You are here: Home > Medical Countermeasures Database > Naloxone
Naloxone - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Naloxone
2. Chemical Defense therapeutic area(s)
— including key possible usesNaloxone may be used as an antidote for fentanyl and fentanyl derivatives such as carfentanyl
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
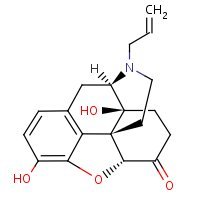
HSDB. Naloxone
Mechanism of action
-
While the mechanism of action of naloxone is not fully understood, the preponderance of evidence suggests that naloxone antagonizes the opioid effects by competing for the same receptor sites.
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
-
Naloxone hydrochloride is essentially a pure opiate antagonist. The precise mechanism of action of the opiate antagonist effects of naloxone is not fully understood. Naloxone is thought to act as a competitive antagonist at mc, κ, and σ opiate receptors in the CNS; it is thought that the drug has the highest affinity for the μ receptor. In contrast to levallorphan or nalorphine, naloxone has little or no agonistic activity. When administered in usual doses to patients who have not recently received opiates, naloxone exerts little or no pharmacologic effect. Even extremely high doses of the drug (10 times the usual therapeutic dose) produce insignificant analgesia, only slight drowsiness, and no respiratory depression, psychotomimetic effects, circulatory changes, or miosis.
-
In patients who have received large doses of morphine or other analgesic drugs with morphine-like effects, naloxone antagonizes most of the effects of the opiate. There is an increase in respiratory rate and minute volume, arterial PCO2 decreases toward normal, and blood pressure returns to normal if depressed. Unlike nalorphine or levallorphan, naloxone antagonizes mild respiratory depression caused by small doses of opiates. Because the duration of action of naloxone is generally shorter than that of the opiate, the effects of the opiate may return as the effects of naloxone dissipate. Naloxone antagonizes opiate-induced sedation or sleep. Reports are conflicting on whether or not the drug modifies opiate-induced excitement or seizures.
-
Naloxone does not produce tolerance or physical or psychological dependence. In patients who are dependent on opiates, parenteral administration of naloxone hydrochloride will precipitate opiate withdrawal symptoms, which may appear within minutes of naloxone administration and subside in about 2 hours. The severity and duration of the withdrawal symptoms are related to the dose of naloxone and the degree and type of opiate dependence. Oral administration of naloxone generally does not precipitate withdrawal symptoms unless the dose exceeds 10 mg. Even a 30-mg oral dose of naloxone usually induces only very mild abstinence symptoms.
-
Naloxone has been shown to increase blood pressure in a limited number of patients with septic shock.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Summary of clinical and non-clinical studies
Antidotes may play an important role in the treatment of poisoning by greatly enhancing the elimination and counteracting the toxic actions of the poison (Jacobsen, 1995). Naloxone (N-allylnoroxymorphine, Narcan) has been used as an antidote to acute opioid poisoning since the 1960's (Clarke et al., 2005). It has been shown to counteract sedation, hypotension, central nervous system (CNS) depression and the potentially fatal complete or partial depression of the respiratory system caused by natural and synthetic opioids and opioid agonist/antagonists. In mice, naloxone (3 mg/kg, intraperitoneal) reversed the respiratory and analgesic effects and precipitated opiate withdrawal when administered 40 minutes after the opioid agonists morphine, methadone, and heroin (Lewanowitsch et al., 2006). Additionally, it completely reversed decreases in respiratory rate and reversed increases in tidal volume 40 minutes and 80 minutes after methadone administration, respectively. In contrast, the depressed respiratory rate and increased tidal volume remained unchanged after saline administration. An intravenous (IV) injection of naloxone (0.4-1.2 mg) resulted in the recovery of consciousness within 1-2 minutes of injection, in patients with narcotic overdosage (Evans et al., 1973). There was also an increase in respiratory rate, respiratory volume, systolic blood pressure and dilation of the pupils following naloxone injection in patients with narcotic poisoning. Naloxone had no effect on the level of consciousness, respiration, blood-pressure, pulse-rate or pupil size in patients poisoned with non-narcotic CNS depressants. Naloxone is the opioid antagonist of choice, but repeated doses or infusion may be required since it has a relatively short duration of action (Bateman, 2011). In a case report, a suicidal 15-year-old girl applied 5 pain relief patches of the narcotic fentanyl (total dose 6 mg, 120 μg) to her body and experienced symptoms of apnea, pinpoint pupils, respiratory depression and decreased conscious level (Lyttle et al., 2012). After repeated doses of naloxone (initially at 6 μg/kg/hour and then increased to 12 μg/kg/hour), she survived with no recurrence of symptoms. In 2002, unknown aerosolized chemical-immobilizing agents, suggested to be fentanyl derivatives, were released during a rescue effort in a Moscow theater containing more than 800 people held captive by several dozen terrorists (Stanley, 2003). It was reported by physicians on the scene that patients had classic signs of opioid intoxication including pinpoint pupils, unconsciousness, depressed respiration and cyanosis. One physician reported that all patients treated at his hospital with naloxone responded; however, another physician said that many of the cases were perplexing. Following the incident, 650 hostages survived, 128 hostages died (123 from the chemical agents), and virtually all the terrorists were killed according to the report.
B. Link to clinical studies
Studies involving multiple populations
-
In late October 2002, several Chechen terrorists commandeered and shortly thereafter controlled and secured a theatre in Moscow containing more than 800 people who were attending a play. The rebels, who had several political demands, threatened to blow up the theater and thus kill themselves and all the hostages if their requests were not met within a short time, a day or two. Apparently, Russian antiterrorist experts and police who had no visual contacts with the terrorists and no way of entering the building without the militants' knowledge made little progress with attempts to talk the terrorists out of their mission and thus had a huge dilemma with little time for alternatives. The issue boiled down to concede to the militants' demands or attempt some sort of rescue that, ideally, would harm few or none of the hostages. The latter approach was risky since some or all of the terrorists claimed to have explosives strapped to their bodies, which they threatened to detonate upon any attempt to rescue the hostages. The former approach was politically unacceptable.
The Russian President, Vladimir Putin, made a decision to rescue the hostages in spite of the huge potential for loss of life if the attempt did not succeed. It is not clear if storming the building forcefully with special forces, antiterrorist experts and/or police was a realistic option. It probably was not. In any event, a decision was made to use a chemical immobilizing agent(s). It appears from reports that the agent(s) was probably nebulized and, using a forced-air system, pumped into the air ducts that led into the auditorium. The aerosolized chemical-immobilizing agents were at first not identified, but later, after significant national and international political and media pressure, suggested to be fentanyl derivatives by the Health Minister Yuri Shevchenko. Shevchenko also said that ‘one thousand antidotes' (which he would not name) were on hand at the time of the rescue.
One report from a laboratory in Germany analyzing some blood from two German hostages suggested that halothane might have been one of the immobilizing agents.
The rescue attempt resulted in the deaths of 128 hostages (123 from the chemical agents). Dozens of other hostages required hospitalization, some with apparently serious but not clearly defined medical problems. Virtually all the terrorists were killed, many by the chemical agents, but some were shot after being subdued by the drugs. The loud outcry and criticism of the Russian decision and rescue attempt in the international media was impressive and prolonged. Somewhat lost in the attention focused on the number of casualties in the rescue was the fact that more that 650 hostages survived, most with no obvious residual medical problems. It is intersting to note that during a news conference at the time, the US President, George W. Bush, pointed out this fact.
It appears from the numerous eyewitness reports, observation of physicians who treated the hostages, statements by Russian officials, and newspaper and magazine articles that a potent opioid was at least one of the chemical agents used. Some unidentified Moscow physicians reported that the patients had classic signs of opioid intoxication: pin point pupils, unconsciousness, depressed respiration and cyanosis. One physician reported that all the hostages treated at his hospital were given naloxone, and all responded. However, at least one other physician said many of cases were perplexing.
The Russian authorities refused specifically to identify the fentanyl derivatives used in the rescue attempt. A group of scientists and immobilization experts in the USA, including Mr Parker Ferguson (a former government chemist, who is now an independent consultant on chemical and biological terrorism), Dr. James H. Woods (Professor of Pharmacology, University of Michigan), and the present author, believe the most likely fentanyl derivative was carfentanil... (Class IV)
Stanley T. Human immobilization: is the experience in Moscow just the beginning? Eur J Anaesthesiol. 2003 Jun;20(6):427-8. [PubMed Citation]
Adults
-
Naloxone (N-allylnoroxymorphone) is a potent narcotic antagonist which is devoid of agonist activity. Nine patients with narcotic analgesic overdosage recovered consciousness immediately after intravenous injection of 0.4-1.2 mg of naloxone given in divided doses over 3 minutes. There was a striking increase in respiratory rate and volume accompanied by a rise in systolic blood-pressure and dilatation of the pupils. In contrast, naloxone did not produce any change in the level of consciousness, respiration, blood-pressure, pulse-rate, or pupil size in thirteen deeply unconscious patients poisoned with a variety of non-narcotic central-nervous-system depressants. Unlike nalorphine, naloxone readily reverses the effects of pentazocine and has no intrinsic respiratory depressant activity. (Class IV)
Evans LE, Swainson CP, Roscoe P, Prescott LF. Treatment of Drug Overdosage with Naloxone, A Specific Narcotic Antagonist. Lancet. 1973. 1(7801):452-455. [PubMed Citation]
-
Naloxone reverses respiratory depression after both anesthesia and overdosage of narcotics and is also recommended in suspected opiate coma. Cardiovascular problems have developed after anesthesia in patients given naloxone to reverse the effects of opiates. We report on a patient addicted to narcotics who suffered ventricular fibrillation on four occasions after treatment with naloxone. (Class IV)
Cuss FM, Colaco CB, Baron JH. Cardiac arrest after reversal of effects of opiates with naloxone. Br Med J (Clin Res Ed). 1984 Feb;288(6414):363-4. [PubMed Citation]
Pediatric studies
-
The use of the fentanyl skin patch to provide pain relief in chronic pain conditions and oncology in adult practice has been common for several years, and an increase in use is now being seen in pediatric practice. Its use in drug misuse and suicide has also increased in recent years. The authors present the case of an adolescent who deliberately overdosed using fentanyl skin patches and describe the implications for management. This report serves to remind clinicians to consider this method of drug administration in children who display signs of opioid toxicity, where overdose may be subsequent to its use in therapy, recreation, or deliberate self-harm. (Class IV)
Lyttle MD, Verma S, Isaac R. Transdermal fentanyl in deliberate overdose in pediatrics. Pediatr Emerg Care. 2012 May;28(5):463-4. [PubMed Citation]
Pregnancy, breastfeeding studies
-
Reproduction studies performed in mice and rats at doses up to 1,000 times the human dose revealed no evidence of impaired fertility or harm to the fetus due to naloxone. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, naloxone hydrochloride should be used during pregnancy only if clearly needed (Class IV).
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
-
Risk-benefit must be considered before naloxone is administered to a pregnant woman who is known or suspected to be opioid-dependent since maternal dependence may often be accompanied by fetal dependence. Naloxone crosses the placenta, and may precipitate withdrawal in the fetus as well as in the mother. Patients with mild to moderate hypertension who receive naloxone during labor should be carefully monitored as severe hypertension may occur (Class IV).
Product label: Naloxone hydrochloride injection, solution [Hospira, Inc.] Last revised: July 2011[DailyMed]
-
Aim: To give new insights into how an infant responded to naloxone, given after acquiring a maternal opiate by recording the breathing pattern directly after birth. Method: A respiratory recording is presented of an infant during resuscitation in the delivery room after receiving naloxone for respiratory depression, resulting from maternal remifentanyl use. Results: The infant was born apneic and bradycardic. Normal resuscitation maneuvres had no effect on the respiratory drive. Directly after administration of naloxone, a tachypneic breathing pattern with sporadic expiratory breaking manoeuvres was observed. Conclusion: The immediate tachypenia is most likely a direct effect of the naloxone causing an immediate rebound response after the release of the opiate-induced inhibition of the respiratory drive (Class IV).
Van Vonderen JJ, Siew ML, Hooper SB, de Boer MA, Walther FJ, te Pas AB. Effects of naloxone on the breathing pattern of a newborn exposed to maternal opiates. Acta Paediatr. 2012 Jul;101(7):e309-12
Clinical reviews
-
Acute opioid intoxication and overdose are common causes of presentation to emergency departments. Although naloxone, a pure opioid antagonist, has been available for many years, there is still confusion over the appropriate dose and route of administration. This article looks at the reasons for this uncertainty and undertakes a literature review from which a treatment algorithm is presented. (Class IV)
Clarke SF J, Dargan PI, Jones AL. Naloxone in opioid poisoning: walking the tightrope. Emerg Med J 2005; 22:612-616. [PubMed Citation]
-
Opioids cause a well-recognized toxidrome including respiratory depression, decreased conscious level, pin point pupils and hypotension. In overdose their toxicity relates to both the amount ingested and speed of absorption. Addicts run into difficulty when a supply changes or adulterants are added, or they have had a period of relative abstinence resulting in loss of tolerance. Some opioids, such as dextropropoxyphene, methadone and tramadol, have additional properties that account for their toxicity. Naloxone is the opioid antagonist of choice and its dose should be titrated according to clinical response. Duration of action is relatively short and repeated doses or infusion may be required. Paradoxical increase in toxicity may be seen if some drug is unabsorbed in the gut before naloxone administration as gut motility recommences. (Class IV)
Bateman DN. Opioids. Medicine 2011;40(3):141-143.
-
For many physicians an antidote is an antidote. According to the International Programme on Chemical Safety definition, an antidote is a therapeutic substance used to counteract the toxic action(s) of a specified xenobiotic. Given this wide definition, the efficacy of an antidote may vary considerably depending on which toxic action(s) being counteracted and the level of counteracting power. An almost 100% efficacy is seen using specific antagonists, such as naloxone in opiate poisoning or flumazenil in benzodiazepine poisoning, e.g. resulting in complete reversal of opiate toxicity unless complications, such as anoxic brain damage, have developed. At the other end of the efficacy scale, we may place chelating agents for heavy metal poisoning and diazepam for organophosphorus insecticide poisoning which are considered only to be an adjuncts to supportive care. When teaching clinical toxicology or recommending the use of antidotes in poisoned patients, the expected efficacy level of the antidote in question should be stressed. This may be particularly important in severe poisonings when the antidote may only be considered as an important adjunct to supportive care, e.g. deferoxamine in acute iron poisoning. Unless this is stressed, the unexperienced physician may rely too much on the antidote and pay insufficient attention to the supportive care. The varying efficacy levels will be discussed based on the presently ongoing International Programme on Chemical Safety/Commission of the European Communities evaluation program on antidotes. (Class IV)
Jacobsen D. The Relative Efficacy of Antidotes. J Tocixol Clin Toxicol. 1995;33(6):705-8. [PubMed Citation]
-
Emergency medical services (EMS) traditionally administer naloxone using a needle. Needleless naloxone may be easier when intravenous (IV) access is difficult and may decrease occupational blood-borne exposure in this high-risk population. Several studies have examined intranasal naloxone, but nebulized naloxone as an alternative needleless route has not been examined in the prehospital setting. To determine whether nebulized naloxone can be used safely and effectively by prehospital providers for patients with suspected opioid overdose. The authors performed a retrospective analysis of all consecutive cases administered nebulized naloxone from January 1 to June 30, 2010, by the Chicago Fire Department. All clinical data were entered in real time into a structured EMS database and data abstraction was performed in a systematic manner. Included were cases of suspected opioid overdose, altered mental status, and respiratory depression; excluded were cases where nebulized naloxone was given for opioid-triggered asthma and cases with incomplete outcome data. The primary outcome was patient response to nebulized naloxone. Secondary outcomes included need for rescue naloxone (IV or intramuscular), need for assisted ventilation, and adverse antidote events. Kappa interrater reliability was calculated and study data were analyzed using descriptive statistics. Out of 129 cases, 105 met the inclusion criteria. Of these, 23 (22%) had complete response, 62 (59%) had partial response, and 20 (19%) had no response. Eleven cases (10%) received rescue naloxone, no case required assisted ventilation, and no adverse events occurred. The kappa score was 0.993. Nebulized naloxone is a safe and effective needleless alternative for prehospital treatment of suspected opioid overdose in patients with spontaneous respirations. (Class IV)
Weber JM, Tataris KL, Hoffman JD, Aks SE, Mycyk MB. Can nebulized naloxone be used safely and effectively by emergency medical services for suspected opioid overdose? Prehosp Emerg Care 2012 Apr-Jun;16(2):289-92. [PubMed Citation]
-
Aims: This paper reviews available literature regarding the effectiveness, safety and utility of intranasal (i.n.) naloxone for the treatment of heroin overdose. Methods Scientific literature in the form of published articles during the period January 1984 to August 2007 were identified by searching several databases including Medline, Cinahl and Embase for the following terms: naloxone, narcan, intranasal, nose. The data extracted included study design, patient selection, numbers, outcomes and adverse events. Results Reports of the pharmacological investigation and administration of i.n. naloxone for heroin overdose are included in this review. Treatment of heroin overdose by administration of i.n. naloxone has been introduced as first-line treatment in some jurisdictions in North America, and is currently under investigation in Australia. Conclusion Currently there is not enough evidence to support i.n. naloxone as first-line intervention by paramedics for treatment of heroin overdose in the pre-hospital setting. Further research is required to confirm its clinical effectiveness, safety and utility. If proved effective, the i.n. route may be useful for drug administration in community settings (including peer-based administration), as it reduces risk of needlestick injury in a population at higher risk of blood-borne viruses. Problematically, naloxone is not manufactured currently in an ideal form for i.n. administration (Class IV).
Kerr D, Dietze P, Kelly AM. Intranasal naloxone for the treatment of suspected heroin overdose. Addiction. 2008 Mar;103(3):379-86. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
Opioid overdose, which is commonly associated with opioid induced respiratory depression, is a problem with both therapeutic and illicit opioid use. While the central mechanisms involved in the effects of opioids are well described, it has also been suggested that a peripheral component may contribute to the effects observed. This study aimed to further characterize the effects of the peripherally acting naloxone methiodide on the respiratory, analgesic and withdrawal effects produced by various opioid agonists. A comparison of the respiratory and analgesic effects of morphine, methadone and heroin in male Swiss-Albino mice was conducted and respiratory depressive ED80 doses of each opioid determined. These doses (morphine 9 mg/kg i.p., methadone 7 mg/kg i.p., and heroin 17 mg/kg i.p.) were then used to show that both naloxone (3 mg/kg i.p.) and naloxone methiodide (30-100 mg/kg i.p.) could reverse the respiratory and analgesic effects of these opioid agonists, but only naloxone precipitated withdrawal. Further investigation in female C57BL/6J mice using barometric plethysmography found that both opioid antagonists could reverse methadone induced decreases in respiratory rate and increases in tidal volume. Its effects do not appear to be strain or sex dependent. It was concluded that naloxone methiodide can reverse the respiratory and analgesic actions of a variety of opioid agonists, without inducing opioid withdrawal.
Lewanowitsch T, Miller JH, Irvine RJ. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Lewanowitsch T, Miller JH, Irvine RJ. Life Sci. 2006 Jan 11;78(7):682-8. [PubMed Citation]
-
Opioid overdose in dogs is manifested by clinical signs such as excessive sedation, bradycardia, and hypothermia. The ability of two different intramuscular (i.m.) naloxone reversal regimens to reverse the opioid-induced effects of a fivefold overdose of long-acting transdermal fentanyl solution was evaluated in dogs. Twenty-four healthy Beagles were administered a single 13 mg/kg dose (fivefold overdose) of transdermal fentanyl solution and randomized to two naloxone reversal regimen treatment groups, hourly administration for 8h of 40 (n=8) or 160 μg/kg i.m. (n=16). All dogs were sedated and had reduced body temperatures and heart rates (HRs) prior to naloxone administration. Both dosage regimens significantly reduced sedation (P<0.001), and the 160 μg/kg naloxone regimen resulted in a nearly threefold lower odds of sedation than that of the 40 μg/kg i.m. naloxone regimen (P<0.05). Additionally, naloxone significantly increased the mean body temperatures and HR (P<0.001), although the 160 μg/kg regimen increased body temperature and HR more (P<0.05). However, the narcotic side effects of fentanyl returned within 1-3 h following termination of the naloxone dosage regimens. The opioid-induced effects of an overdose of transdermal fentanyl solution can be safely and effectively reversed by either 40 or 160 μg/kg i.m. naloxone administered at hourly intervals.
Freise KJ, Newbound GC, Tudan C, Clark TP. Naloxone reversal of an overdose of a novel, long-acting transdermal fentanyl solution in laboratory Beagles. J Vet Pharmacol Ther. 2012 Aug;35 Suppl 2:45-51 [PubMed Citation]
-
Naloxone, when administered for the purpose in reversing opiate-induced respiratory depression after morphine- or fentanyl-anesthesia may precipitate an increase in sympathetic tone with subsequent cardiovascular reactions. In order to investigate which variable of the cardiovascular system is affected most and to compare whether the effects after fentanyl anesthesia (0.05 mg/kg) differ markedly from those after sole equianesthetic meperidine-anesthesia (20 mg/kg), naloxone (5 microgram/kg) was given shortly (15 min) after opiate injection. In the fentanyl group (n = 7) compared to the anesthetic level the antagonist induces an increase in heart-rate by 100%, in LV dP/dt max (inotropic state of the myocardium) by 90%, in mean peripheral blood pressure (afterload of the heart by 80%, in myocardial oxygen consumption by 50% and in left ventricular pressure by 42%. After 32 minutes all variables had returned to anesthesia values. In the meperidine-group (n = 7) naloxone induced an increase in heart-rate by 13%, in LV dP/dt max by 75%, in mean peripheral blood pressure by 65%, in myocardial oxygen consumption by 25% and in left ventricular pressure by 78%. After 25 minutes all increased variables had returned to prenaloxone values. The study indicates that the more potent the opiate agonist, the more naloxone is liable to induce a hyperexcitatory state of the cardiovascular system. This excitatory state is also reflected in an elevated myocardial oxygen consumption. Therefore caution is advised in administering naloxone to patients after sole opiate-anesthesia, who have an impaired myocardial oxygen supply.
Freve E, Hartung E. Naloxone induces excitation of the cardiovascular system and a rise in myocardial oxygen consumption in fentanyl and meperidine-anesthetized dogs. Acta Anaesthesiol Belg. 1982;33(2):89-97[PubMed Citation]
Pregnant animal studies
-
Reproduction studies performed in mice and rats at doses up to 1,000 times the human dose revealed no evidence of impaired fertility or harm to the fetus due to naloxone.
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Absorption: Naloxone is rapidly inactivated following oral administration. Although the drug is effective orally, doses much larger than those required for parenteral administration are required for complete antagonism. In one study, a single 3-g oral dose of naloxone hydrochloride was required to effectively antagonize the effects of 50 mg of heroin for 24 hours. Naloxone has an onset of action within 1-2 minutes following IV administration and within 2-5 minutes following subcutaneous or IM administration. The duration of action depends on the dose and route of administration and is more prolonged following IM administration than after IV administration. In one study, the duration of action was 45 minutes following IV administration of naloxone hydrochloride 0.4 mg/70 kg.
-
Distribution: Following parenteral administration, naloxone is rapidly distributed into body tissues and fluids. Naloxone is weakly bound to plasma proteins (mainly albumin).
-
Elimination: The half-life of naloxone has been reported to be 30-81 minutes in adults
-
Naloxone is rapidly metabolized in the liver, principally by conjugation with glucuronic acid. The major metabolite is naloxone-3-glucuronide. Naloxone also undergoes N-dealkylation and reduction of the 6-keto group followed by conjugation. Limited studies with radiolabeled naloxone indicate that 25-40% of an oral or IV dose of the drug is excreted as metabolites in urine in 6 hours, about 50% in 24 hours, and 60-70% in 72 hours.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Children
-
Following administration of 35 or 70 mcg of naloxone hydrochloride into the umbilical vein in neonates in one study, peak plasma naloxone concentrations occurred within 40 minutes and were 4-5.4 ng/mL and 9.2-20.2 ng/mL, respectively. After IM administration of 0.2 mg to neonates in the same study, peak plasma naloxone concentrations of 11.3-34.7 ng/mL occurred within 0.5-2 hours.
-
Elimination: The half-life of naloxone has been reported to be about 3 hours in neonates.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Pregnancy
-
In humans, the drug readily crosses the placenta. It is not known whether naloxone is distributed into milk.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Animal
-
In rats, high concentrations are observed in the brain, kidney, spleen, lung, heart, and skeletal muscle.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
Opioid Overdose—Known or Suspected: An initial dose of 0.4 mg to 2 mg of naloxone hydrochloride may be administered intravenously. If the desired degree of counteraction and improvement in respiratory functions is not obtained, it may be repeated at 2 to 3 minute intervals. If no response is observed after 10 mg of naloxone hydrochloride have been administered, the diagnosis of opioid induced or partial opioid induced toxicity should be questioned. Intramuscular or subcutaneous administration may be necessary if the intravenous route is not available. Postoperative Opioid Depression: For the partial reversal of opioid depression following the use of opioids during surgery, smaller doses of naloxone hydrochloride are usually sufficient. The dose of naloxone should be titrated according to the patient's response. For the initial reversal of respiratory depression, naloxone hydrochloride should be injected in increments of 0.1 to 0.2 mg intravenously at two to three minute intervals to the desired degree of reversal, i.e., adequate ventilation and alertness without significant pain or discomfort. Larger than necessary dosage of naloxone may result in significant reversal of analgesia and increase in blood pressure. Similarly, too rapid reversal may induce nausea, vomiting, sweating or circulatory stress. Repeat doses of naloxone may be required within one to two hour intervals depending upon the amount, type (i.e., short or long acting) and time interval since last administration of opioid. Supplemental intramuscular doses have been shown to produce a longer lasting effect. Septic Shock: The optimal dosage of Naloxone or duration of therapy for the treatment of hypotension in septic shock patients has not been established.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Children (FDA)
Opioid Overdose—Known or Suspected: The usual initial dose in pediatric patients is 0.01 mg/kg body weight given I.V. If this dose does not result in the desired degree of clinical improvement, a subsequent dose of 0.1 mg/kg body weight may be administered. If an I.V. route of administration is not available, Naloxone Hydrochloride may be administered I.M. or S.C. in divided doses. If necessary, Naloxone Hydrochloride Injection, USP can be diluted with sterile water for injection. Postoperative Opioid Depression: Follow the recommendations and cautions under Adult Postoperative Depression. For the initial reversal of respiratory depression, naloxone hydrochloride should be injected in increments of 0.005 mg to 0.01 mg intravenously at two to three minute intervals to the desired degree of reversal.
When using naloxone hydrochloride injection in neonates a product containing 0.02 mg/mL should be used. Opioid-Induced Depression: The usual initial dose is 0.01 mg/kg body weight administered I.V., I.M., or S.C. This dose may be repeated in accordance with adult administration guidelines for postoperative opioid depression.
Safety and efficacy of naloxone in the management of hypotension associated with septic shock have not been established in pediatric patients. In a study of 2 neonates with septic shock, treatment with naloxone produced positive pressor response; however, one patient subsequently died after intractable seizures.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Pregnancy (FDA)
Pregnancy Category B
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
It is not known if naloxone affects the duration of labor and/or delivery. However, published reports indicate that administration of naloxone during labor did not adversely affect maternal or neonatal status. Patients with mild to moderate hypertension who receive naloxone during labor should be carefully monitored, as severe hypertension may occur.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Nursing Mothers (FDA)
It is not known whether naloxone is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when naloxone is administered to a nursing woman.
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
Geriatric (FDA)
Clinical studies of naloxone did not include sufficient numbers of patients 65 years of age and older to determine whether geriatric patients respond differently from younger patients. While other clinical experience has not revealed age-related differences in response, drug dosage generally should be titrated carefully in geriatric patients, usually initiating therapy at the low end of the dosage range. The greater frequency of decreased hepatic, renal, and/or cardiac function and of concomitant disease and drug therapy observed in the elderly also should be considered.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Naloxone has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Formulation
Naloxone Hydrochloride
Parenteral injection 0.4 mg/mL; 1 mg/mL*
* available from one or more manufacturer, distributor, and/or repackager by generic (nonproprietary) name
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Shelf life
Stability
-
The injections are stable at pH 2.5-5. Following dilution in 5% dextrose or 0.9% sodium chloride injection to a concentration of 0.004 mg/mL (4 mcg/mL), naloxone hydrochloride solutions are apparently stable for 24 hours; after 24 hours, any unused solution should be discarded.
-
Naloxone hydrochloride injection should not be mixed with preparations containing bisulfite, metabisulfite, long-chain or high molecular weight anions, or any solution having an alkaline pH. Drugs or chemical agents should not be added to solutions of naloxone hydrochloride unless their effect on the chemical and physical stability of the solution has been established. Specialized references should be consulted for specific compatibility information.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Storage
-
Naloxone hydrochloride injections should be stored at 15-30°C and protected from light.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Opioid analgesia impairs gastrointestinal motility. Enteral administration of naloxone theoretically allows selective blocking of intestinal opioid receptors caused by extensive presystemic metabolism. Therefore, we studied the effect of enteral naloxone on the amount of gastric tube reflux, the frequency of pneumonia, and the time until first defecation in mechanically ventilated patients with fentanyl analgesia. DESIGN: Prospective, randomized, double-blinded study. SETTING: University hospital intensive care unit. PATIENTS: Eighty-four mechanically ventilated, fentanyl-treated patients without gastrointestinal surgery or diseases. INTERVENTIONS: Patients were assigned to receive 8 mg naloxone or placebo four times daily via a gastric tube during fentanyl administration. MEASUREMENTS AND MAIN RESULTS: Thirty-eight patients received naloxone and 43 placebo; three patients were excluded because of protocol violation. Median gastric tube reflux volume (54 vs. 129 mL, p =0.03) and frequency of pneumonia (34% vs. 56%, p =0.04) were significantly lower in the naloxone group. In both groups, time until first defecation, ventilation time, and length of intensive care unit stay did not differ. There was no difference in fentanyl requirements between the naloxone and the placebo group (7 vs. 6.5 microg/kg/hr, p =0.15). CONCLUSIONS: The results provide evidence that the administration of enteral opioid antagonists in ventilated patients with opioid analgesia might be a simple-and possibly preventive-treatment of increased gastric tube reflux and reduces frequency of pneumonia.
Meissner W, Dohrn B, Reinhart K. Enteral naloxone reduces gastric tube reflux and frequency of pneumonia in critical care patients during opioid analgesia. Crit Care Med. 2003 Mar;31(3):776-80. [PubMed Citation]
Children
-
OBJECTIVE: To describe the effects of enteral naloxone used to treat opioid-induced constipation in pediatric intensive care patients. DESIGN: Retrospective chart review. SETTING: Pediatric intensive care unit. PATIENTS: Twenty-three patients who received opioid therapy and enteral naloxone in our institution from January 2003 to February 2004 were compared with a randomly sampled control group matched for age, weight, sex, and length of stay who received opioids but had not received enteral naloxone. INTERVENTIONS: None. MEASUREMENTS: Daily stool output, daily opiate usage, nutrition, adjuvant laxative use, and side effects were assessed. RESULTS: Patients stayed an average of 5 days (range, 0-13 days) in the pediatric intensive care unit before enteral administration of naloxone was instituted and received it for an average of 9 consecutive days (range, 3-30 days). Mean stool output for study patients before administration of enteral naloxone was 0.14 +/- 0.38 stools per day, whereas after its initiation it was 1.60 +/- 1.14 stools per day (p < 0.001). However, two patients developed significant opiate withdrawal symptoms after receiving enteral naloxone. The average stool output for control patients was 0.53 +/- 1.21 stools per day. CONCLUSIONS: Enteral naloxone may be effective in increasing stool output in opioid-induced constipation but carries the risk of introducing withdrawal symptoms. Further studies are needed to evaluate this agent for opioid-induced constipation in the intensive care unit.
Tofil NM, Benner KW, Faro SJ, Winkler MK. The use of enteral naloxone to treat opioid-induced constipation in a pediatric intensive care unit. Pediatr Crit Care Med. 2006 May;7(3):252-4. [PubMed Citation]
8. Route of Administration/Monitoring
-
Naloxone hydrochloride injection may be administered intravenously, intramuscularly, or subcutaneously. The most rapid onset of action is achieved by intravenous administration, and it is recommended in emergency situations.
-
Since the duration of action of some narcotics may exceed that of naloxone, the patient should be kept under continued surveillance and repeated doses of naloxone should be administered, as necessary.
-
When naloxone hydrochloride is administered intravenously the onset of action is generally apparent within two minutes; the onset of action is only slightly less rapid when it is administered subcutaneously or intramuscularly. The duration of action is dependent upon the dose and route of administration of naloxone hydrochloride. Intramuscular administration produces a more prolonged effect than intravenous administration. The requirement for repeat doses of naloxone, however, will also be dependent upon the amount, type and route of administration of the narcotic being antagonized.
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
-
Naloxone Hydrochloride Injection, USP may be administered intravenously, intramuscularly, or subcutaneously. The most rapid onset of action is achieved by intravenous administration and it is recommended in emergency situations. Since the duration of action of some opioids may exceed that of naloxone, the patient should be kept under continued surveillance. Repeated doses of naloxone should be administered, as necessary. Intravenous Infusion: Naloxone Hydrochloride Injection, USP may be diluted for intravenous infusion in 0.9% sodium chloride injection or 5% dextrose injection. The addition of 2 mg of naloxone hydrochloride in 500 mL of either solution provides a concentration of 0.004 mg/mL. Mixtures should be used within 24 hours. After 24 hours, the remaining unused solution must be discarded. The rate of administration should be titrated in accordance with the patient's response.
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
9. Adverse effects
-
Postoperative: the following adverse events have been associated with the use of naloxone hydrochloride injection in postoperative patients: hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, and cardiac arrest. Death, coma, and encephalopathy have been reported as sequelae of these events. Excessive doses of naloxone in postoperative patients may result in significant reversal of analgesia and may cause agitation.
-
Opioid Depression: abrupt reversal of opioid depression may result in nausea, vomiting, sweating, tachycardia, increased blood pressure, tremulousness, seizures, ventricular tachycardia and fibrillation, pulmonary edema, and cardiac arrest which may result in death.
-
Opioid Dependence: abrupt reversal of opioid effects in persons who are physically dependent on opioids may precipitate an acute withdrawal syndrome which may include, but not limited to the following signs and symptoms: body aches, fever, sweating, runny nose, sneezing, piloerection, yawning, weakness, shivering or trembling, nervousness, restlessness or irritability, diarrhea, nausea or vomiting, abdominal cramps, increased blood pressure, and tachycardia. In the neonate, opioid withdrawal may also include: convulsions, excessive crying, and hyperactive reflexes.
-
Adverse events associated with the postoperative use of naloxone hydrochloride injection are listed by organ system and in decreasing order of frequency as follows:
-
Cardiac Disorders: pulmonary edema, cardiac arrest or failure, tachycardia, ventricular fibrillation, and ventricular tachycardia. Death, coma, and encephalopathy have been reported as sequelae of these events.
-
Gastrointestinal Disorders: vomiting, nausea
-
Nervous System Disorders: convulsions, paraesthesia, grand mal convulsion
-
Psychiatric Disorders: agitation, hallucination, tremulousness
-
Respiratory, Thoracic, and Mediastinal Disorders: dyspnea, respiratory depression, hypoxia
-
Skin and Subcutaneous Tissue Disorders: nonspecific injection site reactions, sweating
-
Vascular Disorders: hypertension, hypotension, hot flashes, or flushing
Product label: Naloxone hydrochloride injection, solution [Hospira, Inc.] Last revised: July 2011[DailyMed]
-
Nausea and vomiting have been reported rarely in postoperative patients who were receiving a parenteral dose of naloxone hydrochloride greater than that usually recommended; however, a causal relationship has not been established.
-
Tremor and hyperventilation associated with an abrupt return to consciousness has occurred in some patients receiving naloxone for opiate overdosage.
-
Although a causal relationship to the drug has not been established, severe cardiopulmonary effects (e.g., hypotension, hypertension, ventricular tachycardia and fibrillation, dyspnea, pulmonary edema, cardiac arrest) resulting in death, coma, and encephalopathy have been reported in patients following postoperative administration of naloxone hydrochloride.
-
Adverse cardiopulmonary effects have occurred most frequently in postoperative patients with preexisting cardiovascular disease or in those receiving other drugs that produce similar adverse cardiovascular effects.
-
Seizures have occurred rarely following administration of naloxone hydrochloride; however, a causal relationship to the drug has not been established.
-
When high oral doses of naloxone have been used in the treatment of opiate addiction, some patients have experienced mental depression, apathy, inability to concentrate, sleepiness, irritability, anorexia, nausea, and vomiting. These adverse effects usually occurred in the first few days of treatment and abated rapidly with continued therapy or dosage reduction. One case of erythema multiforme cleared promptly after naloxone was discontinued.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
10. Contraindication(s)
-
Naloxone hydrochloride injection is contraindicated in patients known to be hypersensitive to it.
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesTitle: Randomized trial of intranasal versus intravenous naloxone in level of consciousness in suspected opioid overdose.
Conditions: Morphinan opioid overdose
Interventions: Drug: intranasal naloxone; Drug: intravenous
Title: Naloxone nasal spray pharmacokinetic study
Condition: Opioid overdose
Interventions: Drug: MVP005; Drug: Naloxone hydrochloride solution for injection with mucosal atomization device
Title: Pharmacokinetics of fentanyl following intravenous and oral routes of administration in healthy volunteers.
Condition: Biological availability
Intervention: Drug: Fentanyl citrate; Naltrexone; Naloxone
Title: Study of single and multiple doses of inhaled AeroLEF (liposome-encapsulated fentanyl) in healthy subjects
Condition: Healthy
Interventions: Drug: i.v. fentanyl; Drug: 3 mL AeroLEF (500 μg/1 mL)
Clinical Trials.gov. Naloxone
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations- No data available at this time.
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations- No data available at this time.
15. Study-related ethical concerns
— including review panel recommendations- No data available at this time.
16. Global regulatory status
U.S.
-
Naloxone Hydrochloride Injection is indicated for the complete or partial reversal of opioid depression, including respiratory depression, induced by natural and synthetic opioids including propoxyphene, methadone, and certain mixed agonist-antagonist analgesics: nalbuphine, pentazocine, butorphanol and cyclazocine. Naloxone hydrochloride is also indicated for the diagnosis of suspected or known acute opioid overdosage.
-
Naloxone may be useful as an adjunctive agent to increase blood pressure in the management of septic shock.
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
E.U.
-
Naloxone IM or IV Initiation: 0.2-0.4 mg repeated until effects observed; up to 2-4 mg for fentanyls. Continued for less than 24 hours for adults: 10-20 mg, for children: 0.1 mg/kg.
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
U.K.
-
Reversal of opioid-induced respiratory depression; reversible of neonatal respiratory depression resulting from opioid administration to mother during labour; overdosage with opioids.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 713
Other
-
Naloxone injection: 400 micrograms (hydrochloride) in 1-mL ampoule
WHO. WHO Model List of Essential Medicines (Adults) 17th list (March 2011)
-
Naloxone injection: 400 micrograms (hydrochloride) in 1-mL ampoule
WHO. WHO Model List of Essential Medicines for Children 3rd list (March 2011)
17. Other potentially useful information
-
To determine the absolute bioavailability of naloxone from oral doses ranging from 5 mg to 120 mg. In this open-label study, 28 healthy subjects received naloxone 1 mg (0.4 mg/ml) as an intravenous infusion (reference treatment), and the following oral doses as prolonged release (PR) naloxone tablets: 5 mg, 20 mg, 40 mg, 80 mg and 120 mg. The pharmacokinetic characteristics of 40 mg administered per rectum were also investigated. Each subject received five of the seven treatments as single doses with a 7 day washout between doses. Pharmacokinetic blood sampling and safety monitoring were performed for 24 h after the intravenous dose, and 72 h after the oral and rectal doses. The mean absolute bioavailability of naloxone from the orally administered PR tablets was very low, ranging from 0.9% for the 5 mg dose to 2% for the 40, 80 and 120 mg doses, based on AUC(t) values. The pharmacokinetics of naloxone were linear across the range of oral doses. Where AUC(inf) values were calculated, these confirmed the results based on AUC(t) values (mean absolute bioavailability ranging from 1.9% to 2.2% for the 20 mg to 120 mg oral doses). The absolute bioavailability of naloxone was higher following rectal administration compared with oral administration, but was still low at 15%. The mean oral absolute bioavailability of naloxone in this study was ≤ 2% at doses ranging from 5 mg to 120 mg.
Smith K, Hopp M, Mundin G, Bond S, Bailey P, Woodward J, Bell D. Low absolute bioavailability of oral naloxone in healthy subjects. Int J Clin Pharmacol Ther. 2012 May;50(5):360-7 [PubMed Citation]
-
Solubilities: In water, 1,400 mg/L at 25 deg C (est)
-
Octanol/Water Partition Coefficient: log Kow = 2.09
HSDB. Naloxone
18. Publications
Bateman DN. Opioids. Medicine 2011;40(3):141-143.
Clarke SF J, Dargan PI, Jones AL. Naloxone in opioid poisoning: walking the tightrope. Emerg Med J 2005; 22:612-616. [PubMed Citation]
Clinical Trials.gov. Naloxone
Cuss FM, Colaco CB, Baron JH. Cardiac arrest after reversal of effects of opiates with naloxone. Br Med J (Clin Res Ed). 1984 Feb;288(6414):363-4. [PubMed Citation]
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
Chemical Agents (April 2003) (EMA)
Evans LE, Swainson CP, Roscoe P, Prescott LF. Treatment of Drug Overdosage with Naloxone, A Specific Narcotic Antagonist. Lancet. 1973. 1(7801):452-455. [PubMed Citation]
Freise KJ, Newbound GC, Tudan C, Clark TP. Naloxone reversal of an overdose of a novel, long-acting transdermal fentanyl solution in laboratory Beagles. J Vet Pharmacol Ther. 2012 Aug;35 Suppl 2:45-51 [PubMed Citation]
Freve E, Hartung E. Naloxone induces excitation of the cardiovascular system and a rise in myocardial oxygen consumption in fentanyl and meperidine-anesthetized dogs. Acta Anaesthesiol Belg. 1982;33(2):89-97[PubMed Citation]
HSDB. Naloxone
Jacobsen D. The Relative Efficacy of Antidotes. J Tocixol Clin Toxicol. 1995;33(6):705-8. [PubMed Citation]
Kerr D, Dietze P, Kelly AM. Intranasal naloxone for the treatment of suspected heroin overdose. Addiction. 2008 Mar;103(3):379-86. [PubMed Citation]
Lewanowitsch T, Miller JH, Irvine RJ. Reversal of morphine, methadone and heroin induced effects in mice by naloxone methiodide. Lewanowitsch T, Miller JH, Irvine RJ. Life Sci. 2006 Jan 11;78(7):682-8. [PubMed Citation]
Lyttle MD, Verma S, Isaac R. Transdermal Fentanyl in Deliberate Overdose in Pediatrics. Pediatr Emerg Care. 2012 May;28(5):463-4. [PubMed Citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 713
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 2236-39
Meissner W, Dohrn B, Reinhart K. Enteral naloxone reduces gastric tube reflux and frequency of pneumonia in critical care patients during opioid analgesia. Crit Care Med. 2003 Mar;31(3):776-80. [PubMed Citation]
Product Label:
Naloxone Hydrochloride (naloxone hydrochloride) injection
[International Medication Systems, Limited]. Last revised. April
2006 [DailyMed]
Product label: Naloxone hydrochloride injection, solution [Hospira, Inc.] Last revised: July 2011[DailyMed]
Smith K, Hopp M, Mundin G, Bond S, Bailey P, Woodward J, Bell D. Low absolute bioavailability of oral naloxone in healthy subjects. Int J Clin Pharmacol Ther. 2012 May;50(5):360-7 [PubMed Citation]
Stanley T. Human immobilization: is the experience in Moscow just the beginning? Eur J Anaesthesiol. 2003 Jun;20(6):427-8. [PubMed Citation]
Tofil NM, Benner KW, Faro SJ, Winkler MK. The use of enteral naloxone to treat opioid-induced constipation in a pediatric intensive care unit. Pediatr Crit Care Med. 2006 May;7(3):252-4. [PubMed Citation]
Van Vonderen JJ, Siew ML, Hooper SB, de Boer MA, Walther FJ, te Pas AB. Effects of naloxone on the breathing pattern of a newborn exposed to maternal opiates. Acta Paediatr. 2012 Jul;101(7):e309-12 [PubMed Citation]
Weber JM, Tataris KL, Hoffman JD, Aks SE, Mycyk MB. Can nebulized naloxone be used safely and effectively by emergency medical services for suspected opioid overdose? Prehosp Emerg Care 2012 Apr-Jun;16(2):289-92 [PubMed Citation]
WHO. WHO Model List of Essential Medicines (Adults) 17th list (March 2011)
WHO. WHO Model List of Essential Medicines for Children 3rd list (March 2011)
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
