pharmacokinetics between oral doses of 0.25 to 1.0 mg/kg (up to a maximum dose of 40 mg) across the age groups ranging from 6 months to <16 years. Linearity was also demonstrated across the doses within the age group of 2 years to <12 years having 18 patients at each of the three doses. The absolute bioavailability of the midazolam HCl syrup in pediatric patients is about 36%, which is not affected by pediatric age or weight. The AUC0-∞ ratio of α-hydroxymidazolam to midazolam for the oral dose in pediatric patients is higher than for an IV dose (0.38 to 0.75 versus 0.21 to 0.39 across the age group of 6 months to <16 years), and the AUC0-∞ ratio of a-hydroxymidazolam to midazolam for the oral dose is higher in pediatric patients than in adults (0.38 to 0.75 versus 0.40 to 0.56).
Product label Midazolam hydrochloride (midazolam hydrochloride syrup) syrup [Roxane] Last revised: June 2008 [DailyMed]
-
In pediatric patients aged 1 year and older, the pharmacokinetic properties following a single dose of midazolam reported in 10 separate studies of midazolam are similar to those in adults. Weight-normalized clearance is similar or higher (0.19 to 0.8 L/hr/kg) than in adults and the terminal elimination half-life (0.78 to 3.3 hours) is similar to or shorter than in adults. The pharmacokinetic properties during and following continuous intravenous infusion in pediatric patients in the operating room as an adjunct to general anesthesia and in the intensive care environment are similar to those in adults. In seriously ill neonates, however, the terminal elimination half-life of midazolam is substantially prolonged (6.5 to 12 hours) and the clearance reduced (0.07 to 0.12 L/hr/kg) compared to healthy adults or other groups of pediatric patients. It cannot be determined if these differences are due to age, immature organ function or metabolic pathways, underlying illness or debility.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
AIMS: To characterize the pharmacokinetics and metabolism of oral midazolam in 15 preterm infants. METHODS: After an oral dose (0.1 mg kg(-1)), blood was drawn up to 24 h after administration. Midazolam and 1-OH-midazolamconcentrations were determined with GC-MS. In 8 out of these 15 patients the pharmacokinetics of intravenous midazolam was also studied. RESULTS: Apparent oral clearance, apparent volume of distribution, plasma half-life and 1-OH-Midazolam/Midazolam AUC ratio were [median (range)]: 2.7 [0.67-15.5] ml kg(-1) min(-1), 1.4 [0.3-12.1] l kg(-1), 7.6 [1.2-15.1], h and 0.03 [0.01-0.96], respectively. Absolute bioavailability was 0.49 [0.12-1.0]. CONCLUSIONS: Midazolam oral clearance is markedly decreased in preterm infants as compared with older children, probably because of immature CYP3A4 activity.
de Wildt SN, Kearns GL, Hop WC, Murry DJ, Abdel-Rahman SM, van den Anker JN; Pharmacokinetics and metabolism of oral midazolam in preterm infants. Br J Clin Pharmacol. 2002 Apr; 53 (4): 390-2. [PubMed Citation]
-
BACKGROUND: Midazolam, a benzodiazepine, is finding expanded use in neonatal intensive care units. We studied the pharmacokinetics and metabolism of midazolam after a single intravenous dose in preterm infants. METHODS: The pharmacokinetics of midazolam and its hydroxylated metabolite (1-OH-midazolam) after a single 0.1 mg/kg intravenous dose of midazolam were determined in 24 preterm infants (gestational age, 26 to 34 weeks; postnatal age, 3 to 11 days). Blood samples were obtained before drug administration and at 0.5, 1, 2, 4, 6, 12, and 24 hours after the start of the infusion. Midazolam and 1-OH-midazolam concentrations were determined by use of gas chromatography-mass spectrometry. RESULTS: Total body clearance, apparent volume of distribution, and plasma half-life of midazolam were (median [range]): 1.8 (0.7-6.7) ml/kg per min, 1.1 (0.4-4.2) L/kg, and 6.3 (2.6-17.7) h, respectively. In 19 of 24 preterm infants, 1-OH-midazolam concentrations could be detected: 1-OH-midazolam (1-OH-M) maximal concentration of drug in plasma (C(max)), time to reach C(max) (T(max)), and 1-OH-M/M area under the concentration-time curve from time zero to the last sampling time point (AUC(0-t)) ratio were [median (range)]: 8.2 (<0.5-68.2) ng/ml, 6 (1-12) h, and 0.09 (<0.001-1), respectively. Midazolam plasma clearance was increased in those infants who had indomethacin (INN, indometacin) exposure. DISCUSSION: Consequent to immature hepatic cytochrome P450 3A4 (CYP3A4) activity, midazolam clearance and 1-OH-midazolamconcentrations are reduced markedly in preterm infants as compared to concentrations in previous reports from studies in older children and adults. Indomethacin exposure and its apparent impact on midazolam clearance support alteration of drug disposition produced by a patent ductus arteriosus or by the direct effects of indomethacin on hemodynamic or renal function.
de Wildt SN, Kearns GL, Hop WC, Murry DJ, Abdel-Rahman SM, van den Anker JN; Pharmacokinetics and metabolism of intravenous midazolam in preterm infants. Clin Pharmacol Ther. 2001 Dec; 70 (6): 525-31. [PubMed Citation]
-
The first-dose pharmacokinetics of midazolam and its primary alpha-hydroxymetabolite were studied after single-dose administration. Eligible study patients were enrolled into one of three study arms: Arm I (midazolam/metabolite pharmacokinetic evaluation after oral administration of a syrup formulation), Arm II (the absolute bioavailability of midazolam syrup), and Arm III (midazolam and metabolite pharmacokinetics after IV administration). Complete blood sampling for pharmacokinetic analysis was available in 87 subjects. Midazolam absorption after administration of the oral syrup formulation was rapid, with adolescents absorbing the drug at approximately half the rate observed in younger children (ages 2 to < 12 years). Furthermore, midazolam t 1/2 was prolonged and CL/F reduced in adolescents as compared with younger children. Although the midazolam Vd/F appeared larger in the youngest age group after oral administration, this observation was not apparent after IV dosing, suggesting subject differences in bioavailability rather than distribution. Like midazolam, the disposition characteristics for alpha-hydroxymidazolam were also highly variable, with the greatest formation of metabolite (reflected by the AUC ratio) observed in children ages 2 to < 12 years. The AUC ratios of alpha-hydroxymidazolam to midazolam after IV dosing were similar across all age groups and were smaller than corresponding values following oral administration. The absolute bioavailability of midazolam averaged 36% with a very broad range (9%-71%). No relationship between midazolam bioavailability and age was observed. Overall, the disposition characteristics of midazolam and its alpha-hydroxy metabolite were highly variable, appeared independent of age and dose administered, and were linear over the dose range studied (0.25 to 1 mg/kg). These data suggest that an initial oral dose of 0.2 to 0.3 mg/kg should be adequate for successful sedation of most pediatric patients. The inherent variability in midazolam bioavailability and metabolism underscores the importance of titrating midazolam dose to desired effect.
Reed MD, Rodarte A, Blumer JL, Khoo KC, Akbari B, Pou S, Pharmd, Kearns GL; The single-dose pharmacokinetics of midazolam and its primary metabolite in pediatric patients after oral and intravenous administration. J Clin Pharmacol. 2001 Dec; 41 (12): 1359-69. [PubMed Citation]
Pregnancy
-
Twenty women were given 0.03 mg/kg of midazolam IV for anesthesia induction before cesarean section. The mean concentrations of midazolam in the mothers' serum and cord blood were 339 and 318 ng/mL (ratio 0.66), respectively. Similar measurements of the metabolite produced values of 22 and 5 (ratio 0.28), respectively. The elimination half-life of midazolam in the newborn infants was 6.3 hours.
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.960-961
Geriatric
-
In three parallel group studies, the pharmacokinetics of midazolam administered IV or IM were compared in young (mean age 29, n=52) and healthy elderly subjects (mean age 73, n=53). Plasma half-life was approximately two-fold higher in the elderly. The mean Vd based on total body weight increased consistently between 15% to 100% in the elderly. The mean Cl decreased approximately 25% in the elderly in two studies and was similar to that of the younger patients in the other.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
OBJECTIVE: We investigated the pharmacologic properties of midazolam with special regard to age using the electroencephalogram (EEG) as a measure of the hypnotic-sedative effect. METHODS: Nine younger (24 to 28 years) and nine elderly (67 to 81 years) male volunteers received midazolam by a computer-controlled device. Two infusion cycles with linearly increasing target plasma levels (slope, 40 ng/mL/min for the younger subjects; 20 ng/mL/min for the elderly subjects) were administered until defined end points were attained (median EEG frequency <4 Hz and loss of responsiveness to acoustic stimuli). An EEG was recorded to quantitate the hypnotic effect, relating the median frequency of the power spectrum to the plasma level by a sigmoid Emax model, including an effect compartment. Pharmacokinetic data were derived from arterial blood samples with use of a three-compartment model. RESULTS: The total doses needed to reach the defined end points were 71+/-9 mg and 35+/-6 mg for the younger and elderly subjects, respectively (P < .001). Pharmacokinetic parameters were similar in both groups (clearance, 399+/-91 and 388+/-97 mL/min; steady-state volume of distribution, 85+/-22 and 104 +/-11 L in young and elderly subjects, respectively). Pharmacodynamic data showed a large difference in half-maximum concentration (EC50; young subjects, 522+/-236 ng/mL; elderly subjects, 223+/-56 ng/mL; P < .05), a steep concentration-response curve, and distinct hysteresis. We found much interindividual variability in the plasma concentrations necessary to achieve the clinical end points, regardless of age. CONCLUSIONS: These results suggest that the lower doses needed to reach sedation in the elderly subjects were attributable to a 50% decrease in EC50, not to changes in pharmacokinetics.
Albrecht S, Ihmsen H, Hering W, Geisslinger G, Dingemanse J, Schwilden H, Schüttler J; The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther. 1999 Jun; 65 (6): 630-9. [PubMed Citation]
-
The effects of age, gender and low-dose (50 mcg or less) oral contraceptive steroids (OCS) on the pharmacokinetics of midazolam were evaluated following a single 7.5 mg intramuscular dose to five groups (8/group) of healthy volunteers consisting of young males, young females, elderly males, elderly females, and young female users of oral contraceptives. Blood samples were collected at specified times over a 24-hour period, and plasma concentrations of midazolam and its 1-hydroxymethyl metabolite were determined by a GC-EC assay. Midazolam was rapidly absorbed following intramuscular administration to the different groups. Comparison of young men vs. elderly men, young women vs. elderly women, young men vs. young women, elderly men vs. elderly women, and young women OCS-users vs. young women non-OCS users indicated no substantial differences in the pharmacokinetic profile of midazolam between groups except for the comparison between the young and elderly men groups. The rate of elimination of midazolam was significantly slower in the elderly males compared to the young men. The pharmacokinetic profile of 1-hydroxymethyl midazolam paralleled that of the parent compound. This is to be expected since this metabolite exhibits formation rate-limited kinetics. Except for one subject who reported hives and itching, considered to be remotely related to test drug, no other adverse experiences or laboratory abnormalities were reported.
Holazo AA, Winkler MB, Patel IH; Effects of age, gender and oral contraceptives on intramuscular midazolam pharmacokinetics. J Clin Pharmacol. 1988 Nov; 28 (11): 1040-5. [PubMed Citation]
-
The pharmacokinetics of midazolam, an imidazo-benzodiazepine derivative, have been studied in 13 subjects over the age of 60 years who received the drug intravenously (0.07 mg kg-1) as an induction agent for endoscopy. Two to three days later, 6 of these subjects received 5 mg of midazolam intramuscularly, and another 6 of the subjects received 10 mg of the drug orally. The plasma concentration-time curves were again studied pharmacokinetically. After intravenous dosing, the mean (+/- SD) elimination half-life (2.14 +/- 1.24 h) showed a statistically significant trend to increase with age in the subjects older than 60 years. While the mean (+/- SD) clearance value (0.30 +/- 0.19 l kg-1h-1) tended to fall with age in the elderly subjects, this trend was not statistically significant. Apparent volume of distribution did not appear to be related to advancing age beyond 60 years, and this parameter (mean +/- SD) did not differ to a statistically significant extent between the aged subjects (0.77 +/- 0.47 l kg-1) and the young subjects studied previously (1.09 +/- 0.58 l kg-1). Atropine premedication did not appear to alter the dispositional parameters of the intravenously administered drug. Intramuscularly administered midazolam was absorbed rapidly. Bioavailability appeared incomplete (F = 0.59 +/- 0.15, mean +/- SD), possibly due to saturable elimination of the drug at the higher plasma levels which were obtained after intravenous midazolam. Oral bioavailability, relative to intravenous, was 0.34 +/- 0.17, (mean +/- SD), with an appreciable but variable lag time (0.74 +/- 0.40 h, mean +/- SD).
Smith MT, Heazlewood V, Eadie MJ, Brophy TO, Tyrer JH; Pharmacokinetics of midazolam in the aged. Eur J Clin Pharmacol. 1984; 26 (3): 381-8. [PubMed Citation]
Renal Impairment
-
Patients with renal impairment may have longer elimination half-lives for midazolam and its metabolites which may result in slower recovery. Midazolam and 1-hydroxy-midazolam pharmacokinetics in 6 ICU patients who developed acute renal failure (ARF) were compared with a normal renal function control group. Midazolam was administered as an infusion (5 to 15 mg/hr). Midazolam clearance was reduced (1.9 vs 2.8 mL/min/kg) and the half-life was prolonged (7.6 vs 13 hours) in the ARF patients. The renal clearance of the 1-hydroxy-midazolam glucuronide was prolonged in the ARF group (4 vs 136 mL/min) and the half-life was prolonged (12 vs > 25 hours). Plasma levels accumulated in all ARF patients to about ten times that of the parent drug. The relationship between accumulating metabolite levels and prolonged sedation is unclear. In a study of chronic renal failure patients (n=15) receiving a single IV dose, there was a two-fold increase in the clearance and volume of distribution but the half-life remained unchanged. Metabolite levels were not studied.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
Fifteen patients with chronic renal failure (CRF) were given midazolam 0.2 mg/kg iv over 15 s. All but one lost consciousness in a time ranging from 22-100 s (mean +/- SD was 55 +/- 26 s) after drug administration. Patients regained consciousness from 6-105 min (mean 53 +/- 32) after drug administration. The calculated mean plasma level of midazolam at arousal was 81 +/- 47 ng/ml. Pharmacokinetics parameters were determined from midazolam plasma levels measured in 16 consecutive venous blood samples. The pharmacokinetic parameters in CRF patients were compared with those of healthy volunteers matched for age, sex, and body size with the CRF patients. Protein binding was determined by equilibrium dialysis. CRF patients had a significantly higher (P less than 0.005) plasma-free drug fraction (6.5% +/- 0.7) compared with the control patients (3.9% +/- 0.1). Total (bound plus unbound) kinetics differed in the two groups: volume of distribution 3.8 +/- .3 1/kg in CRF patients versus 2.2 +/- .2 1/kg in controls (P less than 0.001), and clearance 11.4 +/- 1.6 ml X min-1 X kg-1 in CRF patients versus 6.7 +/- 0.9 ml X min-1 X kg-1 in controls (P less than 0.02). When kinetic parameters were corrected for protein binding, CRF patients unbound volume of distribution (63.5 +/- 6.8 1/kg) and free drug clearance (189 +/- 29 ml X min-1 X kg-1) were not different from the control group's volume of distribution (55.6 +/- 5.7 1/kg) and free drug clearance (176 +/- 24 ml X min-1 X kg-1).
Vinik HR, Reves JG, Greenblatt DJ, Abernethy DR, Smith LR; The pharmacokinetics of midazolam in chronic renal failure patients. Anesthesiology. 1983 Nov; 59 (5): 390-4. [PubMed Citation]
Hepatic Impairment
-
Midazolam pharmacokinetics were studied after an IV single dose (0.075 mg/kg) was administered to 7 patients with biopsy proven alcoholic cirrhosis and 8 control patients. The mean half-life of midazolam increased 2.5 fold in the alcoholic patients. Clearance was reduced by 50% and Vd increased by 20%. In another study in 21 male patients with cirrhosis, without ascites and with normal kidney function as determined by creatinine clearance, no changes in the pharmacokinetics of midazolam or 1-hydroxy-midazolam were observed when compared to healthy individuals.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injecetion [Cardinal Health] Last revised: April 2010 [DailyMed]
-
To study the effects of cirrhosis of the liver on the pharmacokinetics of midazolam single IV (7.5 mg as base) and p.o. (15.0 mg as base) doses of midazolam were administered to seven patients with cirrhosis of the liver and to seven healthy control subjects. One cirrhotic patient did not receive the oral dose. The distribution of midazolam in both study groups was alike as indicated by similar values of t1/2 alpha, V1 and Vss. Also the plasma protein binding of midazolam was unchanged in the patients with cirrhosis. The elimination of midazolam was significantly retarded in the patients as indicated by its lower total clearance (3.34 vs. 5.63 ml/min/kg), lower total elimination rate constant (0.400 vs. 0.721 h-1), and longer elimination half-life (7.36 vs. 3.80 h). The bioavailability of oral midazolam was significantly (P less than 0.05) higher in patients than controls (76% vs. 38%). The antipyrine-half-life was 32.4 h in the patients and 11.8 h in the controls. There were statistically significant (P less than 0.01) correlations between the clearances of the two drugs (r = 0.680) and between their half-lives (r = 0.755). The hypnotic effects of midazolam were similar in both groups. However, on a pharmacokinetic basis a reduced dosage of midazolamto patients with advanced cirrhosis of the liver is recommended.
Pentikäinen PJ, Välisalmi L, Himberg JJ, Crevoisier C; Pharmacokinetics of midazolam following intravenous and oral administration in patients with chronic liver disease and in healthy subjects. J Clin Pharmacol. 1989 Mar; 29 (3): 272-7. [PubMed Citation]
Obesity
-
In a study comparing normals (n=20) and obese patients (n=20) the mean half-life was greater in the obese group (5.9 vs 2.3 hours). This was due to an increase of approximately 50% in the Vd corrected for total body weight. The clearance was not significantly different between groups.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults
Preoperative Sedation, Anxiolysis, and Anterograde Amnesia
-
For preoperative sedation, anxiolysis and anterograde amnesia in good-risk (e.g., ASA Physical Status I and II) adults younger than 60 years of age, the usual IM dose of midazolam is 70-80 mcg/kg (about 5 mg) administered approximately 30-60 minutes prior to surgery.
-
The dosage must be individualized and reduced when IM midazolam is administered to patients with chronic obstructive pulmonary disease, other higher-risk surgical patients, patients 60 years of age or older, and patients who have received opiate agonists or other CNS depressants concomitantly.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Procedural Sedation
-
For procedural sedation in adults, IV midazolam is administered slowly before the procedure. Initial titration of midazolam dosage should begin with a small dose administered over at least a 2-minute period in average healthy adults younger than 60 years of age. The initial IV dose should not exceed 2.5 mg, although some such patients may respond to as little as 1 mg of the drug.
-
... Midazolam dosage may be further titrated in small increments of the initial dose administered over at least a 2-minute period to the desired effect (e.g., onset of slurred speech) if further sedative effect is required.
-
A total dose of up to 5 mg generally is adequate for conscious sedation in an average healthy adult younger than 60 years of age.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Induction and Maintenance of Anesthesia:
-
For sedation of intubated and mechanically ventilated patients as a component of anesthesia, midazolam is administered as a continuous IV infusion. In adults, if a loading dose is necessary to initiate sedation rapidly, 10-50 mcg/kg (approximately 0.5-4 mg for a typical adult) may be given slowly or infused over several minutes. This dose may be repeated at 10- to 15-minute intervals until adequate sedation is achieved. For maintenance of sedation in adults, the usual initial infusion rate is 20-100 mcg/kg per hour (approximately 1-7 mg per hour).
-
For patients who have not been premedicated, an initial midazolam dose of 300-350 mcg/kg is administered IV over 20-30 seconds in healthy adults younger than 55 years of age, allowing approximately 2 minutes for clinical effect; however, some clinicians suggest that a lower initial dose (e.g., 200 mcg/kg) be used in these adults. Supplemental doses of about 25% of the initial dose may be given as necessary to complete induction or for maintenance of sedation or anesthesia. ...Total IV induction doses of up to 600 mcg/kg may be required in some resistant patients, but such doses may prolong recovery from anesthesia.
-
In premedicated patients, especially those who have received an opiate agonist, the usual induction dose ofmidazolam is 150-350 mcg/kg. In premedicated adults younger than 55 years of age, the usual induction dose is 250 mcg/kg administered IV over 20-30 seconds; about 2 minutes should be allowed for clinical effects.
-
For maintenance of anesthesia as a component of balanced anesthesia during short surgical procedures, premedication with an opiate agonist is especially recommended when midazolam is used for maintenance. Midazolam may be administered in incremental IV doses of approximately 25% of the initial induction dose when lightening of anesthesia is evident and repeated, as necessary, according to patient response to maintain the required level of anesthesia.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Sedation in Critical-Care Settings:
-
For sedation in intubated and mechanically ventilated adult patients during treatment in critical-care settings (e.g., an ICU), midazolam is administered as a continuous IV infusion. In adults, if a loading dose is necessary to initiate sedation rapidly, 10-50 mcg/kg (approximately 0.5-4 mg for a typical adult) may be given slowly or infused over several minutes. This dose may be repeated at 10- to 15-minute intervals until adequate sedation is achieved.
-
For maintenance of sedation in adults, the usual initial infusion rate is 20-100 mcg/kg per hour (approximately 1-7 mg per hour).
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Children
-
For all pediatric patients, regardless of the indications for sedation/anxiolysis, it is vital to titrate the midazolam dose and the dose of other concomitant drugs slowly for the desired clinical effect. Pediatric patients generally receive increments of midazolam on a mg/kg basis; drug dose in obese pediatric patients should be calculated on the basis of ideal body weight.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Preoperative Sedation, Anxiolysis, and Anterograde Amnesia:
-
For preoperative sedation in non-neonatal pediatric patients, the usual IM dose of midazolam is 100-150 mcg/kg; doses in this range usually are effective and do not prolong emergence from general anesthesia. Sedation after IM midazolam administration is age and dose dependent; higher doses may result in deeper and more prolonged sedation. For more anxious patients, doses of up to 500 mcg/kg have been used.
-
For preoperative sedation in pediatric patients, the usual IV (as an intermittent injection) dose of midazolam is age dependent; prolonged sedation and risk of hypoventilation may be associated with the higher doses in each recommended range. IV midazolam should be administered over 2-3 minutes. Because midazolam is water soluble, peak EEG effects are not achieved as quickly as with diazepam; it is essential to wait 2-3 minutes to fully evaluate the sedative effect before starting the procedure or administering a repeat dose.
-
In patients 6 months to 5 years of age, an initial IV dose of 50-100 mcg/kg is recommended; a total dose of up to 600 mcg/kg may be required to reach the desired end point, but usually does not exceed a total of 6 mg.
-
In patients 6-12 years of age, an initial IV dose of 25-50 mcg/kg is recommended; a total dose of up to 400 mcg/kg may be required to reach the desired end point, but usually does not exceed a total of 10 mg.
-
Patients 12-16 years of age should be dosed as adults; although some patients in this age range may require higher than recommended adult doses, the total dose usually does not exceed 10 mg.
-
In nonintubated pediatric patients younger than 6 months of age, limited dosing information is available.
-
For preoperative sedation in pediatric patients (6 months up to 16 years of age), a single oral dose of 250-500 mcg/kg is recommended, depending on the status of the patient and the desired effect, up to a maximum of 20 mg.
-
Younger pediatric patients (e.g., 6 months to younger than 6 years of age) and less cooperative patients may require a higher than usual dose of up to 1 mg/kg (up to a maximum of 20 mg).
-
A dose of 250 mcg/kg may be sufficient for children 6-16 years of age or for cooperative patients, especially if the anticipated intensity and duration of sedation is less critical.
-
For pediatric patients (6 months up to 16 years of age) with cardiac or respiratory compromise, other higher-risk surgical pediatric patients, and pediatric patients who have received concomitant opiates or other CNS depressants, an initial dose of 250 mcg/kg should be considered.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Procedural Sedation:
-
For procedural sedation in non-neonatal pediatric patients, the usual IM dose of midazolam is 100-150 mcg/kg. Sedation after IM midazolam administration is age and dose dependent; higher doses may result in deeper and more prolonged sedation. For more anxious patients, doses of up to 500 mcg/kg have been used. The manufacturer states that the total dose usually does not exceed 10 mg.
-
For procedural sedation in pediatric patients, the usual IV (as an intermittent injection) dose of midazolam is age dependent.
-
In patients 6 months to 5 years of age, an initial IV dose of 50-100 mcg/kg is recommended; a total dose of up to 600 mcg/kg may be required to reach the desired end point, but usually does not exceed a total of 6 mg.
-
In patients 6-12 years of age, an initial IV dose of 25-50 mcg/kg is recommended; a total dose of up to 400 mcg/kg may be required to reach the desired end point, but usually does not exceed a total of 10 mg.
-
Patients 12-16 years of age should be dosed as adults; although some patients in this age range may require higher than recommended adult doses, the total dose usually does not exceed 10 mg.
-
In nonintubated pediatric patients younger than 6 months of age, limited dosing information is available.
-
For procedural sedation in pediatric patients (6 months up to 16 years of age), a single oral midazolam dose of 250-500 mcg/kg is recommended, depending on the status of the patient and the desired effect, up to a maximum of 20 mg.
-
Younger pediatric patients (e.g., 6 months to younger than 6 years of age) and less cooperative patients may require a higher than usual dose of up to 1 mg/kg (up to a maximum of 20 mg).
-
A dose of 250 mcg/kg may be sufficient for children 6-16 years of age or for cooperative patients, especially if the anticipated intensity and duration of sedation is less critical.
-
For pediatric patients (6 months up to 16 years of age) with cardiac or respiratory compromise, other higher-risk surgical pediatric patients, and pediatric patients who have received concomitant narcotics or other CNS depressants, an initial oral dose of 0.25 mg/kg should be considered.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Sedation in Critical-Care Settings:
-
For sedation of intubated pediatric (i.e., non-neonatal) patients during treatment in critical-care settings, midazolam generally is initiated with an IV loading dose of 50-200 mcg/kg administered over at least 2-3 minutes; the drug should not be administered as a rapid IV injection.
-
Based on pharmacokinetic parameters and clinical experience, continuous IV infusions of midazolam should be initiated at a rate of 60-120 mcg/kg per hour (1-2 mcg/kg per minute). The infusion rate may be increased or decreased (generally by 25% of the initial or subsequent infusion rate) as required, or supplemental IV doses of midazolam may be administered to increase or maintain the desired effect.
-
For sedation of intubated preterm (i.e., born at less than 32 weeks' gestation) or term (i.e., born at 32 weeks' gestation or later) neonatal patients during treatment in critical-care settings, midazolam generally is initiated as an IV infusion at a rate of 30 mcg/kg per hour (0.5 mcg/kg per minute) or 60 mcg/kg per hour (1 mcg/kg per minute), respectively. IV loading doses should not be used in neonates.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Pregnancy
FDA Pregnancy Risk Category:
-
D; POSITIVE EVIDENCE OF RISK. Studies in humans, or investigational or post-marketing data, have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
Nursing Mothers
Lactation Risk Category:
-
L3 Moderately Safe; There are no controlled studies in breastfeeding women, however, the risk of untoward effects to a breastfed infant is possible; or, controlled studies show only a minimal non-threatening adverse effects. Drugs should be given only if the potential benefit justifies the potential risk to the infant.
-
L4 Possibly Hazardous (if used chronically); There is possible evidence of risk to breastfed infant or to breastmilk production, but the benefits from the use in breastfeeding mothers may be acceptable despite the risk to the infant (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective.)
Hale TW, Medications and Mother's Milk. Amarillo, TX: Hale Publishing L.P., 2006 p.605-606
Geriatric
Preoperative Sedation, Anxiolysis, and Anterograde Amnesia:
-
In a study in patients 60 years of age or older who did not receive concomitant opiate agonist therapy, IM doses of 2-3 mg (20-50 mcg/kg) reportedly produced adequate sedation during the preoperative period; the manufacturer states that an IM midazolam dose of 1 mg may be sufficient in some geriatric patients if the anticipated intensity and duration of sedation is less critical.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Procedural Sedation:
-
Patients 60 years of age or older, chronically ill and/or debilitated patients, and patients with decreased pulmonary reserve should receive 1-1.5 mg as an initial dose over a longer period of injection. The manufacturer recommends that not more than 1.5 mg over at least 2 minutes be administered as an initial dose in these patients.
-
Patients 60 years of age or older, chronically ill and/or debilitated patients, or patients with decreased pulmonary reserve should receive incremental doses of not more than 1 mg of midazolam.
-
A total dose of up to 3.5 mg usually is adequate for patients 60 years of age or older, chronically ill and/or debilitated patients, and patients with decreased pulmonary reserve.
-
In patients 60 years of age and older, these clinicians recommend reducing midazolam dosage by 25% or more.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Induction and Maintenance of Anesthesia:
-
Patients 55 years of age and older who have not been premedicated usually require lower induction doses ofmidazolam; the manufacturer recommends an initial IV induction dose of 300 mcg/kg in these patients. Patients with severe systemic disease or other debilitation who have not been premedicated also usually require lower induction doses. Initial IV doses of 200-250 mcg/kg usually are adequate in such patients, and doses as low as 150 mcg/kg may be adequate for induction in some debilitated patients.
-
In premedicated patients 55 years of age and older who are good risk (e.g., ASA I and II) surgical patients, an initial induction dose of 200 mcg/kg is recommended by the manufacturer. In some premedicated patients with severe systemic disease or debilitation, an initial induction dose of 150 mcg/kg may be sufficient.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
-
Midazolam drug is a potent sedative that requires individualized dosing. Dosage must be carefully adjusted according to individual requirements and response, age, body weight, physical and clinical status, underlying pathologic condition(s), type and amount of premedication or concomitant medication, and the nature and duration of the surgical or other procedure; however, individual response to the drug also may vary independent of these factors. The smallest effective dose should be used, especially in geriatric and/or debilitated patients.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
-
The initial dose should be reduced in geriatric patients, since some degree of impairment in one or more organ systems frequently is present. Dosage requirements in this age group generally appear to decrease with increasing age, and the possibility of profound and/or prolonged effects should be considered in older and/or debilitated patients.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Obesity
-
For all pediatric patients, regardless of the indications for sedation/anxiolysis, it is vital to titrate the midazolam dose and the dose of other concomitant drugs slowly for the desired clinical effect. Pediatric patients generally receive increments of midazolam on a mg/kg basis; drug dose in obese pediatric patients should be calculated on the basis of ideal body weight.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Midiazolam has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (Public Law 108-276).
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
6. Current available formulations/shelf life
Formulation
-
Oral Solution 2 mg/mL (of midazolam)
-
Parenteral Injection 1 mg/mL, 5mg/mL (of midazolam)
FDA; Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Patent and Generic Drug Product Data Last Updated: August 09, 2012
Shelf life
Shelf life
-
When stored as recommended, the commercially available injection has an expiration date of 2 years following the date of manufacture.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Stability
-
Midazolam hydrochloride is reportedly stable at a pH of 3-3.6. The injection is chemically and physically compatible with the following IV solutions: 5% dextrose, 0.9% sodium chloride, or lactated Ringer's. Midazolam hydrochloride injection that has been diluted to a final concentration of 0.5 mg or less per mL is stable for 24 hours in 5% dextrose or 0.9% sodium chloride injection and for 4 hours in lactated Ringer's injection when stored in glass or PVC containers at 25°C. When diluted and stored as recommended, it is not necessary to protect these solutions from light.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Storage
-
Midazolam hydrochloride injection should be stored at 15-30°C and protected from light. Midazolam oral solution should be protected from light and stored at 25°C, but may be exposed to temperatures ranging from 15-30°C.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
The benzodiazepine midazolam, currently FDA-approved as an intravenous sedative and anesthetic, may also be very effective in the treatment of seizures. Midazolam is being investigated to replace diazepam as the immediate anticonvulsant treatment for nerve agent-induced seizures. The potential use of midazolam, administered intramuscularly, to treat nerve agent-induced seizures will require clinical trials to test its effectiveness and gain FDA approval. Different benzodiazepines and other classes of drugs that antagonize various neuronal excitation pathways mediated by the neurotransmitter glutamate and neurosteroids, are also candidates to treat chemically induced seizures. The development of these potential therapies will also require preclinical and clinical studies.
DHHS/NIH; National Institute of Allergy and Infectious Diseases: Medical Countermeasures Against Chemical Threats-Nervous System (NIH/NIAID)
-
Anesthesia, local, adjunct: Midazolam has been used as an adjunct to local or regional anesthesia for some diagnostic and therapeutic procedures. It may be used for sedation of healthy patients receiving subarachnoid or epidural anesthesia.
-
Status epilepticus: Midazolam has been used in the treatment of status epilepticus in adults and children, including children with refractory status epilepticus, treated with benzodiazepine, phenobarbital or phenytoin. Midazolam treatment by intranasal and buccal/sublingual routes has also been used for prolonged seizures (including status epilepticus) when parenteral administration is not feasible.
Thomson/Micromedex 2007; Drug Information for the Health Care Professional: Volume 1, 27th Edition. Greenwood Village, CO. 2007 p.512-535
Children
-
Status epilepticus: Midazolam has been used in the treatment of status epilepticus in adults and children, including children with refractory status epilepticus, treated with benzodiazepine, phenobarbital or phenytoin. Midazolam treatment by intranasal and buccal/sublingual routes has also been used for prolonged seizures (including status epilepticus) when parenteral administration is not feasible.
Thomson/Micromedex 2007; Drug Information for the Health Care Professional: Volume 1, 27th Edition. Greenwood Village, CO. 2007 p.512-535
8. Route of Administration/Monitoring
-
Routine observation of vital signs, especially Glasgow Coma Scale (GCS) and airway patency, is indicated. Measurement of partial pressure of carbon dioxide via expired air or arterial blood gases is the best way to assess respiratory compromise from sedation.
Dart RC (ed): Medical Toxicology, 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2004 p.811-823
9. Adverse effects
-
Intravenous midazolam has been associated with respiratory depression and respiratory arrest, especially when used for sedation in noncritical care settings. In some cases, where this was not recognized promptly and treated effectively, death or hypoxic encephalopathy has resulted. Intravenous midazolam should be used only in hospital or ambulatory care settings, including physicians' and dental offices, that provide for continuous monitoring of respiratory and cardiac function, i.e., pulse oximetry. Immediate availability of resuscitative drugs and age- and size-appropriate equipment for bag/valve/mask ventilation and intubation, and personnel trained in their use and skilled in airway management should be assured. For deeply sedated pediatric patients, a dedicated individual, other than the practitioner performing the procedure, should monitor the patient throughout the procedure.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
The initial intravenous dose for sedation in adult patients may be as little as 1 mg, but should not exceed 2.5 mg in a normal healthy adult. Lower doses are necessary for older (over 60 years) or debilitated patients and in patients receiving concomitant narcotics or other central nervous system (CNS) depressants. The initial dose and all subsequent doses should always be titrated slowly; administer over at least 2 minutes and allow an additional 2 or more minutes to fully evaluate the sedative effect. The use of the 1 mg/mL formulation or dilution of the 1 mg/mL or 5 mg/mL formulation is recommended to facilitate slower injection. Doses of sedative medications in pediatric patients must be calculated on a mg/kg basis, and initial doses and all subsequent doses should always be titrated slowly. The initial pediatric dose of midazolam for sedation/anxiolysis/amnesia is age, procedure and route dependent.
-
Midazolam should not be administered by rapid injection in the neonatal population. Severe hypotension and seizures have been reported following rapid IV administration, particularly with concomitant use of fentanyl
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
Midazolam can depress respiration. Relatively small doses, such as those used for preoperative sedation, usually do not substantially impair respiratory function; however, relatively large doses (e.g., more than 100-200 mcg/kg) may substantially depress the ventilatory response to carbon dioxide (CO2) stimulation.
-
Patients with chronic obstructive pulmonary disease appear to be particularly sensitive to the respiratory depressant effects of midazolam (e.g., impairment of the ventilatory response to CO2), exhibiting more marked and prolonged depression than patients with healthy lungs.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
-
Hypotensive episodes requiring treatment have been reported rarely during or following diagnostic or surgical manipulation in patients receiving midazolam. Concomitant administration of an opiate agonist (e.g., as premedication for moderate sedation [formerly known as conscious sedation]) appears to increase the risk of severe hypotension associated with midazolam administration.
-
Other adverse respiratory effects of midazolam include hiccups and coughing, which occur in up to 4 and 1% of patients, respectively, receiving the drug IV. Laryngospasm, bronchospasm, dyspnea, hyperventilation, wheezing, shallow respiration, airway obstruction, and tachypnea occur in less than 1% of patients, principally those receiving the drug IV.
-
Other adverse cardiovascular effects of midazolam include decreases in systemic vascular resistance, cardiac index, and stroke index. Bradycardia has been reported in up to 1% of pediatric patients receiving midazolam oral solution. Bigeminy, ventricular premature complexes, vasovagal episodes, tachycardia, and nodal rhythms reportedly occur in less than 1% of patients. Trigeminy also has been reported in patients receiving midazolam.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
-
Adverse nervous system effects of midazolam generally are extensions of the pharmacologic actions of the drug; however, some reactions (e.g., agitation, involuntary movements) may be paradoxical in nature, manifestations of an underlying iatrogenic disorder (e.g., cerebral hypoxia), and/or associated with the surgical or other procedures employed
-
Excessive sedation, headache, and drowsiness occur in 1-2% of patients following parenteral administration of midazolam. Adverse reactions manifested as agitation, involuntary movements (e.g., tonic/clonic movements, muscle tremor), hyperactivity, and combativeness have occurred in less than 1% of patients receiving parenteral midazolam, principally in those receiving the drug IV.
-
Other adverse nervous system effects occur in less than 1% of patients receiving midazolam, either orally or IV. Such effects include retrograde amnesia, euphoria, hallucination, dysphoria, prolonged emergence from anesthesia, emergence delirium or agitation, dreaming during emergence, prolonged sedation, sleep disturbance, insomnia, nightmares, paresthesia, adverse behavior, mood swings, aggression, excitation, disinhibition, argumentativeness, nervousness, anxiety, restlessness, seizure-like activity, dysarthria, and athetoid movements. Confusion, grogginess, ataxia, dizziness, loss of balance or vertigo, lightheadedness, lethargy, yawning, faint feeling, weakness, slurred speech, blurred vision, strabismus, diplopia, and dysphonia have also been reported in less than 1% of patients.
-
Midazolam has reduced cerebral blood flow and oxygen consumption in animals and humans. In patients without intracranial pathology, induction of anesthesia with midazolam results in a moderate decrease in CSF pressure, similar to that occurring with thiopental.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
-
Nausea and/or vomiting occur in 2-3% of adult patients receiving midazolam IV and in up to 4 or 8%, respectively, of pediatric patients receiving the drug orally. Acid taste, excessive salivation, gagging, drooling, and retching occur in less than 1% of patients. Other adverse GI effects include metallic taste, dry mouth, and constipation. Hypersensitivity reactions reported in less than 1% of patients receiving midazolam include anaphylactoid reactions, urticaria, rash, and pruritus.
-
Tenderness at the site of injection and pain during injection occur in 5-6% of patients receiving midazolam IV. Erythema and induration occur at the IV site in 2-3% of patients, and phlebitis occurs in less than 1%. Pain at the site of IM injection occurs in about 4% of patients, and local induration, erythema, and muscle stiffness occur in less than 1% of patients receiving midazolam IM.
-
Urticaria-like elevation at the injection site, swelling or feeling of burning, and warmth or coldness at the injection site occur in less than 1% of patients receiving midazolam parenterally, principally in those receiving the drug IV.
-
Adverse ocular effects occur in less than 1% of patients receiving parenteral midazolam, principally in those receiving the drug IV, and include blurred vision, diplopia, nystagmus, pinpoint pupils, cyclic movements of eyelids, visual disturbances, and focusing difficulty.
-
Chills, toothache, blockage of ears, and hematoma occur in less than 1% of patients receiving midazolam parenterally, principally in those receiving the drug IV. Limited data suggest that administration of midazolam as an adjunct to anesthesia may result in transient decreases in renal blood flow and glomerular filtration rate.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
10. Contraindication(s)
-
Injectable midazolam is contraindicated in patients with a known hypersensitivity to the drug.
-
Benzodiazepines are contraindicated in patients with acute narrow-angle glaucoma.
-
Benzodiazepines may be used in patients with open-angle glaucoma only if they are receiving appropriate therapy. Measurements of intraocular pressure in patients without eye disease show a moderate lowering following induction with midazolam; patients with glaucoma have not been studied.
-
Midazolam Injection is not intended for intrathecal or epidural administration due to the presence of the preservative benzyl alcohol in the dosage form.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues-
Title: Phase I Trial for Intramuscular Administration of Midazolam Using an Autoinjector.
Condition: Seizures
Intervention: Drug: Midazolam
"Study completed but no results posted"
ClinicalTrials.gov; Phase I Trial for Intramuscular Administration of Midazolam Using an Autoinjector. (NIH)
-
Intranasal Midazolam Versus Rectal Diazepam for Treatment of Seizures.
Condition: Seizures
Intervention: Drug: Midazolam; Drug: Diazepam
"Study completed"
ClinicalTrials.gov; Intranasal Midazolam Versus Rectal Diazepam for Treatment of Seizures. (NIH)
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations-
Sufficient data to support immediate use of midazolam; can and should be used to treat nerve agent exposure; should be made immediately available in the SNS and CHEMPACKs as a nerve agent treatment.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Critical nerve agent antidotes should be evaluated to determine if they can be safely and effectively administered to infants, children and adults via the intravenous and aerosol routes.
-
The accelerated development of promising antidotes for chemical nerve agents should be pursued.
-
Expanded labeling of currently licensed anticonvulsants for use against chemical nerve agents and their utilization in autoinjectors should be pursued.
-
Objective screening tests that can evaluate behavior and cognitive ability, and can differentiate chemically-induced neurological injury from pre-existing neurological illness need to be developed.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
Treatment of organophosphate/pesticide poisoning, treatment of pediatric seizures.
-
Gather data for a pediatric labeling application for treatment of refractory seizures.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
15. Study-related ethical concerns
— including review panel recommendationsEthical concerns include:
-
Informed consent for data collection in disaster/mass casualty situation
-
Central institutional review boards (IRBs)
-
How to conduct multicenter studies
Poison centers
Pediatric Emergency Care Applied Research Network
Hospital consortia.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
16. Global regulatory status
U.S.
-
Midazolam Injection
Midazolam Injection is indicated intramuscularly or intravenously for preoperative sedation/anxiolysis/amnesia;
Midazolam Injection is indicated intravenously as an agent for sedation/anxiolysis/amnesia prior to or during diagnostic, therapeutic or endoscopic procedures, such as bronchoscopy, gastroscopy, cystoscopy, coronary angiography, cardiac catheterization, oncology procedures, radiologic procedures, suture of lacerations and other procedures either alone or in combination with other CNS depressants;
Midazolam Injection is indicated intravenously for induction of general anesthesia, before administration of other anesthetic agents. With the use of narcotic premedication, induction of anesthesia can be attained within a relatively narrow dose range and in a short period of time. Intravenous midazolam can also be used as a component of intravenous supplementation of nitrous oxide and oxygen (balanced anesthesia);
Midazolam Injection is indicated continuous intravenous infusion for sedation of intubated and mechanically ventilated patients as a component of anesthesia or during treatment in a critical care setting.
Midazolam is associated with a high incidence of partial or complete impairment of recall for the next several hours
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
Midazolam HCl Syrup
Midazolam HCl Syrup is indicated for use in pediatric patients for sedation, anxiolysis and amnesia prior to diagnostic, therapeutic or endoscopic procedures or before induction of anesthesia.
Midazolam HCl syrup is intended for use in monitored settings only and not for chronic or home use. MIDAZOLAM HCL SYRUP MUST BE USED AS SPECIFIED IN THE LABEL.
Midazolam is associated with a high incidence of partial or complete impairment of recall for the next several hours
Product label Midazolam hydrochloride (midazolam hydrochloride syrup) syrup [West-Ward Pharmaceuticals Corp.] Last revised: June 2008 [DailyMed]
U.K.
-
Status epilepticus; febrile convulsions [unlicensed]; other indications.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.267
17. Other potentially useful information
-
Midazolam injection is classified by the Drug Enforcement Administration as a schedule IV controlled substance.
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
-
The anticonvulsant midazolam has been shown to be effective against both convulsant (e.g., picrotoxin, hydrazine, strychnine, tetramethylenedisulfotetramine) and cholinergic (e.g., sarin (GB), soman (GD), cyclosarin (GF), tabun (GA), VX, and organophosphorus pesticides) agents by enhancing the inhibitory effect of gamma-aminobutyric acid at GABA(a) receptors and limiting excess central nervous system excitation and seizures.
Department of Homeland Security (DHS). Chemical Security Analysis Center (CSAC). 2011. Chemical Segregation by Toxidrome for the Chemical Terrorism Risk Assessment. PowerPoint available in Report on the Toxic Chemical Syndrome Definition and Nomenclature Workshop (May, 2012)
-
Log P (octanol-water) = 4.330 (est)
US NLM. ChemIDplus Lite. Midazolam
18. Publications
Albrecht S, Ihmsen H, Hering W, Geisslinger G, Dingemanse J, Schwilden H, Schüttler J; The effect of age on the pharmacokinetics and pharmacodynamics of midazolam. Clin Pharmacol Ther. 1999 Jun; 65 (6): 630-9. [PubMed Citation]
Anderson DR, Harris LW, Chang FC, Baze WB, Capacio BR, Byers SL, Lennox WJ; Antagonism of soman-induced convulsions by midazolam, diazepam and scopolamine. Drug Chem Toxicol. 1997 Aug; 20 (3): 115-31. [PubMed Citation]
Baze WB; Soman-induced morphological changes: an overview in the non-human primate. J Appl Toxicol. 1993 May-Jun; 13 (3): 173-7. [PubMed Citation]
Behne M, Janshon G, Asskali F, Förster H; The pharmacokinetics of midazolam following intramuscular administration. Anaesthesist. 1989 Jun; 38 (6): 278-84. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Bokonjić D, Rosić N; Anticonvulsive and protective effects of diazepam and midazolam in rats poisoned by highly toxic organophosphorus compounds.
Arh Hig Rada Toksikol. 1991 Dec; 42 (4): 359-65. [PubMed Citation]
Briggs GG, Freeman RK, Yaffe SJ, eds.: Drugs in Pregnancy and Lactation, Ninth Edition. Lippincott Williams & Wilkins, Philadelphia, PA 2008 p.960-961
Brunton, L.L, et al. (eds.). (2006). (Goodman & Gilman's) The Pharmacological Basis of Therapeutics (11th Ed.). McGraw-Hill-Medical Publishing Division, New York, NY. p.402-407.
Chong WS, Hyun CL, Park MK, Park JM, Song HO, Park T, Lim YS, Cho CK, Kang PS, Kwon HU; Midazolam protects B35 neuroblastoma cells through Akt-phosphorylation in reactive oxygen species derived cellular injury. Korean J Anesthesiol. 2012 Feb; 62 (2): 166-71.
ClinicalTrials.gov; Phase I Trial for Intramuscular Administration of Midazolam Using an Autoinjector. (NIH)
Dart RC (ed): Medical Toxicology, 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2004 p.811-823
de Wildt SN, Kearns GL, Hop WC, Murry DJ, Abdel-Rahman SM, van den Anker JN; Pharmacokinetics and metabolism of intravenous midazolam in preterm infants. Clin Pharmacol Ther. 2001 Dec; 70 (6): 525-31. [PubMed Citation]
de Wildt SN, Kearns GL, Hop WC, Murry DJ, Abdel-Rahman SM, van den Anker JN; Pharmacokinetics and metabolism of oral midazolam in preterm infants. Br J Clin Pharmacol. 2002 Apr; 53 (4): 390-2. [PubMed Citation]
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
DHHS/NIH; National Institute of Allergy and Infectious Diseases: Medical Countermeasures Against Chemical Threats-Nervous System (NIH/NIAID)
FDA; Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Patent and Generic Drug Product Data Last Updated:
August 09, 2012
Ghilain S, Rijckevorsel-Harmant K, Harmant J, de Barsy TH; Midazolam in the treatment of epileptic seizures. J Neurol Neurosurg Psych. 1988; 51 (5): 732.
Gilat E, Goldman M, Lahat E, Levy A, Rabinovitz I, Cohen G, Brandeis R, Amitai G, Alkalai D, Eshel G; Nasal midazolam as a novel anticonvulsive treatment against organophosphate-induced seizure activity in the guinea pig. Arch Toxicol. 2003 Mar; 77 (3): 167-72. [PubMed Citation]
Gilat E, Kadar T, Levy A, Rabinovitz I, Cohen G, Kapon Y, Sahar R, Brandeis R; Anticonvulsant treatment of sarin-induced seizures with nasal midazolam: an electrographic, behavioral, and histological study in freely moving rats. Toxicol Appl Pharmacol. 2005 Nov 15; 209 (1): 74-85. [PubMed Citation]
Hale TW, Medications and Mother's Milk. Amarillo, TX: Hale Publishing L.P., 2006 p.605-606
Hayward IJ, Wall HG, Jaax NK, Wade JV, Marlow DD, Nold JB; Decreased brain pathology in organophosphate-exposed rhesus monkeys following benzodiazepine therapy. J Neurol Sci. 1990 Aug; 98 (1): 99-106. [PubMed Citation]
Holazo AA, Winkler MB, Patel IH; Effects of age, gender and oral contraceptives on intramuscular midazolam pharmacokinetics. J Clin Pharmacol. 1988 Nov; 28 (11): 1040-5. [PubMed Citation]
Holsti M, Dudley N, Schunk J, Adelgais K, Greenberg R, Olsen C, Healy A, Firth S, Filloux F; Intranasal Midazolam vs. Rectal Diazepam for the Home Treatment of Acute Seizures in Pediatric Patients With Epilepsy. Arch Pediatr Adolesc Med. 2010; 164 (8): 747-753.
HSDB. Midazolam Hydrochloride
Koplovitz I, Schulz S, Shutz M, Railer R, Macalalag R, Schons M, McDonough J; Combination anticonvulsant treatment of soman-induced seizures. J Appl Toxicol. 2001 Dec; 21 Suppl 1:S53-5. [PubMed Citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.267
McDonough JH Jr, McMonagle J, Copeland T, Zoeffel D, Shih TM. Comparative evaluation of benzodiazepines for control of soman-induced seizures. Arch Toxicol. 1999 Nov;73(8-9):473-8. [PubMed Citation]
McDonough, JH; Pharmacology Division, U.S. Army Medical Research Institute of Chemical Defense. Midazolam: An Improved Anticonvulsant Treatment For Nerve Agent-Induced Seizures. Proceedings of the 2001 ECBC Scientific Conference on Chemical and Biological Defense Research, 6-8 March , Marriott's Hunt Valley Inn, Hunt Valley, MD.
McDonough JH, McMonagle J, Gonzales M, Rowland R, Shih TM; Studies on anticonvulsants against nerve agent-induced seizures. Pharmacology Division U.S. Army Medical Research Institute of Chemical Defense Aberdeen Proving Ground, MD 21010-5400 USA 2003
McDonough JH, Van Shura KE, LaMont JC, et al; Comparison of the intramuscular, intranasal or sublingual routes of midazolam administration for the control of soman-induced seizures. Basic Clin Pharmacol Toxicol, 2009 Jan; 104(1):27-34. [PubMed Citation]
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2627-2636
Nitsun M, Szokol JW, Saleh HJ, Murphy GS, Vender JS, Luong L, Raikoff K, Avram MJ; Pharmacokinetics of midazolam, propofol, and fentanyl transfer to human breast milk. Clin Pharmacol Ther. 2006 Jun; 79 (6): 549-57. [PubMed Citation]
Pentikäinen PJ, Välisalmi L, Himberg JJ, Crevoisier C; Pharmacokinetics of midazolam following intravenous and oral administration in patients with chronic liver disease and in healthy subjects. J Clin Pharmacol. 1989 Mar; 29 (3): 272-7. [PubMed Citation]
Product label Midazolam hydrochloride (Midazolam hydrocloride) injection [Cardinal Health] Last revised: April 2010 [DailyMed]
Product label Midazolam hydrochloride (midazolam hydrochloride syrup) syrup [West-Ward Pharmaceuticals Corp.] Last revised: June 2008 [DailyMed]
Reed MD, Rodarte A, Blumer JL, Khoo KC, Akbari B, Pou S, Pharmd, Kearns GL; The single-dose pharmacokinetics of midazolam and its primary metabolite in pediatric patients after oral and intravenous administration. J Clin Pharmacol. 2001 Dec; 41 (12): 1359-69. [PubMed Citation]
Reichard DW, Atkinson AJ, Hong SP, Burback BL, Corwin MJ, Johnson JD; Human safety and pharmacokinetic study of intramuscular midazolam administered by autoinjector. J Clin Pharmacol. 2010 Oct; 50(10):1128-35. [PubMed Citation]
Shih TM, Koviak TA, Capacio BR; Anticonvulsants Anticonvulsants for poisoning by the organophosphorus compound soman: pharmacological mechanisms. Neurosci Biobehav Rev. 1991 Fall; 15 (3): 349-62.
Shih TM, McDonough JH, McMonagle J, Koplovitz I.; Anticonvulsants for soman-induced seizure activity. J Biomed Sci, 1999 Mar-Apr; 6(2):86-96. [PubMed Citation]
Shih TM, Duniho SM, McDonough JH. Control of nerve agent-induced seizures is critical for neuroprotection and survival. Toxicol Appl Pharmacol. 2003 Apr 15;188(2):69-80. [PubMed Citation]
Shih TM, Rowland TC, McDonough JH; Anticonvulsants for nerve agent-induced seizures: The influence of the therapeutic dose of atropine. J Pharmacol Exp Ther. 2007 Jan; 320 (1): 154-61. [PubMed Citation]
Silbergleit R, Durkalski V, Lowenstein D et al; Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med, 2012; 366(7):591-600. [PubMed Citation]
Skovira JW, McDonough JH, Shih TM; Protection against sarin-induced seizures in rats by direct brain microinjection of scopolamine, midazolam or MK-801. J Mol Neurosci. 2010 Jan; 40 (1-2): 56-62. [PubMed Citation]
Smith MT, Heazlewood V, Eadie MJ, Brophy TO, Tyrer JH; Pharmacokinetics of midazolam in the aged. Eur J Clin Pharmacol. 1984; 26 (3): 381-8. [PubMed Citation]
Sumi Y, Oode Y, Tanaka H; Chinese Dumpling Scare Hits Japan--A Case of Methamidophos Poisoning. J Toxicol Sci, 2008; 33(4):485-6. [PubMed Citation]
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
Talukdar B, Chakrabarty B; Efficacy of buccal midazolam compared to intravenous diazepam in controlling convulsions in children: A randomized controlled trial. Brain & Development 2009; 31 (10): 744-749.
Thomson/Micromedex 2007; Drug Information for the Health Care Professional: Volume 1, 27th Edition. Greenwood Village, CO. 2007 p.512-535
Towne AR, DeLorenzo RJ; Use of Intramuscular Midazolam for Status Epilepticus. The Journal of Emergency Medicine, 1999; 17 (2): 323-328.
US NLM. ChemIDplus Lite. Midazolam
Vinik HR, Reves JG, Greenblatt DJ, Abernethy DR, Smith LR; The pharmacokinetics of midazolam in chronic renal failure patients. Anesthesiology. 1983 Nov; 59 (5): 390-4. [PubMed Citation]
Department of Homeland Security (DHS). Chemical Security Analysis Center (CSAC). 2011. Chemical Segregation by Toxidrome for the Chemical Terrorism Risk Assessment. PowerPoint available in Report on the Toxic Chemical Syndrome Definition and Nomenclature Workshop (May, 2012)
Zilker T; Medical management of incidents with chemical warfare agents. Toxicology 2005 Oct; 214 (3): 221-31. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
