You are here: Home > Medical Countermeasures Database > Pralidoxime
Pralidoxime - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Pralidoxime
2. Chemical Defense therapeutic area(s)
— including key possible usesAntidote for organophosphorous nerve agent poisoning including chlorosarin, cyclosarin (GF), R-33 (VR), R-VX, sarin (GB), tabun (GA), VX, chlorosoman, soman (GD), and organophosphorous pesticides
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
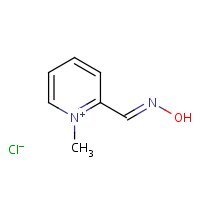
US NLM. ChemIDplus Lite. Pralidoxime
Mechanism of action
-
The principal action of pralidoxime is to reactivate cholinesterase (mainly outside of the central nervous system) which has been inactivated by phosphorylation due to an organophosphate pesticide or related compound. The destruction of accumulated acetylcholine can then proceed and neuromuscular junctions will again function normally. Pralidoxime also slows the process of aging of phosphorylated cholinesterase to a non-reactivatable form, and detoxifies certain organophosphates by direct chemical reaction. The drug has its most critical effect in relieving paralysis of the muscles of respiration. Because pralidoxime is less effective in relieving depression of the respiratory center, atropine is always required concomitantly to block the effect of accumulated acetylcholine at this site. Pralidoxime relieves muscarinic signs and symptoms, salivation, bronchospasm, etc., but this action is relatively unimportant since atropine is adequate for this purpose.
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
-
There is convincing evidence that the antidotal potency of oximes is primarily attributed to their abilities to reactivate the phosphorylated cholinesterases. Oximes reactivate phosphorylated cholinesterases by displacing the phosphoryl moiety from the enzyme... The rate of reactivation depends on the structure of the phosphoryl moiety bound to the enzyme, the source of the enzyme, the structure and concentration of oxime which is present at the active site, and the rate of post-inhibitory dealkylation known as aging. Phosphorylated oximes are formed during reactivation reaction and some of the appear to be potent inhibitors of /acetylcholinesterase/... In addition to performing /acetylcholinesterase/ reactivation in /organophosphates/ poisoning, oximes might also show some direct pharmacological effects.
Gupta RC, ed. Handbook of Toxicology of Chemical Warfare Agents. Oxford, UK: Elsevier, Academic Press, 2009 p. 985-96
-
Some effects of oximes are not well understood. Their quaternary ammonium compound structures are thought to reduce their passage across the blood-brain barrier and prevent CNS effects. However, obidoxime has been detected in cerebrospinal fluid (CSF)and at least one case report describes pralidoxime-induced improvements in mental status and electroencephalograms not attributable to improved ventilation or perfusion.
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1450-66
-
The efficacy of oximes depends on (1) the chemical structure of organophosphate, (2) the delay in treatment due to the ageing of the phosphorylated enzyme, (3) the endpoints used to assess their efficacy.
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
Summary of clinical and non-clinical studies
Organophosphates (OP) are commonly used as pesticides and as military nerve agents; the latter include sarin, soman, tabun, and VX. OP intoxication is the result of irreversible inhibition of acetylcholinesterase (AChE) via phosphorylation of the active-site serine (Jokanovic and Prostran, 2009). As a result, acetylcholine accumulates at synapses, inducing convulsions, behavioral impairments, and eventually death, if untreated. Standard treatment for acute OP intoxication involves pre-treatment with the anticonvulsant pyridostigmine bromide (when possible), and the concomitant administration of atropine and oximes. Oximes reactivate AChE via nucleophilic attack at the phosphorus atom, generating free, active enzyme and a phosphorylated oxime (Jokanovic and Prostran, 2009). The oxime pralidoxime (2-PAM) has been used against OP poisoning, including, notably, the Tokyo subway sarin attack (Yanagisawa et al., 2006). It is available for adults in combination with atropine in autoinjector format, and the Mark 1 autoinjector is currently used by the United States Army and stocked by municipal emergency medical service squads. Preclinical data suggests that individual oximes may be selectively more effective against certain OP compounds. In vivo studies exposing guinea pigs to lethal doses of various OPs indicated that subsequent injection of pralidoxime restored AChE function in blood and peripheral tissues after VX and sarin, and in peripheral tissue after Russian VX; it did not reactivate AChE at all after cyclosarin exposure (Shih et al., 2010). Similarly, while a high dose (1 mM) of pralidoxime in vitro can reactivate AChE from rat brain homogenate after VX, Russian VX, sarin, or chlorpyrifos exposure, it showed no efficacy against cyclosarin, tabun, or soman (Kuca et al., 2005; Kuca et al., 2007). On the other hand, pralidoxime effectively restored the function (TSA, tetanus sustaining ability) of rat diaphragm muscles exposed to tabun, sarin, and VX, suggesting that factors other than AChE reactivation may also contribute to pralidoxime's potential clinical benefit (Reddy et al., 1991).
Clinical trials of pralidoxime in OP-intoxicated patients have yielded varied results (Buckley et al., 2011). In a multi-arm clinical trial of Iranian patients aged 14-60 who accidentally orally ingested OP pesticide and showed moderate to severe OP intoxication symptoms, all 8 patients in the pralidoxime/atropine group survived and saw some AChE recovery, whereas 6 of 12 (50%) patients on the obidoxime/atropine arm and 4 of 43 (9%) patients receiving atropine alone expired (Balali-Mood and Shariat, 1998). In that trial, only the pralidoxime/atropine group demonstrated some AChE recovery, although it was statistically non-significant (r=0.4747). On the other hand, a randomized, controlled (vs. placebo) clinical trial of pralidoxime in patients accidentally poisoned by OP pesticides failed to show any significant improvement in survival and morbidity, in spite of a statistically significant improvement in AChE activity (Eddleston et al., 2009). There was a statistically non-significant (p=0.12) increase in the number of deaths on the pralidoxime arm (30/121 patients, 24.8%) compared to the placebo arm (18/114, 15.8%), and similar numbers of patients required intubation on both arms (26/121 and 24/114, respectively). Although pralidoxime is currently approved for human use, investigators are testing other oximes including a pro-form of pralidoxime (pro-2-PAM), in anticipation of finding an oxime with activity against a broader range of OPs and more robust efficacy (Shih, 2011; Demar, 2010).
B. Link to clinical studies
Studies involving multiple populations
-
Acute organophosphorus pesticide poisoning causes tens of thousands of deaths each year across the developing world. Standard treatment involves administration of intravenous atropine and oxime to reactivate inhibited acetylcholinesterase. The clinical usefulness of oximes, such as pralidoxime and obidoxime, has been challenged over the past 20 years by physicians in many parts of the world. /The objective of this study was/ to quantify the effectiveness and safety of the administration of oximes in acute organophosphorus pesticide-poisoned patients.
The authors searched both English and Chinese databases: Cochrane Injuries Group Specialized Register, Cochrane Central Register of Controlled Trials (The Cochrane Library), MEDLINE (Ovid SP), EMBASE (Ovid SP), ISI Web of Science: Science Citation Index Expanded (SCI-EXPANDED), ISI Web of Science: Conference Proceedings Citation Index-Science (CPCI-S) and the Chinese language databases CNKI and WANGFANG. All searches were run in September 2009. Articles that could possibly be randomized controlled trial (RCT), were retrieved to determine if they were randomized. The published methodology of three RCTs was not clear. The investigators contacted the principal authors of these, but did not obtain further information. Seven pralidoxime RCTs were found. Three RCTs including 366 patients studied pralidoxime vs placebo and four RCTs including 479 patients compared two or more different doses. These trials found quite disparate results with treatment effects ranging from benefit to harm. However, many studies did not take into account several issues important for outcomes. In particular, baseline characteristics were not balanced, oxime doses varied widely, there were substantial delays to treatment, and the type of organophosphate was not taken into account. Only one RCT compared the World Health Organization (WHO) recommended doses with placebo. This trial showed no clinical benefits and a trend towards harm in all sub-groups, despite clear evidence that these doses reactivated acetylcholinesterase in the blood. Current evidence is insufficient to indicate whether oximes are harmful or beneficial. The WHO recommended regimen (30 mg/kg pralidoxime chloride bolus followed by 8 mg/kg/hr infusion) is not supported. Further RCTs are required to examine other strategies and regimens. There are many theoretical and practical reasons why oximes may not be useful, particularly for late presentations of dimethyl OP and those with a large excess of OP that simply re-inhibits reactivated enzymes. Future studies should screen for patient sub-groups that may benefit and may need flexible dosing strategies as clinical effectiveness and doses may depend on the type of OP. (Class IV)
Buckley N.A., M. Eddleston, Li Y, Bevan M, Roberstson J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev. 2011 Feb;(2):CD005085. [PubMed Citation]
-
Two terrorist attacks with the nerve agent sarin affected citizens in Matsumoto and Tokyo, Japan in 1994 and 1995, killing 19 and injuring more the 6000. Sarin, a very potent organophosphate nerve agent, inhibits acetylcholinesterase (AchE) activity within the central, peripheral, and autonomic nervous systems. Acute and long-term sarin effects upon humans were well documented in these two events. Sarin gas inhalation caused instantaneous death by respiratory arrest in 4 victims in Matsumoto. In Tokyo, two died in station yards and another ten victims died in hospitals within a few hours to 3 months after poisoning. Six victims with serum ChE below 20% of the lowest normal were resuscitated from cardiopulmonary arrest (CPA) or coma with generalized convulsion. Five recovered completely and one remained in vegetative state due to anoxic brain damage. EEG abnormalities persisted for up to 5 years. Miosis and copious secretions from the respiratory and GI tracts (muscarinic effects) were common in severely to slightly affected victims. Weakness and twitches of muscles (nicotinic effects) appeared in severely affected victims. Neuropathy and ataxia were observed in small number (less than 10%) of victims, which findings disappeared between 3 days and 3 months. Leukocytosis and high serum CK levels were common. Hyperglycemia, ketonuria, low serum triglyceride, hypopotassemia were observed in severely affected victims, which abnormalities were attributed to damage of the adrenal medulla. Oximes, atropine sulfate, diazepam and ample intravenous infusion were effective treatments. Pralidoxime iodide IV reversed cholinesterase and symptoms quickly even if administered 6 h after exposure. Post Traumatic Stress Disorder (PTSD) was less than 8% after 5 years. However, psychological symptoms continue in victims of both incidents. In summary, both potent toxicity and quick recovery from critical ill conditions were prominent features. Conventional therapies proved effective in sarin incidents in Japan. (Class IV)
Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: Acute toxicity and long-term effects. Journal of the Neurological Sciences 2006 Nov;249(1):76-85. [PubMed Citation]
-
Poisoning with organophosphorus (OP) insecticides is a major global public health problem, causing an estimated 200,000 deaths each year. Although the World Health Organization recommends use of pralidoxime, this antidote's effectiveness remains unclear. The authors aimed to determine whether the addition of pralidoxime chloride to atropine and supportive care offers benefit. They performed a double-blind randomized placebo-controlled trial of pralidoxime chloride (2 g loading dose over 20 min, followed by a constant infusion of 0.5 g/h for up to 7 d) versus saline in patients with organophosphorus insecticide self-poisoning. Mortality was the primary outcome; secondary outcomes included intubation, duration of intubation, and time to death. The authors measured baseline markers of exposure and pharmacodynamic markers of response to aid interpretation of clinical outcomes. Two hundred thirty-five patients were randomized to receive pralidoxime (121) or saline placebo (114). Pralidoxime produced substantial and moderate red cell acetylcholinesterase reactivation in patients poisoned by diethyl and dimethyl compounds, respectively. Mortality was nonsignificantly higher in patients receiving pralidoxime: 30/121 (24.8%) receiving pralidoxime died, compared with 18/114 (15.8%) receiving placebo (adjusted hazard ratio [HR] 1.69, 95% confidence interval [CI] 0.88-3.26, p = 0.12). Incorporating the baseline amount of acetylcholinesterase already aged and plasma OP concentration into the analysis increased the HR for patients receiving pralidoxime compared to placebo, further decreasing the likelihood that pralidoxime is beneficial. The need for intubation was similar in both groups (pralidoxime 26/121 [21.5%], placebo 24/114 [21.1%], adjusted HR 1.27 [95% CI 0.71-2.29]). To reduce confounding due to ingestion of different insecticides, the authors further analyzed patients with confirmed chlorpyrifos or dimethoate poisoning alone, finding no evidence of benefit. Despite clear reactivation of red cell acetylcholinesterase in diethyl organophosphorus pesticide poisoned patients, we found no evidence that this regimen improves survival or reduces need for intubation in patients with organophosphorus insecticide poisoning. The reason for this failure to benefit patients was not apparent. Further studies of different dose regimens or different oximes are required. (Class II)
Eddleston, M., P. Eyer, F. Worek, Juszczak E, Alder N, Mohamed F, Senarathna L, Hittarage A, Azher S, Jeganathan K, Jayamanne S, von Meyer L, Dawson AH, Sheriff MH, Buckley NA. Pralidoxime in acute organophosphorus insecticide poisoning--a randomised controlled trial. PLoS Med. 2009 Jun;6(6):e1000104. [PubMed Citation]
Adult
-
The neuromuscular transmission failure in acute organophosphate (OP) poisoning occurs because of the irreversible inactivation of the enzyme acetylcholinesterase located in the neuromuscular junction, and is distinguished neuroelectrophysiologically by single electrical stimulus-induced repetitive responses and either a decremental or a decrement-increment response upon high-rate repetitive nerve stimulation (RNS). Understandably, the administration of pharmacological agents with actions at different sites in the neuromuscular junction would alter the neuroelectrophysiological findings in acute OP poisoning. Methods: The effect of several pharmacological agents including pralidoxime (10 patients), magnesium sulfate (4 patients) and pancuronium (7 patients) on the neuroelectrophysiological abnormalities was studied in 21 patients with acute OP poisoning. Pralidoxime administration produced neurophysiological amelioration in 11 out of 15 occasions. In those cases where it produced a beneficial effect, pralidoxime administration was continued and its neuroelectrophysiological effects were studied daily. The efficacy of pralidoxime administration was demonstrated by neuroelectrophysiological testing for a maximum of 6 days after poisoning. Three types of neuroelectrophysiological responses to pralidoxime were noted: (i) lack of neuroelectrophysiological improvement (two patients); (ii) initial improvement with subsequent lack of improvement (two patients); and (iii) initial improvement with subsequent normalization of neuromuscular transmission (5 patients). Normalization of the electrodiagnostic tests and the failure of pralidoxime to ameliorate the neuromuscular transmission abnormalities were neuroelectrophysiological indications for the discontinuation of pralidoxime treatment. The administration of magnesium sulfate (MgSO4.7H2O, 4 g intravenous) resulted in a decrease in the Compound Muscle Action Potential (CMAP) amplitude, loss of the repetitive response and conversion of the decrement-increment response at high-rate RNS to an incremental response. Repetitive responses and the decremental response at high-rate RNS also disappeared after the administration of pancuronium (0.5 mg intravenous) to 6 patients. However, in one case where pancuronium administration was preceded by pralidoxime, there occurred a dramatic worsening of the neuromuscular transmission defect. While the administration of all 3 agents - pralidoxime, magnesium sulfate and pancuronium - resulted in the reversion of the neuroelectrophysiological defects, only pralidoxime is contended to be therapeutically useful. The therapeutic benefit due to its administration is limited by a short duration of action, and hence it is recommended that it should be administered for a longer period of time under neuroelectrophysiolgical guidance. (Class IV)
Singh G, Avasthi G, Khurana D, Whig J, Mahajan R. Neurophysiological monitoring of pharmacological manipulation in acute organophosphate (OP) poisoning. The effects of pralidoxime, magnesium sulphate and pancuronium. Electroencephalography and clinical Neurophysiology 1998 Aug;107(2):140-8. [PubMed Citation]
-
Organophosphate (OP) compounds have been used as pesticides and in chemical warfare (nerve agents). Two nerve agents, tabun and sarin, were used by the Iraqi army against Iranian troops and innocent people. Hundreds of the exposed combatants died in the field. Atropine sulfate has been used successfully in large doses to counteract the muscarinic effects of OP poisoning. The effects of oximes in human OP poisoning have not been well studied. The aim was to study the effects of obidoxime and pralidoxime in OP pesticide poisoning. The patients were divided into three groups: atropine (A), obidoxime + atropine (OA) and pralidoxime + atropine (PA). Sixty-three patients (33 males, 30 females) with a mean age of 25 years were studied in different groups (43 A, 22 OA and 8 PA I. There were no statistical significant differences in major clinical findings and acetylcholinesterase (AChE) activity on admission between the groups. Significant changes were observed during the treatment. Notwithstanding the severity of intoxication - particularly respiratory complications were more observed in the OA and PA groups - there were no fatalities in the PA group, whereas 4 (9%) and 6 (50%) patients in the A and OA groups died, respectively. AChE reactivation was only observed in the PA group, although it was not statistically significant (r = 0.4747). There was a good relationship between the AChE reactivation and outcome of the patients. High doses of obidoxime (8 mg/kg followed by 2 mg/kg/h) were found to be hepatotoxic and should be avoided. High doses of pralidoxime(30 mg/kg followed by 8 mg/kg/h) did not induce serious side effects and may be effective in some OP pesticides poisoning. (Class III)
Balali-Mood M, Shariat M. Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J Physiology (Paris) 1998 Oct-Dec;92(5-6):375-8. [PubMed Citation]
Pediatric studies
-
No formal clinical trials have been conducted using pralidoxime in pediatric patients. A retrospective analysis of the scientific literature from 2001 to 2004 was performed to search for cases of pediatric pralidoxime usage; also included in this analysis were detailed data drawn from the American Association of Poison Control Centers' Toxic Exposure Surveillance System from 1999 to 2001 (Quail and Shannon, 2007). In total, 81 children (16 years of age or younger) were found to meet the criteria for suspected OP intoxication and pralidoxime treatment. Two children (2.5%) expired, and three (3.7%) were identified as having a potential adverse drug reaction; all were mild (Class IV).
-
The American Academy of Pediatrics (AAP) issued a 2006 statement (reaffirmed in 2011) noting that the pralidoxime/atropine autoinjector uses adult dosing only, and that while the FDA had approved an atropine pediatric autoinjector, no such pediatric autoinjector has yet been approved for pralidoxime. The AAP recommends following consensus guidelines developed by the AAP Committee on Pediatric Emergency Medicine, Task Force on Terrorism: children weighing 13 kg or more (2-3 years or older) receive a 600-mg (adult) dose of pralidoxime from an autoinjector, on the basis of the belief that this pralidoxime dose falls within the range of safety for the drug. Children weighing less than 13 kg should receive the customary weight-based (20-50 mg/kg) dose, administered from a multidose vial; if unavailable, an autoinjector should be used (Class IV).
-
Children remain potential victims of chemical or biological terrorism. In recent years, children have even been specific targets of terrorist acts. Consequently, it is necessary to address the needs that children would face after a terrorist incident. A broad range of public health initiatives have occurred since September 11, 2001. Although the needs of children have been addressed in many of them, in many cases, these initiatives have been inadequate in ensuring the protection of children. In addition, public health and health care system preparedness for terrorism has been broadened to the so-called all-hazards approach, in which response plans for terrorism are blended with plans for a public health or health care system response to unintentional disasters (eg, natural events such as earthquakes or pandemic flu or manmade catastrophes such as a hazardous-materials spill). In response to new principles and programs that have appeared over the last 5 years, this policy statement provides an update of the 2000 policy statement. The roles of both the pediatrician and public health agencies continue to be emphasized; only a coordinated effort by pediatricians and public health can ensure that the needs of children, including emergency protocols in schools or child care centers, decontamination protocols, and mental health interventions, will be successful. (Class IV)
Chemical-biological terrorism and its impact on children. Pediatrics 2006 Sep; 118(3):1267-78. [PubMed Citation]
-
Currently the safety of pralidoxime administration via adult autoinjectors for pediatric patients has not been established. Up until 2000, the published literature did not recommend its usage for children less than 12 kg or under the age of 10 years old. Since 2000, limited published articles have emerged validating adult autoinjector usage for the pediatric victim, in extreme circumstances. The authors sought to determine whether adverse drug reactions (ADR) from pralidoxime administration to children occur. Recurrent PubMed Medline literature search of all years were performed from 2001 to 2004 inclusive. The main search criteria were articles pertaining to U.S. children 16 years or younger who received pralidoxime. In addition, a review of 3 years (1999-2001) of detailed retrospective TESS exposure annual poison center data was obtained from the AAPCC. Eighty-one children met inclusion criteria and received pralidoxime for suspected organophosphate poisoning. Two children (2.5%) expired. Three children (3.7%) were identified as having a potential adverse drug reaction; all were mild. The author's recognize this study possesses limitations that require its findings be interpreted with caution. The data suggest that adverse drug reactions to pralidoxime treatment in children are rare. However, further investigation is needed to more firmly establish the safety of this antidote in children and for its use in the prehospital environment. (Class IV)
Quail MT, Shannon MW. Pralidoxime safety and toxicity in children. Prehosp Emerg Care. 2007 Jan-Mar;11(1):36-41. [PubMed Citation]
Clinical reviews
-
During more than five decades, pyridinium oximes have been developed as therapeutic agents used in the medical treatment of poisoning with organophosphorus compounds. Their mechanism of action is reactivation of acetylcholinesterase (AChE) inhibited by organophosphorus agents. Organophosphorus compounds (OPC) are used as pesticides and developed as warfare nerve agents such as tabun, soman, sarin, VX and others. Exposure to even small amounts of an OPC can be fatal and death is usually caused by respiratory failure resulting from paralysis of the diaphragm and intercostal muscles, depression of the brain respiratory center, bronchospasm, and excessive bronchial secretions. The mechanism of OPC poisoning involves phosphorylation of the serine hydroxyl group at the active site of AChE leading to the inactivation of this essential enzyme, which has an important role in neurotransmission. AChE inhibition results in the accumulation of acetylcholine at cholinergic receptor sites, producing continuous stimulation of cholinergic fibers throughout the central and peripheral nervous systems. Presently, a combination of an antimuscarinic agent, e.g. atropine, AChE reactivator such as one of the standard pyridinium oximes (pralidoxime, trimedoxime, obidoxime, HI-6) and diazepam has been used for the treatment of organophosphate poisoning in humans. Despite enormous efforts devoted to synthesis and development of new pyridinium oximes as potential antidotes against poisoning with OPC, only four compounds have found their application in human medicine so far. However, they differ in their activity in poisoning with warfare nerve agents and pesticides and there is still no universal broad-spectrum oxime capable of protecting against all known OPC. In this article, we review data on structure-activity relationship of pyridinium oximes and discuss their pharmacological and toxicological significance. (Class IV)
Jokanovic M, Prostran M. Pyridinium oximes as cholinesterase reactivators. structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds. Current Medicinal Chemistry 2009;16:2177-88. [PubMed Citation]
-
Three oximes currently being evaluated for adoption as replacement nerve agent therapy by various countries were compared for therapeutic efficacy against the toxic organophosphate inhibitors soman and tabun under a standard set of conditions. These oximes together with PAM-Cl and toxogonin, were also compared for efficacy against GF, an agent weaponized by Iraq. The order of effectiveness against soman was HI-6 > HLo 7> pyrimidoxime were of moderate value against GF HI-6 and HLo 7 were extremely effective, toxogonin was moderately effective, and PAM-Cl and pyrimidoxime were the least effective. HI-6 provided a high level of protection against all of the agents tested as did HLo 7 to a slightly lesser degree. The other oximes suffered from their lack of effects against one or more of the organophosphates. (Class IV)
Lundy PM, Hansen AS, Hand BT, Boulet CA. Comparison of several oximes against poisoning by soman, tabun and GF. Toxicology 1992;72:99-105. [PubMed Citation]
-
Organophosphate (OP) poisoning poses great danger to both military and civilian populations. OP induced brain injury is characterized by rapid loss of consciousness, seizures, central respiratory inhibition as well as long-term behavioral changes in sub-lethal injuries. The pharmacological treatment of OP poisoning is based on anticholinergic and anticonvulsant drugs as well as oximes, which reactivate the non-aged inhibited enzyme. The commonly used oximes are quaternary compounds with questionable capacity to penetrate through the blood-brain barrier. This implies that the main beneficial effect of oximes may result from reactivation of AChE activity in respiratory muscles rather than in the brain. Importantly, data accumulated over the last few decades suggests a potential beneficial role for oximes in the brain, despite their polarity. Albeit the concentration of oximes in the central nervous system is significantly lower than in the plasma, they do gain access into the brain and are able to reactivate inhibited local AChE. Oximes may also attenuate OP-induced brain insult via different mechanisms other than AChE reactivation. In this review, we focus on the ability of oximes to act in the brain and protect the central nervous system from OP-induced injury, either by direct reactivation of AChE or by other pharmacological mechanisms. While this is a poorly investigated field we believe that the data supports the potential role of oximes in mitigating OP-induced neuronal injury, thus making them valuable in the treatment of severe casualties (Class IV).
Shrot S , Markel G, Dushnitsky T, Krivoy A. The possible use of oximes as antidotal therapy in organophosphate-induced brain damage. NeuroToxicology 2009 Mar;30(2):167-73. [PubMed Citation]
-
This paper reviews the mechanisms of interaction of organophosphorus compounds with cholinesterases and clinical signs of acute poisoning. Further, the authors describe the current understanding of the mechanisms of action of pyridinium oximes pralidoxime (PAM-2), trimedoxime (TMB-4), obidoxime (LuH-6, Toxogonin), HI-6 and HLo 7 which are used as cholinesterase reactivators in the treatment of poisoning with organophosphorus compounds. The authors also review the most important literature data related to the efficacy of these oximes in the treatment of poisoning with warfare nerve agents soman, sarin, tabun, VX and cyclosarin and organophosphorus insecticides. Finally, the authors discuss the criteria for selection of oximes intended for further development as antidotes in poisoning with organophosphorus compounds and auto-injectors for their application in urgent situations (Class IV).
Jokanovic M, Stojiljkovic MP. Current understanding of te application of pyridinium oximes as cholinesterase reactivators in treatment of organophosphate poisoning. European Journal of Pharmacology 2006;553:10-7. [PubMed Citation]
-
Chemical agents have been used previously in wartime on numerous occasions, from World War I to the Gulf War. In 1994 and 1995, sarin nerve gas was used first in peacetime as a weapon of terrorism in Japan. The Tokyo subway sarin attack was the first large-scale disaster caused by nerve gas. A religious cult released sarin gas into subway commuter trains during morning rush hour. Twelve passengers died and about 5500 people were harmed. Sarin is a highly toxic nerve agent that can be fatal within minutes to hours. It causes the clinical syndrome of cholinergic hyperstimulation by inhibition of the crucial enzyme acetylcholinesterase. Therapy of nerve agent toxicity is divided into three categories, decontamination, respiratory support, and antidotes. All of these therapies may be given simultaneously. This article reviews toxicology and management of this acute chemical emergency. To help minimize the possible catastrophic impact on the public, we make several recommendations based on analysis of the Tokyo subway sarin attack and systematically review the current scientific literature (Class IV).
Tokuda Y, Kikuchib M, Takahashib O, Stein GH. Prehospital management of sarin nerve gas terrorism in urban settings: 10 years of progress after the Tokyo subway sarin attack. Resuscitation 2006;68:193-202. [PubMed Citation]
-
Nerve agents (NAs) are the most lethal chemical weapons. The authors review the pathophysiology and management of NA poisoning of children. NAs cause cholinergic crisis. Children may manifest signs of cholinergic poisoning differently than adults. Children may be less likely to manifest miosis and glandular secretions. They may present with neurologic derangements alone. The goals of treatment should be to limit additional exposure, to provide respiratory support, and to prevent neurologic morbidity. Autoinjectors are optimal delivery vehicles for intramuscular antidotes and are likely to be used in civilian prehospital care. Antidotes include anticholinergics, oximes, and benzodiazepines. Several medications may be available within each class of antidotes. Clinicians will select an antidote based on the status of the individual victim, the accessibility of supportive care, and the availability of the drug. Atropine is well-tolerated and high doses may be required. The oxime pralidoxime chloride has a longer half-life in children. Currently, diazepam is the standard NA anticonvulsant. Midazolam may be the most effective intramuscular anticonvulsant after NA exposure, but, despite its efficacy, it is not an approved agent for seizures. Supportive care and long-term complications are summarized (Class IV).
Rotenberg JS, Newmark J. Nerve agent attacks on children: diagnosis and management. Pediatrics 2003 Sep;112(3 Pt 1):648-58 [PubMed Citation]
-
The cholinesterase-inhibiting organophosphorus compounds referred to as nerve agents (soman, sarin, tabun, GF agent, and VX) are particularly toxic and are considered to be among the most dangerous chemical warfare agents. Included in antidotal medical countermeasures are oximes to reactivate the inhibited cholinesterase. Much experimental work has been done to better understand the properties of the oxime antidotal candidates including the currently available pralidoxime and obidoxime, the H oximes HI-6 and HLo 7, and methoxime. There is no single, broad-spectrum oxime suitable for the antidotal treatment of poisoning with all organophosphorus agents. If more than one oxime is available, the choice depends primarily on the identity of the responsible organophosphorus compound. The H oximes appear to be very promising antidotes against nerve agents because they are able to protect experimental animals from toxic effects and improve survival of animals poisoned with supralethal doses. They appear more effective against nerve agent poisoning than the currently used oximes pralidoxime and obidoxime, especially in the case of soman poisoning. On the other hand, pralidoxime and especially obidoxime seem sufficiently effective to treat poisonings with organophosphorus insecticides that have relatively less toxicity than nerve agents (Class IV).
Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. Journal of Toxicology Clinical Toxicology 2002;40(6):803-16 [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animals
-
This study examined whether pro-2-PAM, a pro-drug dihydropyridine derivative of the oxime 2-pralidoxime (2-PAM) that can penetrate the brain, could prevent or reverse the central toxic effects of three nerve agents; sarin, cyclosarin, and VX. The first experiment tested whether pro-2-PAM could reactivate guinea pig cholinesterase (ChE) in vivo in central and peripheral tissues inhibited by these nerve agents. Pro-2-PAM produced a dose-dependent reactivation of sarin- or VX-inhibited ChE in both peripheral and brain tissues, but with substantially greater reactivation in peripheral tissues compared to brain. Pro-2-PAM produced 9-25% reactivation of cyclosarin-inhibited ChE in blood, heart, and spinal cord, but no reactivation in brain or muscle tissues. In a second experiment, the ability of pro-2-PAM to block or terminate nerve agent-induced electroencephalographic seizure activity was evaluated. Pro-2-PAM was able to block sarin- or VX-induced seizures (16-33%) over a dose range of 24-32 mg/kg, but was ineffective against cyclosarin-induced seizures. Animals that were protected from seizures showed significantly less weight loss and greater behavioral function 24 h after exposure than those animals that were not protected. Additionally, brains were free from neuropathology when pro-2-PAM prevented seizures. In summary, pro-2-PAM provided modest reactivation of sarin- and VX-inhibited ChE in the brain and periphery, which was reflected by a limited ability to block or terminate seizures elicited by these agents. Pro-2-PAM was able to reactivate blood, heart, and spinal cord ChE inhibited by cyclosarin, but was not effective against cyclosarin-induced seizures.
Shih T-M, Guarisco JA, Myers TM, Kan RK, McDonough JH. The oxime pro-2-PAM provides minimal protection against the CNS effects of the nerve agents sarin, cyclosarin, and VX in guinea pigs. Toxicol Mech Methods 2011 Jan; 21(1):53-62. [PubMed Citation].
-
Novel therapeutics to overcome the toxic effects of organophosphorus (OP) chemical agents are needed due to the documented use of OPs in warfare (e.g. 1980-1988 Iran/Iraq war) and terrorism (e.g. 1995 Tokyo subway attacks). Standard OP exposure therapy in the United States consists of atropine sulfate (to block muscarinic receptors), the acetylcholinesterase (AChE) reactivator (oxime) pralidoxime chloride (2-PAM), and a benzodiazepine anticonvulsant to ameliorate seizures. A major disadvantage is that quaternary nitrogen charged oximes, including 2-PAM, do not cross the blood brain barrier (BBB) to treat brain AChE. Therefore, the investigators synthesized and evaluated pro-2-PAM (a lipid permeable 2-PAM derivative) that can enter the brain and reactivate CNS AChE, preventing seizures in guinea pigs after exposure to OPs. The protective effects of the pro-2-PAM after OP exposure were shown using (a) surgically implanted radiotelemetry probes for electroencephalogram (EEG), (b) neurohistopathology of brain, (c) cholinesterase activities in the PNS and CNS, and (d) survivability. The PNS oxime 2-PAM was ineffective at reducing seizures/status epilepticus (SE) in diisopropylfluorophosphate (DFP)-exposed animals. In contrast, pro-2-PAM significantly suppressed and then eliminated seizure activity. In OP-exposed guinea pigs, there was a significant reduction in neurological damage with pro-2-PAM but not 2-PAM. Distinct regional areas of the brains showed significantly higher AChE activity 1.5h after OP exposure in pro-2-PAM treated animals compared to the 2-PAM treated ones. However, blood and diaphragm showed similar AChE activities in animals treated with either oxime, as both 2-PAM and pro-2-PAM are PNS active oximes. In conclusion, pro-2-PAM can cross the BBB, is rapidly metabolized inside the brain to 2-PAM, and protects against OP-induced SE through restoration of brain AChE activity. Pro-2-PAM represents the first non-invasive means of administering a CNS therapeutic for the deleterious effects of OP poisoning by reactivating CNS AChE.
Demar JC, Clarkson ED, Ratcliffe RH, Campbell AJ, Thangavelu SG, Herdman CA, Leader H, Schultz CM, Marek E, Medynetcs MA, Ku TC, Evans SA, Khan FA, Owens RR, Nambiar MP, Gordon RK. Pro-2-PAM therapy for central and peripheral cholinesterases. Chem Biol Interact. 2010 Sep;187(1-3):191-8 [PubMed Citation].
-
Three experiments are reported: 1) a feasibility study on using laboratory primates repeatedly in behavioral toxicity studies of organophosphate (OP) agents or of chemical countermeasures against OPs; 2) a study of the efficacy of pyridostigmine pretreatment and 2-PAM therapy; and 3) a study to determine the effects of these treatments on soman-induced cholinesterase (ChE) inhibition and its recovery. In rhesus monkeys, three repeated acute low-dose (2.1 to 2.8 micrograms/kg) soman exposures, separated by intervals >5 weeks, did not change baseline compensatory tracking performance or the soman ED50. Atropine therapy (97 micrograms/kg) alone had no effect on soman ED50. Addition of pyridostigmine pretreatment (150 micrograms/kg) and 2-PAM therapy (17 mg/kg) to atropine therapy increased the soman ED50 for a performance decrement from 2.27 micrograms/kg to 2.58 micrograms/kg, an insignificant protective effect. At the soman ED50 for behavioral decrements, pyridostigmine pretreatment increased the inhibition of serum ChE observed immediately after soman exposure, but reduced the extent of permanent inhibition. The 2-PAM therapy reduced serum ChE inhibition from about 80% to less than 70%. These effects on the time course of ChE inhibition following soman exposure appear to combine additively. These chemical countermeasures do not prevent soman-induced performance decrements, even though they are effective in protecting lives after much higher doses. The soman doses used produce only small, transient performance decrements; animals so exposed can, thus, be used repeatedly in such studies.
Blick DW, Murphy MR, Brown GC, Hartgraves SL. Primate performance decrements following acute soman exposure: Failure of chemical countermeasures. Pharmacol Biochem Behav 1994;49(3):503-10 [PubMed Citation]
-
This study compared the efficacy of HI-6 and 2-PAM against nerve agent (soman, tabun, sarin, and VX) induced lethality in the atropinesterase-fry rabbits pretreated with vehicle (controls) or pyridostigmine. Treatment was administered at signs or 2 min after agent challenge and consisted of oxime (100 μmol/kg) + atropine (13 mg/kg) (alone or together with diazepam). Twenty-four-h LD50 values were calculated for soman- and tabun-intoxicated animals, whereas 24-h survival was noted in animals given 10 LD50s of sarin or VX. In pyridostigmine and control rabbits intoxicated with soman and treated with oxime + atropine (alone or together with diazepam), HI-6 was 3-5 times more effective than 2-PAM. In contrast, HI-6 was less effective than 2-PAM against tabun poisoning. In pyridostigmine-pretreated animals exposed to tabun, efficacy was increased more than 3-fold when compared to tabun-challenged animals treated with atropine + HI-6 alone. Both oximes were highly effective against sarin and VX. These findings suggest that HI-6 could replace 2-PAM as therapy for nerve agent poisoning, because it is superior to 2-PAM against soman, and when used in pyridostigmine-pretreated animals, it affords excellent protection against all four nerve agents when used in combination with atropine (alone or together with diazepam) therapy.
Koplovitz I, Stewart JR. A comparison of the efficacy of HI6 and 2-PAM against soman, tabun, sarin, and VX in the rabbit. Toxicology Letters 1994 Feb;70(3): 269-79. [PubMed Citation]
-
Non-human primates are valuable animal models that are used for the evaluation of nerve agent toxicity as well as antidotes and results from animal experiments are extrapolated to humans. It has been demonstrated that the efficacy of an oxime primarily depends on its ability to reactivate nerve agent-inhibited acetylcholinesterase (AChE). If the in vitro oxime reactivation of nerve agent-inhibited animal AChE is similar to that of human AChE, it is likely that the results of an in vivo animal study will reliably extrapolate to humans. Therefore, the goal of this study was to compare the aging and reactivation of human and different monkey (Rhesus, Cynomolgus, and African Green) AChEs inhibited by GF, GD, and VR. The oximes examined include the traditional oxime 2-PAM, two H-oximes HI-6 and HLo 7, and the new candidate oxime MMB4. Results indicate that oxime reactivation of all three monkey AChEs was very similar to human AChE. The maximum difference in the second order reactivation rate constant between human and three monkey AChEs or between AChEs from different monkey species was 5-fold. Aging rate constants of GF-, GD-, and VR-inhibited monkey AChEs were very similar to human AChE except for GF-inhibited monkey AChEs, which aged 2-3 times faster than the human enzyme. The results of this study suggest that all three monkey species are suitable animal models for nerve agent antidote evaluation since monkey AChEs possess similar biochemical/pharmacological properties to human AChE.
Luo C, Tong M, Maxwell DM, Saxena A. Comparison of oxime reactivation and aging of nerve agent-inhibited monkey and human acetylcholinesterases. Chemico-Biological Interactions 2008 Sep;175(1-3):261-6. [PubMed Citation]
-
This study compared the ability of nine oximes (HI-6, HLo 7, MMB-4, TMB-4, carboxime, ICD585, ICD692, ICD3805, and 2-PAM) to reactivate in vivo cholinesterase (ChE) in blood, brain, and peripheral tissues in guinea pigs intoxicated by one of four organophosphorus nerve agents. Two bis-pyridinium compounds without an oxime group, SAD128 and ICD4157, served as non-oxime controls. Animals were injected subcutaneously with 1.0×LD50 of the nerve agents sarin, cyclosarin, VR or VX and treated intramuscularly 5 min later with one of these oximes. Toxic signs and lethality were monitored; tissue ChE activities were determined at 60 min after nerve agent. Some animals exposed to sarin or cyclosarin, with or without nonoxime treatment, died within 60 min; however, no animal treated with an oxime died. For VR or VX, all animals survived the 60 min after exposure, with or without non-oxime or oxime therapy. The four nerve agents caused differential degrees of inhibition in blood, brain regions and peripheral tissues. The tested oximes exhibited differential potency in reactivating nerve agent-inhibited ChE in various peripheral tissues, but did not affect ChE activity in the brain regions. There was no direct relation between blood and peripheral tissues in the reactivating efficacy of oxime treatments. ChE inhibited by sarin was the most susceptible to oxime reactivation while cyclosarin the least susceptible. There was no difference in the ChE reactivating potency between the dimethanesulfonate and dichloride salts of HI-6. MMB-4 significantly reactivated the ChE inhibited by these four nerve agents in blood and all three peripheral tissues of the guinea pig, and among all the oximes tested it was the most effective in vivo ChE reactivator against all four nerve agents.
Shih T-M, Skovira JW, O'Donnell JC, McDonough. JH. In vivo reactivation by oximes of inhibited blood, brain and peripheral tissue cholinesterase activity following exposure to nerve agents in guinea pigs. Chemico-Biological Interactions 2010 Sep;187(1-3):207-14. [PubMed Citation]
Other non-clinical studies
Human non-clinical studies-
According to current knowledge, inhibition of acetylcholinesterase (AChE) is a very important toxic action of organophosphorus compounds (OP). Hence, it is obvious to follow the AChE activity in order to quantify the degree of inhibition and to assess possible reactivation. Red blood cell (RBC)-AChE provides an easily accessible source to follow the AChE status also in humans. There are many reports underlining the appropriateness of RBC-AChE as a surrogate parameter that mirrors the synaptic enzyme. With this tool at hand, we can study the kinetic parameters of inhibition, spontaneous and oxime-induced reactivation, as well as aging with human RBCs under physiological conditions in vitro. Moreover, we can simulate the influence of inhibitor and reactivator on enzyme activity and can calculate what happens when both components change with time. Finally, we can correlate under controlled conditions the AChE-status in intoxicated patients with the clinical signs and symptoms and determine the time-dependent changes of the oxime and OP concentration. Data of a clinical trial performed in Munich to analyze the value of obidoxime has elucidated that obidoxime worked as expected from in vitro studies. Following a 250 mg bolus, obidoxime was administered by continuous infusion at 750 mg/24 h aimed at maintaining a plasma concentration of 10-20 microM obidoxime. This oxime concentration reactivated RBC-AChE > 20% of normal in most cases of OP poisoning by diethylphosphoryl compounds within a few hours. The degree of reactivation fitted theoretical calculations very well when the obidoxime and paraoxon concentrations were fed into the model. Only in a few cases reactivation was much lower than expected. The reason for this effect is probably based on the polymorphism of paraoxonase (PON1) in that the 192arginine phenotype does hardly hydrolyze the arising diethylphosphoryl obidoxime. While this variable may complicate a proper assessment even more, the authors are confident that the in vitro evaluation of all relevant kinetic data enables the prediction of probable effectiveness in humans. These studies also help to understand therapeutic failures and to define scenarios where oximes are virtually ineffective. These include poisonings with rapidly aging phosphylated AChE, late start with an effective oxime and too early discontinuation of oximes in poisonings with a persistent OP. The experience gathered with the experimental and therapeutic approaches to human poisoning by OP pesticides may be helpful when oximes have to be selected against nerve agents. (Class IV)
Eyer P, Szinicz L, Thiermann H, Worek F, Zilker T. Testing of antidotes for organophosphorus compounds: Experimental procedures and clinical reality. Toxicology 2007 Apr;233(1-3):108-19. [PubMed Citation]
-
Organophosphorus pesticides (e.g. chlorpyrifos, malathion, and parathion) and nerve agents (sarin, tabun, and VX) are highly toxic organophosphorus compounds with strong inhibition potency against two key enzymes in the human body-acetylcholinesterase (AChE; EC 3.1.1.7) and butyrylcholinesterase (BuChE; EC 3.1.1.8). Subsequent accumulation of acetylcholine at synaptic clefts can result in cholinergic crisis and possible death of intoxicated organism. For the recovery of inhibited AChE, derivatives from the group of pyridinium or bispyridinium aldoximes (called oximes) are used. Their efficacy depends on their chemical structure and also type of organophosphorus inhibitor. In this study,we have tested potency of selected cholinesterase reactivators (pralidoxime, obidoxime, trimedoxime, methoxime and H-oxime HI-6) to reactivate human erythrocyte AChE and human plasma BuChE inhibited by pesticide paraoxon. For this purpose, modified Ellman's method was used and two different concentrations of oximes (10 and 100 microM), attainable in the plasma within antidotal treatment of pesticide intoxication were tested. Results demonstrated that obidoxime (96.8%) and trimedoxime (86%) only reached sufficient reactivation efficacy in case of paraoxon-inhibited AChE. Other oximes evaluated did not surpassed more than 25% of reactivation. In the case of BuChE reactivation, none of tested oximes surpassed 12.5% of reactivation. The highest reactivation efficacy was achieved for trimedoxime (12.4%) at the concentration 100 microM. From the data obtained, it is clear that only two from currently available oximes (obidoxime and trimedoxime) are good reactivators of paraoxon-inhibited AChE. In the case of BuChE, none of these reactivators could be used for its reactivation.
Juna D, Musilova L, Kuca K, Kassa J, Bajgar J. Potency of several oximes to reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon in vitro. Chemico-Biological Interactions 2008 Sep;175(1-3):421-4. [PubMed Citation]
-
Acetylcholinesterase (AChE) reactivators are employed for the prophylaxis and treatment of intoxications with organophosphorus AChE inhibitors, including nerve agents and pesticides. For the recovery of inhibited enzyme, derivatives from the group of pyridinium or bispyridinium aldoximes (called oximes) are used. Adverse effects of these substances are not well elucidated, because of their narrow and one-shot usage. Owing to this fact, the study evaluated the influence of some currently applied oximes on human platelet aggregation in vitro. The antiplatelet activity of pralidoxime, obidoxime, HI-6, methoxime and HLo 7 was assayed in human platelet rich plasma (2.5 × 10+8 platelets/ml) at a concentration of 1.35 mM. Arachidonic acid (AA), adenosine diphosphate (ADP), collagen (COL) and thrombin (TR) were used as agonists of platelet aggregation. All tested substances, except pralidoxime and methoxime, caused a significant inhibition of the aggregation process induced by AA, ADP and COL. Of the oximes assayed, none was found to influence TR triggered aggregation. Since reduced platelet aggregation can play an important role as an adverse effect in reactivator administration, further evaluation is needed for the estimation of the real impact of active oximes to the aggregation process in humans.
Jun D, Kuca K, Hronek M, Opletal L. Effect of some acetylcholinesterase reactivators on human platelet aggregation in vitro. J. Appl. Toxicol. 2006 May-June;26(3):258-61 [PubMed Citation]
-
The investigators in vitro tested the ability of common, commercially available, cholinesterase reactivators (pralidoxime, obidoxime, methoxime, trimedoxime and HI-6) to reactivate human acetylcholinesterase (AChE), inhibited by five structurally different organophosphate pesticides and inhibitors (paraoxon, dichlorvos, DFP, leptophos-oxon and methamidophos). They also tested reactivation of human butyrylcholinesterase (BChE) with the aim of finding a potent oxime, suitable to serve as a —pseudocatalytic bioscavenger in combination with this enzyme. Such a combination could allow an increase of prophylactic and therapeutic efficacy of the administered enzyme. According to our results, the best broad-spectrum AChE reactivators were trimedoxime and obidoxime in the case of paraoxon, leptophos-oxon, and methamidophos-inhibited AChE. Methamidophos and leptophos-oxon were quite easily reactivatable by all tested reactivators. In the case of methamidophos-inhibited AChE, the lower oxime concentration (10—5 M) had higher reactivation ability than the 10—4 M concentration. Therefore, we evaluated the reactivation ability of obidoxime in a concentration range of 10—3 to 10—7 M. The reactivation of methamidophos-inhibited AChE with different obidoxime concentrations resulted in a bell shaped curve with maximum reactivation at 10—5 M. In the case of BChE, no reactivator exceeded 15% reactivation ability and therefore none of the oximes can be recommended as a candidate for —pseudocatalytic bioscavengers with BChE.
Jun D, Musilova L, Musilek K, Kuca K. In vitro ability of currently available oximes to reactivate organophosphate pesticide-inhibited human acetylcholinesterase and butyrylcholinesterase. Int. J. Mol. Sci. 2011;12(3): 2077-87 [PubMed Citation]
Comparative human and animal in vitro studies-
Previous in vitro studies showed marked species differences in the reactivating efficiency of oximes between human and animal acetylcholinesterase (AChE) inhibited by organophosphorus (OP) nerve agents. These findings provoked the present in vitro study which was designed to determine the inhibition, aging, spontaneous and oxime-induced reactivation kinetics of the pesticide paraoxon, serving as a model compound for diethyl-OP, and the oximes obidoxime, pralidoxime, HI-6 and MMB-4 with human, Rhesus monkey, swine, rabbit, rat and guinea pig erythrocyte AChE. Comparable results were obtained with human and monkey AChE. Differences between human, swine, rabbit, rat and guinea pig AChE were determined for the inhibition and reactivation kinetics. A six-fold difference of the inhibitory potency of paraoxon with human and guinea pig AChE was recorded while only moderate differences of the reactivation constants between human and animal AChE were determined. Obidoxime was by far the most effective reactivator with all tested species. Only minor species differences were found for the aging and spontaneous reactivation kinetics. The results of this study underline the necessity to determine the inhibition, aging and reactivation kinetics in vitro as a basis for the development of meaningful therapeutic animal models, for the proper assessment of in vivo animal data and for the extrapolation of animal data to humans.
Worek F, Aurbek N, Wille T, Eyer P, Thiermann H. Kinetic analysis of interactions of paraoxon and oximes with human, Rhesus monkey, swine, rabbit, rat and guinea pig acetylcholinesterase. Toxicology Letters 2011 Jan;200(1-2):19-23. [PubMed Citation]
-
The reactivation of nerve agent-inhibited acetylcholinesterase (AChE) by oxime is the most important step in the treatment of nerve agent poisoning. Since the evaluation of nerve agent antidotes cannot be conducted in humans, results from animal experiments are extrapolated to humans. Guinea pig is one of the animal models that is frequently used for conducting nerve agent antidote evaluations. Several investigations have demonstrated that the efficacy of an oxime primarily depends on its ability to reactivate nerve agent-inhibited AChE. If the in vitro oxime reactivation of nerve agent-inhibited animal AChE is similar to that of human AChE, it is likely that the results of an in vivo animal study will reliably extrapolate to humans. Therefore, the goal of this study was to compare the reactivation of guinea pig and human AChEs inhibited by six different G and V type nerve agents. Reactivation kinetic studies with five mono and bis-pyridinium oximes showed that oxime reactivation of nerve agent-inhibited human AChE in most cases was faster than guinea pig AChE. The most significant enhancement was observed in the reactivation of human AChE inhibited by nerve agents containing bulky side chains GF, GD, and VR, by H-series oximes HLo-7, HI-6, and ICD-585. In these cases, species-related differences observed between the two AChEs, based on the second-order reactivation rate constants, were 90- to over 400-fold. On the other hand, less than 3-fold differences were observed in the rates of aging of nerve agent-inhibited guinea pig and human AChEs. These results suggest that the remarkable species-related differences observed in the reactivation of nerve agent-inhibited guinea pig and human AChEs were not due to differences in the rates of aging. These results also suggest that guinea pig may not be an appropriate animal model for the in vivo evaluation of oxime therapy.
Luo C, Tong M, Chilukuri N, Brecht K, Maxwell DM, Saxena A. An in vitro comparative study on the reactivation of nerve agent-inhibited guinea pig and human acetylcholinesterases by oximes. Biochemistry 2007 Oct;46(42):11771-9. [PubMed Citation]
-
Organophosphorus compounds such as nerve agents inhibit, practically irreversibly, cholinesterases by their phosphorylation in the active site of these enzymes. Current antidotal treatment used in the case of acute nerve agent intoxications consists of combined administration of anticholinergic drug (usually atropine) and acetylcholinesterase (AChE, EC 3.1.1.7) reactivator (HI-6, obidoxime, pralidoxime), which from a chemical view is a derivative from the group of pyridinium or bispyridinium aldoximes (commonly called oxime). Oximes counteract acetylcholine increase, resulting from AChE inhibition. In the human body environment these compounds are powerful nucleophiles and are able to break down the bond between AChE and nerve agent molecule. This process leads to renewal of enzyme functionality to its reactivation. The usefulness of oxime in the reactivation process depends on its chemical structure and on the nerve agent whereby AChE is inhibited. Due to this fact, selection of suitable reactivator in the treatment of intoxications is very important. In this work, the investigators compared differences in the in vitro inhibition potency of VX and Russian VX on rat, pig and human brain, and subsequently tested reactivation of rat brain cholinesterase inhibited by these agents using oxime HI-6, obidoxime, pralidoxime, trimedoxime and methoxime. The results showed that no major differences in the reactivation process of both VX and Russian VX-inhibited cholinesterase. The similarity in reactivation was caused by analogous chemical structure of either nerve agent; and that oxime HI-6 seems to be the most effective reactivator tested, which confirms that HI-6 is currently the most potent reactivator of AChE inhibited by nerve agents.
Kuca K, Jun D, Cabal J, Hrabinova M, Bartosova L, Opletalova V. Russian VX: inhibition and reactivation of acetylcholinesterase compared with VX agent. Basic & Clinical Pharmacology & Toxicology 2006 Apr;98(4):389-94. [PubMed Citation]
Animal in vitro studies-
Administration of acetylcholinesterase (AChE) reactivators (oximes) is usually used in order to counteract the poisoning effects of nerve agents. The possibility was suggested that oximes may show some therapeutic and/or adverse effects through their action in central nervous system. There are no sufficient data about interaction of oximes with monoaminergic neurotransmitter's systems in the brain. Oxime-type AChE reactivators pralidoxime, obidoxime, trimedoxime, methoxime and HI-6 were tested for their potential to affect the activity of monoamine oxidase of type A (MAO-A) and type B (MAO-B) in crude mitochondrial fraction of pig brains. The compounds were found to inhibit fully MAO-A with half maximal inhibitory concentration (IC50) of 0.375 mmol/L (pralidoxime), 1.53 mmol/L (HI-6), 2.31 mmol/L (methoxime), 2.42 mmol/L (obidoxime) and 4.98 mmol/L (trimedoxime). Activity of MAO-B was fully inhibited by HI-6 and pralidoxime only with IC50 4.81 mmol/L and 11.01 mmol/L, respectively. Methoxime, obidoxime and trimedoxime displayed non-monotonic concentration dependent effect on MAO-B activity. Because oximes concentrations effective for MAO inhibition could not be achieved in vivo at the cerebral level, we suppose that oximes investigated do not interfere with brain MAO at therapeutically relevant concentrations.
Fisar Z, Hroudova J, Korabecny J, Musilek K, Kuca K. In vitro effects of acetylcholinesterase reactivators on monoamine oxidase activity. Toxicology Letters 2011 Mar;201(2):176-80. [PubMed Citation]
-
A comparison of one mono- and seven bisquaternary acetylcholinesterase (AChE) reactivators of acetylcholinesterase inhibited by VX agent was performed. As a source of the acetylcholinesterase, a rat brain homogenate was taken. There were significant differences in reactivation potency of all tested oximes. The oxime TO205 seems to be the most efficacious followed by TO046, HI-6, HS-6, K027, obidoxime, MMC and 2-PAM. In addition, the results of this study showed that the reactivation potency of the tested reactivators depends on many factors such as the number of pyridinium rings, the number of oxime groups and their position, as well as the length and the shape of linkage bridge between two pyridinium rings.
Kuca K, Kassa J. Oximes-induced reactivation of rat brain acetylcholinesterase inhibited by VX agent. Human & Experimental Toxicology 2004;23:167-71. [PubMed Citation]
-
Six acetylcholinesterase (AChE) reactivators (pralidoxime, obidoxime, HI-6, trimedoxime, BI-6 and HLo 7) were tested for reactivation of sarin- and cyclosarin-inhibited AChE using an in vitro reactivation test. Rat brain homogenate was used as the suitable source of enzyme. All oximes are able to reactivate sarin-inhibited AChE. On the other hand, only HI-6 is able to reactivate satisfactorily cyclosarin-inhibited AChE.
Kuca K, Cabal J, Jun D, Kassa J, Bartosova L, Kunesova G. In vitro reactivation potency of some acetylcholinesterase reactivators against sarin- and cyclosarin-induced inhibitions. J. Appl. Toxicol. 2005 Jul-Aug;25(4):296-300. [PubMed Citation]
-
In vitro comparison of reactivation efficacy of five currently used oximes - pralidoxime, obidoxime, trimedoxime, methoxime, and HI-6 (at two concentrations: 10—5 and 10—3 M)- against acetylcholinesterase (AChE; E.C. 3.1.1.7) inhibited by six different nerve agents (VX, Russian VX (R-VX), sarin, cyclosarin, tabun, soman) and organophosphorus insecticide chlorpyrifos was the aim of this study. As a source of AChE in the experiments, rat brain homogenate was used. According to the results obtained, no AChE reactivator was able to reach sufficient potency for AChE inhibited by all nerve agents used. Moreover, oxime HI-6 (the most effective one) was not able to reactivate tabun- and soman-inhibited AChE. Due to this fact, it could be designated as a partially broadspectrum reactivator.
Kuca K, Jun D, Bajgar J. Currently used cholinesterase reactivators against nerve agent intoxication: comparison of their effectivity in vitro. Drug and Chemical Toxicology 2007;30(1):31-40. [PubMed Citation]
-
The efficacy of H oximes (HI-6, HLo 7), the oxime BI-6, and currently used oximes (pralidoxime, obidoxime, trimedoxime) to reactivate acetylcholinesterase inhibited by two nerve agents (tabun, VX agent) was tested in vitro. Both H oximes (HI-6, HLo-7) and the oxime BI-6 were found to be more efficacious reactivators of VX-inhibited acetylcholinesterase than pralidoxime and obidoxime. On the other hand, their potency to reactivate tabun-inhibited acetylcholinesterase was low and did not reach the reactivating efficacy of trimedoxime and obidoxime. Thus, none of these compounds can be considered to be a broad-spectrum reactivator of nerve agent-inhibited acetylcholinesterase in spite of high potency to reactivate acetylcholinesterase inhibited by some nerve agents. More than one oxime may be necessary for the antidotal treatment of nerve agent-exposed individuals.
Kuca K, Cabal J, Kassa J, Jun D, Hrabinova M. In vitro potency of H oximes (HI-6, HLo-7), the oxime BI-6, and currently used oximes (pralidoxime, obidoxime, trimedoxime) to reactivate nerve agent-inhibited rat brain acetylcholinesterase. Journal of Toxicology and Environmental Health, Part A 2006 Aug;69(15):1431-40. [PubMed Citation]
-
The in vivo rat brain microdialysis technique with HPLC/UV was used to determine the blood-brain barrier (BBB) penetration of pralidoxime iodide (2-PAM), which is a component of the current nerve agent antidote therapy. After intravenous dosage of 2-PAM (10, 50, 100 mg/kg), 2-PAM appeared dose-dependently in the dialysate; the striatal extracellular/blood concentration ratio at 1 h after 50 mg/kg dosage was 0.093 +/- 0.053 (mean +/- SEM). This finding offered conclusive evidence of the BBB penetration of 2-PAM. Whether the BBB penetration of 2-PAM was mediated by a certain specific transporter, such as a neutral or basic amino acid transport system was also examined. Although it was unclear, the neural uptake of 2-PAM was Na+ dependent. The mean BBB penetration by 2-PAM was approximately 10%, indicating the intravenous administration of 2-PAM might be to a degree effective to reactivation of the blocked cholinesterase in the brain.
Sakurada K, Matsubara K, Shimizu K, Shiono H, Seto Y, Tsuge K, Yoshino M, Sakai I, Mukoyama H, Takatori T. Pralidoxime iodide (2-PAM) penetrates across the blood-brain barrier. Neurochemical Research 2003 Sep;28(9):1401-7. [PubMed Citation]
-
Phrenic nerve diaphragm muscles of young adult rats were used to study the ability of the oximes 2-PAM and HI-6 to recover muscle function depressed by organophosphate (OP) agents. The single twitch of diaphragm muscles which were exposed to soman (0.2 PM) recovered after washing with saline for 3 hr, but the muscles pretreated with sarin (0.4 PM), VX (0.2 PM), or tabun (0.4 PM) showed only partial recovery. In addition, after 3 hr washing, the muscles pretreated with soman as well as with tabun did not recover the tetanus sustaining ability (TSA), yet complete recovery was observed with muscles pretreated with sarin and VX. These results indicate that the OPs have different effects on muscle contractile properties and that VX- and sarin-pretreated muscles recover equally well after wash with physiological solution. The recovery of twitch tension of diaphragm muscles by 2-PAM and HI-6 was similar to that achieved by washing with saline for 3 hr for sarin- and soman-exposed muscles. The most remarkable differences were seen in the recovery of TSA. Both 2-PAM and HI-6 recovered the TSA of muscles that were pretreated with sarin and VX. Although 2-PAM recovered the TSA after tabun pretreatment, HI-6 had no discernible effect. On the other hand, HI-6 recovered the TSA of soman-pretreated muscles but 2-PAM did not. The effectiveness of muscle function recovery was not related to the oximes' ability to reactivate AChE, thus indicating that the recovery of muscle contractility may be attributed to a direct effect of these compounds on the muscle.
Reddy VK, Deshpande SS, Cintra WM, Scoble GT, Albuquerque EX. Effectiveness of oximes 2-PAM and HI-6 in recovery of muscle function depressed by organophosphate agents in the rat hemidiaphragm: an in vitro study. Fundamental and Applied Toxicology 1991 Nov;17(4):746-60. [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Pralidoxime is distributed throughout the extracellular water, it is not bound to plasma protein; its apparent volume of distribution at steady state has been reported to range from 0.60 to 2.7 L/kg. The drug is rapidly excreted in the urine partly unchanged, and partly as a metabolite produced by the liver. Consequently, pralidoxime is relatively short acting and repeated doses may be needed, especially where there is any evidence of continuing absorption of the poison.
-
Pralidoxime chloride is relatively short acting and repeated doses may be needed, unless continuous intravenous infusion is selected. Simulations suggest that after a dose of 1000 mg given intravenously, concentrations fall below 4 μg/mL in about 1.5 hours. The short duration of action of pralidoxime chloride and the necessity for repeated doses should be considered especially where there is any evidence of continuing absorption of the poison. The apparent half-life of pralidoxime is 74 to 77 minutes. The drug is rapidly excreted in the urine by renal tubular secretion, partly unchanged, and partly as a metabolite produced by the liver. After intramuscular administration of 1000 mg of pralidoxime chloride, the renal clearance has been reported to be 7.2 +/- 2.9 mL/min/kg in healthy volunteers and 3.6 +/-1.5 mL/min/kg in organophosphate-poisoned patients.
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
-
In one study of healthy adult volunteers and patients self-poisoned with organophosphate compounds, a single intramuscular injection of 1000 mg of pralidoxime chloride resulted in mean peak plasma levels of 7.5 ± 1.7 μg/mL and 9.9 ± 2.4 μg/mL, respectively. Time to reach the mean peak plasma levels in both groups was similar, 34 minutes in healthy adults and 33 minutes in poisoned patients. Mean half-life was about 3 hours in both groups.
-
Some evidence suggests that a loading dose followed by continuous intravenous infusion of pralidoxime chloride may maintain therapeutic levels longer than short intermittent infusion therapy. In a cross-over study of seven healthy adults (18 - 50 years) a short intravenous infusion dose of 16 mg/kg over 30 minutes was compared to an intravenous loading dose of 4 mg/kg over 15 minutes, followed by 3.2 mg/kg/hr for 3.75 hours (for a total dose of 16 mg/kg). Results showed that the mean time over which plasma levels were maintained above 4 μg/mL was prolonged in the volunteers who received a loading dose followed by continuous infusion as compared to those who received short infusion therapy (257.5 ± 50.5 min vs. 118.0 ± 52.1 min). Use of continuous intravenous infusion in adult patients with organophosphate poisoning has been described in several case reports, with and without loading doses. Infusion rates ranged from 400 - 600 mg/hr. In one case the blood levels were 11.6 - 13.7 μg/mL when given 400 mg/hr over 5 days (measured at 5, 10 and 18 hours). In another case following an initial loading dose of 1000 mg, blood levels were 11.79 μg/mL when given 500 mg/hr and 17.26 μg/mL when given 600 mg/hr. In the latter case the pralidoxime elimination half-life was 4 hours. In two other cases blood levels were not measured.
Product label: PROTOPAM CHLORIDE (pralidoxime chloride) injection, powder, lyophilized, for solution [Baxter Healthcare Corporation] Last Revised: December 2010 [DailyMed]
Children
-
There are no adequate and well-controlled clinical trials that establish the effectiveness of pralidoxime chloride in pediatric patients. Efficacy has been extrapolated from the adult population and is supported by nonclinical studies, pharmacokinetic studies in adults and experience in the pediatric population.
-
In one study of 11 organophosphate-poisoned pediatric patients (age, 0.8 to 18 years), an intravenous loading dose of 15-50 mg/kg (mean 29 mg/kg) of pralidoxime chloride followed by a continuous infusion of 10-16 mg/kg/hr (mean 14 mg/kg/hr) over 12 to 43 hours (mean 27 ± 8 hours) resulted in an average steady state plasma concentration of 22.2 mg/L (6.9 to 47.4 mg/L) and an average body clearance of 0.88 L/kg/hr (0.28 to 2.20 L/kg/hr). After the continuous infusion was discontinued, determinations of the apparent volume of distribution and half-life ranged from 1.7 to 13.8 L/kg and from 2.4 to 5.3 hours, respectively
Product label: PROTOPAM CHLORIDE (pralidoxime chloride) injection, powder, lyophilized, for solution [Baxter Healthcare Corporation] Last Revised: December 2010 [DailyMed]
Geriatric
-
Clinical studies of PROTOPAM did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Product label: PROTOPAM CHLORIDE (pralidoxime chloride) injection, powder, lyophilized, for solution [Baxter Healthcare Corporation] Last Revised: December 2010 [DailyMed]
Animal
-
The pharmacokinetics of 2-PAM, a component of the current nerve agent antidote therapy for U.S. military forces was compared to the pharmacokinetics of another acetylcholinesterase reactivator HI-6. Additionally, the effects of these compounds on muscle tissue following intramuscular injection were examined. Plasma concentrations of the oximes were determined by HPLC. Plasma concentration-time profiles for both oximes fit a one-compartment open model with first-order absorption and elimination. The results demonstrate that the half-time of absorption of the two oximes are nearly the same while the maximum plasma concentration of HI-6 was significantly higher than that for 2-PAM. Musculoirritancy was assessed on the basis of quantitative histological examinations of the injection sites and by the measurement of serum creatinine phosphokinase. Comparison of the scores from the histological sections demonstrates no difference between the two oximes. Serum creatinine phosphokinase values were elevated following injections of HI-6, but were not consistently elevated following the 2-PAM injections.
Moore DH, Hayward IJ, Tucker FS, Lukey B. HI-6 and 2-PAM in sheep: pharmacokinetics and effects on muscle tissue following intramuscular injection. Biopharmaceutics & Drug Disposition 1991 Apr;12(3): 223-32. [PubMed Citation]
-
Animal studies suggest that the minimum therapeutic concentration of pralidoxime in plasma is 4 ug/mL; this level is reached in about 16 minutes after a single injection of 600 mg pralidoxime chloride.
Product label: PROTOPAM CHLORIDE (pralidoxime chloride) injection, powder, lyophilized, for solution [Baxter Healthcare Corporation] Last Revised: December 2010 [DailyMed]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
Organophosphate Pesticide Poisoning:
For the treatment of toxic exposure to organophosphate cholinesterase inhibitors, pralidoxime therapy should be initiated at the same time as atropine. The usual initial IV dose of pralidoxime chloride is 1-2 g given over 15 to 30 minutes for adults. The dose of pralidoxime chloride may be repeated in about 1 hour if muscle weakness has not been relieved. Additional doses may be administered cautiously if muscle weakness continues. Alternatively, some clinicians recommend continuous IV infusion of 500 mg of the drug per hour. In severe cases, especially after ingestion of the poison, the manufacturer recommends electrocardiographic monitoring because the anticholinesterase may cause heart block. Continued absorption of the anticholinesterase from the lower bowel constitutes new exposure; in such cases, additional doses of pralidoxime may be needed every 3-8 hours. As in all cases of organophosphate poisoning, the patient should be observed closely for at least 24 hours.
To facilitate out-of-hospital administration, pralidoxime is available in a prefilled auto-injector containing atropine 2.1 mg and pralidoxime chloride 600 mg (e.g., DuoDote); the auto-injector should be used by emergency medical service personnel. For administration in an out-of-hospital setting, the dose of pralidoxime and atropine (DuoDote) is based on severity of symptoms. For the treatment of adults with 2 or more mild symptoms of pesticide exposure (e.g., miosis or blurred vision, tearing, runny nose, hypersalivation or drooling, wheezing, muscle fasciculations, nausea/vomiting), administer contents of one auto-injector (atropine 2.1 mg and pralidoxime chloride 600 mg) by IM injection. If the patient develops any severe symptoms (behavioral changes, severe breathing difficulty, severe respiratory secretions, severe muscle twitching, involuntary defecation or urination, seizures, unconsciousness), administer contents of two additional auto-injectors IM in rapid succession. For the treatment of adults who present with any severe symptoms, administer contents of three auto-injectors (total dose: atropine 6.3 mg and pralidoxime chloride 1800 mg) IM in rapid succession. Additional doses should not be administered unless definitive medical care is available.
Chemical Warfare Agents:
The dose and route of administration of pralidoxime chloride for the treatment of nerve agent (e.g., sarin, soman, tabun, VX [methylphosphonothiotic acid]) poisoning in the context of chemical warfare or terrorism is based on the severity of symptoms (i.e., mild/moderate or severe), the victim's age, and the treatment setting. Mild to moderate symptoms include localized sweating, muscle fasciculations, nausea, vomiting, weakness, and/or dyspnea; severe symptoms include apnea, flaccid paralysis, seizures, and/or unconsciousness. Pralidoxime chloride must be administered within minutes to hours following exposure to nerve agents to be effective. Pralidoxime is administered concomitantly with atropine.
For the immediate treatment of nerve agent poisoning in an out-of-hospital setting, pralidoxime chloride usually is administered IM. The usual out-of-hospital IM adult dose is 600 mg for those with mild to moderate symptoms and 1800 mg for those with severe symptoms;.
To facilitate out-of-hospital administration, pralidoxime chloride injection is available in a prefilled auto-injector; the auto-injector should be used by individuals who have received adequate training in the recognition and treatment of nerve agent poisoning. For the initial treatment of adults with symptoms of nerve agent poisoning, one 600-mg IM dose of pralidoxime chloride should be administered; pralidoxime is administered after atropine. If symptoms are still present after 15 minutes, another dose of atropine and another 600-mg dose of pralidoxime chloride should be administered. If symptoms persist after an additional 15 minutes, another dose of atropine and another 600-mg dose of pralidoxime chloride should be administered. If symptoms persist after the third doses, medical care should be sought.
Another option for out-of-hospital administration is to administer atropine and pralidoxime using a prefilled auto-injector containing atropine 2.1 mg and pralidoxime chloride 600 mg (e.g., DuoDote, ATNAA). For the treatment of adults with 2 or more mild symptoms of nerve agent poisoning (e.g., miosis or blurred vision, tearing, runny nose, hypersalivation or drooling, wheezing, muscle fasciculations, nausea/vomiting), the contents of one auto-injector (atropine 2.1 mg and pralidoxime chloride 600 mg) should be administered by IM injection. If the patient develops any severe symptoms (behavioral changes, severe breathing difficulty, severe respiratory secretions, severe muscle twitching, involuntary defecation or urination, seizures, unconsciousness), the contents of 2 additional auto-injectors should be administered by IM injection in rapid succession. For the treatment of adults who present with any severe symptoms, the contents of 3 auto-injectors (total dose: atropine 6.3 mg and pralidoxime chloride 1800 mg) should be administered by IM injection in rapid succession. Additional doses should not be administered unless definitive medical care is available.
In an emergency department or similar setting, pralidoxime chloride generally is administered by slow IV injection. When pralidoxime chloride is administered IV in such a setting for the treatment of nerve agent poisoning, the usual adult dose is 15 mg/kg (maximum 1 g) for those with mild to moderate or severe symptoms.
Diazepam may be administered for seizure control.
Other Uses:
As an antagonist to carbamate anticholinesterase agents used in the treatment of myasthenia gravis (e.g., ambenonium, neostigmine, pyridostigmine), 1-2 g of pralidoxime chloride has been given IV initially, followed by 250 mg every 5 minutes.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
Children (FDA)
Safety and effectiveness in children have not been established.
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
Treatment of toxic exposure to organophosphate cholinesterase inhibitors: pralidoxime therapy should be initiated at the same time as atropine. The usual initial IV dose of pralidoxime chloride is 20-40 mg/kg given over 30 minutes for children.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
In an out-of-hospital setting: the usual IM dose of pralidoxime chloride for children 0-10 years of age and adolescents older than 10 years of age with mild to moderate symptoms is 15 mg/kg, and the usual dose for children 0-10 years of age and adolescents older than 10 years of age with severe symptoms is 25 mg/kg.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
In an emergency department setting: the usual IV dose of pralidoxime chloride for children 0-10 years of age and adolescents older than 10 years of age with mild to moderate or severe symptoms is 15 mg/kg. Atropine is administered concomitantly with pralidoxime.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
Pregnancy (FDA)
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
Nursing Mothers (FDA)
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when pralidoxime is administered to a nursing woman.
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
Geriatric (FDA)
In an emergency department or similar setting: pralidoxime chloride generally is administered by slow IV injection. When pralidoxime chloride is administered IV in such a setting for the treatment of nerve agent poisoning, the usual adult dose is 15 mg/kg (maximum 1 g) for those with mild to moderate or severe symptoms; frail geriatric adults with mild to moderate or severe symptoms may receive 5-10 mg/kg.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
Immediate treatment of nerve agent poisoning in an out-of-hospital setting: pralidoxime chloride usually is administered IM. The usual out-of-hospital IM adult dose is 600 mg for those with mild to moderate symptoms and 1800 mg for those with severe symptoms; frail geriatric adults with mild to moderate symptoms may receive 10 mg/kg and those with severe symptoms may receive 25 mg/kg.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
Renal Impairment (FDA)
Treatment of toxic exposure to organophosphate cholinesterase inhibitors: pralidoxime therapy should be initiated at the same time as atropine. The usual initial IV dose of pralidoxime chloride is 1-2 g given over 15 to 30 minutes for adults. Dosage of pralidoxime chloride should be reduced in patients with renal insufficiency.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Pralidoxime has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Formulation
Pralidoxime Chloride
Injection 1 g Protopam Chloride; Injection 600 mg* Generic Name: Pralidoxime Chloride Injection Auto-Injector
Pralidoxime Chloride and Atropine
600 mg/2 mL Pralidoxime Chloride and 2.1 mg/0.7 mL Atropine; Auto-Injector (each drug in a separate chamber)
* available from one or more manufacturer, distributor, and/or repackager by generic (nonproprietary) name
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
Shelf life
Stability
-
Study objective: Pralidoxime is indicated to treat patients poisoned with nerve agents. It is available in intravenous formulation for more seriously ill hospitalized patients and intramuscular formulation for field treatment and less seriously ill patients. This study describes a method to convert the intramuscular formulation for intravenous use and determines the stability and sterility of the resulting formulation over time and under various environmental conditions. Methods: An inventory was taken of all intravenous (Protopam) and intramuscular (Mark I Autoinjector kits) pralidoxime available in Franklin County, Ohio hospitals, and out-of-hospital stockpiles. A method was devised to safely convert the intramuscular pralidoxime to an intravenous formulation, which was then tested for stability and sterility under a variety of environmental temperatures over time. Results: In Franklin County, Ohio (population 1.1 million), the 10 acute care hospitals and out-of hospital community have 36 g of intravenous pralidoxime and 4,398 g (7,270 Mark I kits) of intramuscular pralidoxime. The reformulated pralidoxime retained greater than 90% stability and remained sterile at all environmental temperatures through day 28. Conclusion: Available pralidoxime in Franklin County is predominantly in the intramuscular preparation. Conversion of intramuscular to intravenous pralidoxime results in a stable and sterile solution for up to 28 days under a variety of environmental conditions and should be considered in a mass casualty situation in which additional intravenous supplies are needed.
Corvino TF, Nahata MC, Angelos MG, Tschampel MM, Morosco RS Zerkle J, Nelson RN. Availability, stability, and sterility of pralidoxime for mass casualty use. Ann Emerg Med. 2006 Mar;47(3):272-77. [PubMed Citation]
Storage
-
Store at 25°C (77°F); Excursions permitted to 15-30°C (59-86°F). Keep from freezing.
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data- No data available at this time.
8. Route of Administration/Monitoring
-
Pralidoxime Chloride Injection (auto-injector) provides pralidoxime chloride in a sterile solution for intramuscular injection.
-
Each prefilled auto-injector provides a dose of the antidote, pralidoxime chloride in a self-contained unit, specially designed for automatic self- or buddy- administration by military personnel. Pralidoxime in the auto-injector may also be administered by qualified civilian emergency responders who have had adequate training in the on-site recognition and treatment of nerve agent intoxication in the event of an accidental release of nerve agent. The recommended procedure is to inject the contents of the auto-injector into the muscles of an outer thigh.
-
Treatment of organophosphate poisoning should be instituted without waiting for the results of laboratory tests. Red blood cell, plasma cholinesterase, and urinary paranitrophenol measurements (in the case of parathion exposure) may be helpful in confirming the diagnosis and following the course of the illness. A reduction in red blood cell cholinesterase concentration to below 50% of normal has been seen only with organophosphate ester poisoning.
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
-
Administration of PROTOPAM should be carried out slowly and, preferably, by infusion. If intravenous administration is not feasible, intramuscular or subcutaneous injection should be used.
Product label: PROTOPAM CHLORIDE (pralidoxime chloride) injection, powder, lyophilized, for solution [Baxter Healthcare Corporation] Last Revised: December 2010 [DailyMed]
-
Experimental and clinical studies suggest that the plasma concentration of oxime would be a key parameter to determine its efficacy. However, the measurement of plasma oxime concentration is limited to a small number of laboratories and is thus not generally available in an emergency.
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
9. Adverse effects
-
Although pralidoxime is generally well-tolerated, dizziness, blurred vision, diplopia and impaired accommodation, headache, drowsiness, nausea, tachycardia, hyperventilation, maculopapular rash, and muscular weakness have been reported following administration of the drug.
-
However, it is difficult to differentiate the toxic effects produced by atropine or organophosphates from those of pralidoxime, and the condition of patients suffering from organophosphate intoxication will generally mask minor signs and symptoms reported in normal subjects who receive pralidoxime. When atropine and pralidoxime are used concomitantly, signs of atropinism may occur earlier than when atropine is used alone, especially if the total dose of atropine is large and administration of pralidoxime is delayed.
-
Excitement, confusion, manic behavior, and muscle rigidity have been reported following recovery of consciousness, but these symptoms have also occurred in patients who were not treated with pralidoxime.
-
Rapid IV injection of pralidoxime has produced tachycardia, laryngospasm, muscle rigidity, and transient neuromuscular blockade; therefore, the drug should be administered slowly, preferably by IV infusion.
-
IV administration of pralidoxime reportedly may also cause hypertension which is related to the dose and rate of infusion. Some clinicians recommend that the patient's blood pressure be monitored during pralidoxime therapy. For adults, IV administration of 5 mg of phentolamine mesylate reportedly quickly reverses pralidoxime-induced hypertension.
-
IM administration of pralidoxime may produce mild pain at the injection site.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
-
Forty to 60 minutes after intramuscular injection, mild to moderate pain may be experienced at the site of injection.
-
Pralidoxime may cause blurred vision, diplopia and impaired accommodation, dizziness, headache, drowsiness, nausea, tachycardia, increased systolic and diastolic blood pressure, hyperventilation, and muscular weakness when given parenterally to normal volunteers who have not been exposed to anticholinesterase poisons. In patients it is very difficult to differentiate the toxic effects produced by atropine or the organophosphate compounds from those of the drug.
-
Elevations in SGOT and/or SGPT enzyme levels were observed in 1 of 6 normal volunteers given 1200 mg of pralidoxime chloride intramuscularly, and in 4 of 6 volunteers given 1800 mg intramuscularly. Levels returned to normal in about 2 weeks.
-
Transient elevations in creatine phosphokinase were observed in all normal volunteers given the drug.
-
A single intramuscular injection of 330 mg in 1 mL in rabbits caused myonecrosis, inflammation and hemorrhage.
-
When atropine and pralidoxime are used together, the signs of atropinization may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime has been delayed.
-
Excitement and manic behavior immediately following recovery of consciousness have been reported in several cases. However, similar behavior has occurred in cases of organophosphate poisoning that were not treated with pralidoxime.
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
10. Contraindication(s)
-
Pralidoxime is contraindicated in the treatment of toxic exposure to pesticides of the carbamate class since it appears to increase the toxicity of carbaryl.
-
Pralidoxime should be used with caution in patients with myasthenia gravis who are receiving anticholinesterase agents, since the drug may precipitate a myasthenic crisis.
-
Pralidoxime should be used with caution and in reduced dosage in patients with impaired renal function.
-
The use of succinylcholine, theophylline, aminophylline, reserpine, and respiratory depressants (e.g., barbiturates, morphine, phenothiazines) should be avoided in patients with toxic exposure to anticholinesterase compounds.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
11. Clinical studies in progress or completed
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesTitle: Study to know the efficacy of higher doses of pralidoxime in patients of organophosphorus poisoning. - Trial completed 6/29/2011
Condition: Acute organophosphorus pesticide poisoning
Intervention: Drug:Pralidoxime
ClinicalTrials.gov. Pralidoxime
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesNext generation oxime therapeutic for chemical agent inhibited CNS (brain) ChEs
Current treatment of acute pesticide or organophosphate (OP) poisoning includes a combined administration of a cholinesterase reactivator (oxime), a muscarinic receptor antagonist (atropine) and an anticonvulsant (diazepam). Since the oxime does not penetrate the blood brain barrier (BBS), removal of excessive accumulation of acetylcholine (ACh) is not accomplished by acetylcholinesterase (AChE). Pesticides and OPs are significant terrorist threats to civilian populations. The chemical agent sarin was used in the Tokyo subway terrorist event in 1995 and resulted in long-term neuronal sequelae. Clearly, a new formulation of oxime is required to improve CNS therapy. Because of their chemical structure, the positively charged small molecule oximes do not penetrate the BBB and therefore cannot treat pesticide- and OP- induced toxicity in the brain. The long term goal is to transport oxime (2-PAM, MMB-4) quickly and non-invasively into the CNS. CNS-penetrating oxime therapy will reactivate the brain AChE in a timely manner, reduce the requirement for anticonvulsant drug regimens, and improve the long term recovery of the exposed individual by reducing or eliminating CNS neuronal damage, (a) The investigators will synthesize and evaluate more lipoidal forms (pro-oximes), that can be converted to their active (charged) form in the brain, (b) In addition, the investigators will develop carrier(s) of oximes that will come from a class of FDA approved/phase 1 trial Pharmaceuticals that pass the BBB... including Stealth liposomes, nanoparticles, or cyclodextrins. (c) Two additional advantages to the carriers: should permit a sustained delivery of oxime into the systemic circulation through the skin (via skin patch) and prolong the circulatory time of the oxime(s), which are rapidly cleared by the renal system (T1/2<2 hr). (d) For formulations that are successful in in vitro BBB tissue culture and pig skin penetration models, the investigators will determine reactivation kinetics of the encapsulated oximes with pesticide- and OP-inhibited AChE and BuChE, the release rates of the oxime from the carrier in the peripheral and CNS of animal model (guinea pig), and the pharmacokinetics of the pro-oxime and oxime carrier and distribution in diaphragm, blood, and brain, (d) The most efficacious formulation will be validated in a status epilepticus guinea pig model as a therapy for CNS chemical agent OP-induced toxicity of DFP, GB, GD, and VX using EEC radiotelemetry probe techniques. This program will address an important and potential threat to civilian populations, for post-exposure CNS treatment in response to exposure to chemical threat agents. Further, the outcome of this work could provide a non-invasive means of administrating appropriate therapeutics for deleterious effects of OP poisoning, directly relevant to emergency and preparedness response to chemical threats for Public Health.
RePORTER. NIH. Next generation oxime therapeutic for chemical agent inhibited CNS (brain) ChEs.
Rapid development of in vivo models for countermeasure discovery: organophosphate
A major problem in chemical countermeasure discovery is the potential emergence of novel agents for which there are no known antidotes or post exposure therapies. Classic countermeasures have often been discovered only serendipitously or have taken years to develop. As novel chemical threats emerge (in the form of novel chemical warfare agents or environmental pollutants), the speed with which we are able to understand and counteract each threat will determine the magnitude of its societal impact. One promising approach for rapidly identifying and manipulating the molecular pathways underlying the response to any chemical threat is the use of phenotype-based chemical screens. Models can be developed in which specific toxicants result in reproducible phenotypes in cells or whole organisms. By subjecting these models to high-throughput screening (HTS), small molecules could be identified that reverse the phenotype through a variety of novel mechanisms. Small molecules discovered by these screens would be excellent lead compounds for novel countermeasures and powerful tools for dissecting toxicity pathways, which may in turn point to additional countermeasure targets. In this application, the investigators outline a process for rapid development of in vivo organophosphate toxicity models and their use in discovery of novel organophosphate countermeasures. Although the investigators expect the proposed project will lead directly to development of novel organophosphate countermeasures, they also expect it to serve as a model for rapid countermeasure development that can be applied broadly to other existing and emerging chemical threats. Specifically, the investigators propose: Aim 1. To develop and validate in vivo assays for organophosphate toxicity in the zebrafish. They will seek to identify physiological responses that are surrogates for known human responses to organophosphates and that can be scaled for high throughput in vivo screening. Aim 2. To scale these novel assays for automated high-throughput screening in multiwell plates. The investigators will develop assays for organophosphate toxicity that can be performed automatically in 96-well format. Aim 3. To identify novel compounds that counteract the effects of prior organophosphate exposure. High-throughput screening will identify compounds that facilitate recovery from organophosphate exposure
RePORTER. NIH. Rapid development of in vivo models for countermeasure discovery: organophosphate.
Oxime-assisted catalysis of organophosphates and reactivation of AChE
The proposed project is directed to improving prophylaxis and therapy for organophosphate nerve agent organophosphate exposure through the design of mutant human acetylcholinesterases (AChEs), novel oximes and related reactivating nucleophiles. This strategy of oxime-assisted catalysis modifies AChE such that, when placed in the circulation, the oxime and AChE become a catalytic, rather than a stoichiometric, scavenger of organophosphates. Proof of principle has already been established for hydrolysis of a series of organophosphates, and the investigators will refine AChE mutations and oxime structure to enhance catalytic rates for hydrolysis and retention in plasma space. Since oxime therapy, 2-PAM and HI-6, also reactivates inhibited AChE at the target site and the therapeutic benefits of long term oxime therapy have become fully appreciated recently, the investigators propose to design new reactivators based on contemporary knowledge of AChE structure to which they and other groups have contributed to over the past two decades. This approach considers impaction of the active center gorge and angle of access of the oxime as limiting constraints in the design strategy. Moreover, the /research/ groups have applied a novel freeze-frame, click chemistry to the development of reactivating agents using AChE-phosphonate conjugates as the template for synthesis of the triazoles. In addition to oxime assisted catalysis in plasma, new oximes and other nucleophiles will be developed as novel reactivating agents at the target site. This chemistry also enjoys the advantage of combinatorial screening for medium to high throughput in the synthesis-screening paradigm. Nucleophiles and nucleophile AChE combinations will be optimized for enhancing therapeutic efficacy to the particular offending organophosphate. Hence, the investigators approach will both augment catalytic scavenging of organophosphates in the circulation and enhance reactivation at the target tissues.
RePORTER. NIH. Oxime-assisted catalysis of organophosphates and reactivation of AChE.
Synthesis of methylating ligands that reactivate aged acetylcholinesterase
Organophosphorus (OP) chemical warfare agents, such as sarin and soman, are acutely toxic compounds that act by inhibiting the activity of the enzyme acetylcholinesterase (AChE) at nerve-nerve and neuromuscular junctions in the central and peripheral nervous systems, respectively. Inhibition of the enzyme occurs by phosphylation of the active site serine nucleophile that is normally involved in catalysis. Prompt administration of oximes can lead to dephosphylation of the phosphyl-AChE adduct and hence is an antidote strategy. However, and particularly with the chemical warfare agent soman, the initial phosphyl-AChE adduct undergoes a dealkylation reaction that leads to what is called the aged adduct, for which there is no known antidote. This application proposes to address this perplexing problem by synthesis and evaluation of AChE ligands that can bind in the active site of the aged enzyme adduct, and subsequently serve as methyl transfer agents to realkylate the aged enzyme. This in turn will resurrect the susceptibility of the adduct to nucleophilic dephosphylation by oximes and hence lead to recovery of enzyme activity. Success in this endeavor should therefore open the door to the development of efficacious drugs for antidote therapy against currently intractable OP chemical warfare agents. PUBLIC HEALTH RELEVANCE: This application endeavors to synthesize and evaluate ligands of the enzyme acetylcholinesterase that can serve as antidotes against organophosphorus (OP) chemical warfare agents. This work is motivated by the concern that terrorist organizations may aspire to inflict mass casualties by use of these agents. It is anticipated that as OP agent antidote therapy improves, the health and security risks that these agents pose to our society will be ameliorated.
RePORTER. NIH. Synthesis of methylating ligands that reactivate aged acetylcholinesterase.
Optimization of nonpyridinium oximes for BChE hydrolysis of OPs in plasma
The principal aim of this study is to optimize the efficient and affordable butyrylcholinesterase (BChE) based catalytic scavenger system developed in vitro for treatment of acute organophosphate (OP) intoxication. Currently used oxime and atropine combination therapy is inefficient for treatment of higher exposure OP poisoning due to constant acetylcholinesterase (AChE) reinhibition by excess OP. Novel approaches in therapy based on BChE as stoichiometric scavenger appear prohibitively expensive with a four figure price tag per single application. Conversion of BChE from stoichiometric to catalytic scavenger by combining it with an oxime reactivator has been hampered by lack of efficient BChE reactivators. In the investigators preliminary studies they were able to identify a novel class of superior specific BChE reactivators of distinct nontraditional structural scaffold. The proposed study deals with optimization of this novel class of reactivators using six step optimization iterative cycle that include detailed kinetic characterization of both reactivation and OP hydrolytic properties of oxime/BChE catalytic scavenger systems in buffer medium, extracorporeal human blood and in vivo in mouse animal model. Results of preliminary studies indicate that BChE in combination with optimized oxime reactivators should completely and rapidly, in a several minute timeframe, hydrolyze OPs in plasma space following exposure to an order of magnitude higher than LD50 OP doses at a fraction of cost of currently developed BChE based stoichiometric scavenger system. The proposed optimized catalytic scavenger system technology will thus enable an effective treatment of large OP intoxicated populations and serve as deterrent to the use of OP based nerve agents as terrorist or combat weapons in closed ventilation systems. PUBLIC HEALTH RELEVANCE: This project will develop optimized, efficient and affordable butyrylcholinesterase based catalytic scavenger system for rapid degradation of toxicants in the plasma space of patients exposed to OP agents.
RePORTER. NIH. Optimization of nonpyridinium oximes for BChE hydrolysis of OPs in plasma.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations-
Develop a pediatric size autoinjector and appropriate dosing
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
The development of promising scavenger molecules effective against a wide variety of chemical nerve agents should be pursued.
-
The development of oximes that are broader acting than 2-PAM, and have the ability to reverse the permanent binding that can occur with some nerve agents should be pursued.
-
Critical nerve agent antidotes should be evaluated to determine if they can be safely and effectively administered to infants, children and adults via the intravenous and aerosol routes.
-
The accelerated development of promising antidotes for chemical nerve agents should be pursued.
-
Expanded labeling of currently licensed anticonvulsants for use against chemical nerve agents and their utilization in autoinjectors should be pursued.
-
Objective screening tests that can evaluate behavior and cognitive ability, and can differentiate chemically-induced neurological injury from pre-existing neurological illness need to be developed.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
Develop a pediatric size autoinjector and appropriate dosing
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
The development of promising scavenger molecules effective against a wide variety of chemical nerve agents should be pursued.
-
The development of oximes that are broader acting than 2-PAM, and have the ability to reverse the permanent binding that can occur with some nerve agents should be pursued.
-
Critical nerve agent antidotes should be evaluated to determine if they can be safely and effectively administered to infants, children and adults via the intravenous and aerosol routes.
-
The accelerated development of promising antidotes for chemical nerve agents should be pursued.
-
Expanded labeling of currently licensed anticonvulsants for use against chemical nerve agents and their utilization in autoinjectors should be pursued.
-
Objective screening tests that can evaluate behavior and cognitive ability, and can differentiate chemically-induced neurological injury from pre-existing neurological illness need to be developed.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
15. Study-related ethical concerns
— including review panel recommendations- No data available at this time.
16. Global regulatory status
U.S.
-
This auto-injector for pralidoxime chloride is specifically indicated for intramuscular use as an adjunct to atropine in the treatment of poisoning by nerve agents having anticholinesterase activity.
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
-
PROTOPAM is indicated as an antidote:
In the treatment of poisoning due to those pesticides and chemicals (e.g., nerve agents) of the organophosphate class which have anticholinesterase activity and
In the control of overdosage by anticholinesterase drugs used in the treatment of myasthenia gravis.
The principal indications for the use of PROTOPAM are muscle weakness and respiratory depression. In severe poisoning, respiratory depression may be due to muscle weakness.
Product label: PROTOPAM CHLORIDE (pralidoxime chloride) injection, powder, lyophilized, for solution [Baxter Healthcare Corporation] Last Revised: December 2010 [DailyMed]
U.K.
-
Adjunct to atropine in the treatment of poisoning by organophosphorus insecticide or nerve agent.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 36
E.U.
-
Adults: Initiate as soon as possible; slow IV injection, or IM 1-2 g. Repeat up to a total of 30 mg/kg. Continuation up to 24 h about 2000-4000 mg max. Treatment may be needed for several days, especially for highly toxic OP.
-
Children: Initiate as soon as possible; slow IV injection, 25-40 mg/kg (max 1 g). Continuation of infusion of 10-20 mg/kg h for 18 h or longer.
Treatment may be needed for several days, especially for highly toxic OP.
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
Other
-
Formulations: Contrathion (Argentina, Brasil, France, Greece, Italy, Turkey), Protopam (Canada, USA), Neopam (India), Pampara (Malaysia), Nerve Agent Antidote L4A1(U.K., multi-ingredient)
Sweetman SC (ed). Martindale, The Complete Drug Reference. London: Pharmaceutical Press (2009) p.1460
17. Other potentially useful information
-
The authors sought to determine the feasibility of discharging Mark 1 atropine and pralidoxime autoinjectors into small, sterile vials to facilitate the potential intramuscular injection of these antidotes, particularly pralidoxime, on a milligram per kilogram basis to small children. Methods: Autoinjectors were swabbed with isopropyl alcohol and then discharged into emptied, sterile, plastic 10-mL vials. This was repeated with the investigator garbed in standard personal protective gloves and full face mask and hood. The autoinjector injection surfaces were cultured. The autoinjectors were easily discharged into the vials without need for practice or special dexterity, even when investigators were garbed in protective gear. A small core of rubber stopper might be injected into the vial, and thus, the vial contents need to be withdrawn through a filter needle before reinjection. The autoinjector injection surfaces were sterile after alcohol swabbing. Autoinjectors might be a readily available source of concentrated pralidoxime for potential intramuscular use in small children.
Henretig FM, Mechem C, Jew R. Potential use of autoinjector-packaged antidotes for treatment of pediatric nerve agent toxicity. Ann Emerg Med. 2002 Oct;40(4):405-8. [PubMed Citation]
-
The pertinent threat of using organophosphorus compound (OP)-type chemical warfare agents (nerve agents) during military conflicts and by non-state actors requires the continuous search for more effective medical countermeasures. OP inhibit acetylcholinesterase (AChE) and therefore standard treatment of respective poisoning includes AChE reactivators (oximes) in combination with antimuscarinic agents. Hereby, standard oximes, 2-PAM and obidoxime, are considered to be rather insufficient against various nerve agents. Numerous experimental oximes have been investigated in the last decades by in vitro and in vivo models. The authors studied the reactivating potency of several oximes with human AChE inhibited by structurally different OP and observed remarkable differences depending on the OP and oxime. In order to investigate structure-activity relationships the authors determined the various kinetic constants (inhibition, reactivation, aging) for a series of sarin analogues bearing a methyl, ethyl, n-propyl, n-butyl, i-propyl, i-butyl, cyclohexyl or pinacolyl group with human AChE and BChE. The rate constants for the inhibition of human erythrocyte AChE and plasma BChE by these OP (ki), for the spontaneous dealkylation (ka) and reactivation (ks) of OP-inhibited AChE and BChE as well as for the oxime-induced reactivation of OP-inhibited AChE and BChE by the oximes obidoxime, 2-PAM, HI-6, HLo 7 and MMB-4 were determined. With compounds bearing an n-alkyl group the inhibition rate constant increased with chain length. A relation between chain length and spontaneous reactivation velocity was also observed. In contrast, no structure-activity dependence could be observed for the oxime-induced reactivation of AChE and BChE inhibited by the compounds tested. In general, OP-inhibited AChE and BChE were susceptible towards reactivation by oximes. HLo 7 was the most potent reactivator followed by HI-6 and obidoxime while 2-PAM and MMB-4 were rather weak reactivators. These data indicate a potential structure-activity relationship concerning inhibition and spontaneous reactivation but not for oxime-induced reactivation.
Bartling A, Worek F, Szinicz L, Thiermann H. Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology 2007;233:166-72. [PubMed Citation]
-
Organophosphorus compound-based (OP) chemical warfare agents (nerve agents) represent a continuing threat to military forces and the civilian population. OPs act primarily by inhibiting acetylcholinesterase (AChE), the standard treatment for which includes AChE reactivators (oximes) in combination with antimuscarinic drugs. In the last decades, the efficacy of oximes has been investigated mostly in small animal models. In order to increase the predictive value of animal studies it is desirable to measure numerous physiological and biochemical parameters. This is difficult in small animals. Large animal models fulfill these requirements and swine are increasingly being used in toxicology studies. Swine breeds generally show considerable variability in different characteristics which may be minimized by the use of specially bred minipigs which have a known genetic background and health status. A comparative study was, therefore, initiated to investigate the kinetic properties of the White Landrace pig and Gottingen minipig AChE in respect of inhibition by the pesticide paraoxon; the nerve agents cyclosarin, VX and VR; the reactivation of inhibited AChE by oximes (obidoxime, pralidoxime and HI 6); and the aging and spontaneous reactivation of inhibited AChE. The determination of the respective kinetic constants found similarities between pig and minipig AChE which showed marked differences in comparison with human AChE values. This has to be considered in designing meaningful models for the investigation of oxime efficacy in pig or minipig experiments. The generated data indicate comparable kinetic properties of pig and minipig AChE and may provide a kinetic basis for extrapolation of data from pig studies to humans.
Worek F, Aurbek N, Wetherell J, Pearce P, T. Mann T, Thiermann H. Inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds: pig versus mini pig acetylcholinesterase. Toxicology 2008 Feb;244:35-41. [PubMed Citation]
-
Searching for new potent acetylcholinesterase (AChE; E.C. 3.1.1.7) reactivators (oximes) is a very time-consuming process. The authors are able to synthesize more than 50 new AChE reactivators per year. Owing to this fact, these investigators have to select promising reactivators using an in vitro method (potentiometric titration, pH 8 and temperature 25°C; source of cholinesterases, rat brain homogenate; time of inhibition by nerve agents, 30 min; time of reactivation, 10 min) prior to in vivo experiments. For this purpose, we are using two-phase in vitro evaluation of reactivator potency. In the first phase, reactivation potency of all newly synthesized AChE reactivators is tested at two concentrations: 10—3 M and 10—5 M. Afterwards, all reactivators achieving reactivation potency over 15% (especially at the concentration 10—5 M) are tested. The second phase consists of the measurement of the relationship between concentration of the oxime and its reactivation ability. In most cases, the reactivation bell-shaped curve is obtained. The most potent AChE reactivators are selected and provided for further experiments during our development process.
Kuca K, Cabal J, Jun D, Hrabinova M. In vitro evaluation of acetylcholinesterase reactivators as potential antidotes against tabun nerve agent poisonings. Drug and Chemical Toxicology 2006;29(4):443-49. [PubMed Citation]
-
The widespread use of organophosphorus compounds (OP) as pesticides and the repeated misuse of highly toxic OP as chemical warfare agents (nerve agents) emphasize the necessity for the development of effective medical countermeasures. Standard treatment with atropine and the established acetylcholinesterase (AChE) reactivators, obidoxime and pralidoxime, is considered to be ineffective with certain nerve agents due to low oxime effectiveness. From obvious ethical reasons only animal experiments can be used to evaluate new oximes as nerve agent antidotes. However, the extrapolation of data from animal to humans is hampered by marked species differences. Since reactivation of OP-inhibited AChE is considered to be the main mechanism of action of oximes, human erythrocyte AChE can be exploited to test the efficacy of new oximes. By combining enzyme kinetics (inhibition, reactivation, aging) with OP toxicokinetics and oxime pharmacokinetics a dynamic in vitro model was developed which allows the calculation of AChE activities at different scenarios. This model was validated with data from pesticide-poisoned patients and simulations were performed for intravenous and percutaneous nerve agent exposure and intramuscular oxime treatment using published data. The model presented may serve as a tool for defining effective oxime concentrations and for optimizing oxime treatment. In addition, this model can be useful for the development of meaningful therapeutic animal models.
Worek F, Szinicz L, Eyer P, Thiermann H. Evaluation of oxime efficacy in nerve agent poisoning: Development of a kinetic-based dynamic model.
Toxicology and Applied Pharmacology 2005 Dec;209(3):193-202. [PubMed Citation]
-
The toxicity of organophosphorus (OP) nerve agents is manifested through irreversible inhibition of acetylcholinesterase (AChE) at the cholinergic synapses, which stops nerve signal transmission, resulting in a cholinergic crisis and eventually death of the poisoned person. Oxime compounds used in nerve agent antidote regimen reactivate nerve agent-inhibited AChE and halt the development of this cholinergic crisis. Due to diversity in structures of OP nerve agents, none of the currently available oximes is able to reactivate AChE inhibited by different nerve agents. To understand the mechanism for the differential activities of oximes toward AChE inhibited by diverse nerve agents in order to aid the design of new broad-spectrum AChE reactivators, the authors undertook site-directed mutagenesis and molecular modeling studies. Recombinant wild-type and mutant bovine (Bo) AChEs were inhibited by two bulky side-chain nerve agents, GF and VR, and used for conducting reactivation kinetics with five oximes. A homology model for wild-type Bo AChE was built using the recently published crystal structure of human AChE and used to generate models of 2-PAM and HI-6 bound to the active-sites of GF- and VR-inhibited Bo AChEs before nucleophilic attack. Results revealed that the peripheral anionic site (PAS) of AChE as a whole plays a critical role in the reactivation of nerve agent-inhibited AChE by all 4 bis-pyridinium oximes examined, but not by the mono-pyridinium oxime 2-PAM. Of all the residues at the PAS, Y124 appears to be critical for the enhanced reactivation potency of H oximes.
Luo C, Chambers C, Pattabiraman N, Tong M, Tipparaju P, Saxena A. Y124 at the peripheral anionic site is important for the reactivation of nerve agent-inhibited acetylcholinesterase by H oximes. Biochemical Pharmacology 2010 Nov;80(9): 1427-36. [PubMed Citation]
-
The potential replacement of the established oximes obidoxime and pralidoxime by HI-6 or MMB-4 will not result in the availability of an antidote covering the whole spectrum of threat agents... The major drawback of HI-6 is its weak reactivating potency with pesticide inhibited /acetylcholinesterase/ and its failure to reactivate tabun-inhibited /acetylcholinesterase/. MMB-4 was shown to be superior to pralidoxime but mostly less potent than obidoxime and HI-6. Hence the next generation of oxime antidotes (HI-6 or MMB-4) will give some improvement but will not fulfill the requirement as broad-spectrum reactivators...
-
At present, none of the oximes tested so far can be considered as real broad spectrum reactivator covering the whole range of threat agents... A short-term alternative could be the combined use of two or more oximes having a complimentary spectrum...
Worek F, Thiermann H. Strategies for the development of effective broad-spectrum oximes. In defense against the effects of chemical hazards: toxicology, diagnosis and medical countermeasures 2007 p. 30-1-30-6
-
Every new oxime could be tested for its broad spectrum potency. However, the probability of finding such an oxime is low. Alternatively, we can use a combination of two oximes. Such an approach was already applied in the former Yugoslavia...
Gupta RC, ed. Handbook of Toxicology of Chemical Warfare Agents. Oxford, UK: Elsevier, Academic Press, 2009 p. 997-1021
-
logP (octanol-water) = -3.440 (est)
US NLM. ChemIDplus Lite. Pralidoxime
18. Publications
Balali-Mood M, Shariat M. Treatment of organophosphate poisoning. Experience of nerve agents and acute pesticide poisoning on the effects of oximes. J Physiology (Paris) 1998 Oct-Dec;92(5-6):375-8. [PubMed Citation]
Bartling A, Worek F, Szinicz L, Thiermann H. Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology 2007;233:166-72. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Blick DW, Murphy MR, Brown GC, Hartgraves SL. Primate performance decrements following acute soman exposure: Failure of chemical countermeasures. Pharmacol Biochem Behav 1994;49(3):503-10 [PubMed Citation]
Buckley N.A., M. Eddleston, Li Y, Bevan M, Roberstson J. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev. 2011 Feb;(2):CD005085. [PubMed Citation]
Chemical-biological terrorism and its impact on children. Pediatrics 2006 Sep; 118(3):1267-78. [PubMed Citation]
ClinicalTrials.gov. Pralidoxime
Corvino TF, Nahata MC, Angelos MG, Tschampel MM, Morosco RS Zerkle J, Nelson RN. Availability, stability, and sterility of pralidoxime for mass casualty use. Ann Emerg Med. 2006 Mar;47(3):272-77. [PubMed Citation]
Demar JC, Clarkson ED, Ratcliffe RH, Campbell AJ, Thangavelu SG, Herdman CA, Leader H, Schultz CM, Marek E, Medynetcs MA, Ku TC, Evans SA, Khan FA, Owens RR, Nambiar MP, Gordon RK. Pro-2-PAM therapy for central and peripheral cholinesterases. Chem Biol Interact. 2010 Sep;187(1-3):191-8 [PubMed Citation].
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
Eddleston, M., P. Eyer, F. Worek, Juszczak E, Alder N, Mohamed F, Senarathna L, Hittarage A, Azher S, Jeganathan K, Jayamanne S, von Meyer L, Dawson AH, Sheriff MH, Buckley NA. Pralidoxime in acute organophosphorus insecticide poisoning--a randomised controlled trial. PLoS Med. 2009 Jun;6(6):e1000104. [PubMed Citation]
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
Eyer P, Szinicz L, Thiermann H, Worek F, Zilker T. Testing of antidotes for organophosphorus compounds: Experimental procedures and clinical reality. Toxicology 2007 Apr;233(1-3):108-19. [PubMed Citation]
Fisar Z, Hroudova J, Korabecny J, Musilek K, Kuca K. In vitro effects of acetylcholinesterase reactivators on monoamine oxidase activity. Toxicology Letters 2011 Mar;201(2):176-80. [PubMed Citation]
Gupta RC, ed. Handbook of Toxicology of Chemical Warfare Agents. Oxford, UK: Elsevier, Academic Press, 2009 p. 985-96
Gupta RC, ed. Handbook of Toxicology of Chemical Warfare Agents. Oxford, UK: Elsevier, Academic Press, 2009 p. 997-1021
Henretig FM, Mechem C, Jew R. Potential use of autoinjector-packaged antidotes for treatment of pediatric nerve agent toxicity. Ann Emerg Med. 2002 Oct;40(4):405-8. [PubMed Citation]
Jokanovic M, Prostran M. Pyridinium oximes as cholinesterase reactivators. structure-activity relationship and efficacy in the treatment of poisoning with organophosphorus compounds. Current Medicinal Chemistry 2009;16:2177-88. [PubMed Citation]
Jokanovic M, Stojiljkovic MP. Current understanding of the application of pyridinium oximes as cholinesterase reactivators in treatment of organophosphate poisoning. European Journal of Pharmacology 2006;553:10-7. [PubMed Citation]
Juna D, Musilova L, Kuca K, Kassa J, Bajgar J. Potency of several oximes to reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon in vitro. Chemico-Biological Interactions 2008 Sep;175(1-3):421-4. [PubMed Citation]
Jun D, Kuca K, Hronek M, Opletal L. Effect of some acetylcholinesterase reactivators on human platelet aggregation in vitro. J. Appl. Toxicol. 2006 May-June;26(3):258-61 [PubMed Citation]
Jun D, Musilova L, Musilek K, Kuca K. In vitro ability of currently available oximes to reactivate organophosphate pesticide-inhibited human acetylcholinesterase and butyrylcholinesterase. Int. J. Mol. Sci. 2011;12(3): 2077-87 [PubMed Citation]
Kassa J. Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents. Journal of Toxicology Clinical Toxicology 2002;40(6):803-16 [PubMed Citation]
Koplovitz I, Stewart JR. A comparison of the efficacy of HI6 and 2-PAM against soman, tabun, sarin, and VX in the rabbit. Toxicology Letters 1994 Feb;70(3): 269-79. [PubMed Citation]
Kuca K, Cabal J, Jun D, Hrabinova M. In vitro evaluation of acetylcholinesterase reactivators as potential antidotes against tabun nerve agent poisonings. Drug and Chemical Toxicology 2006;29(4):443-49. [PubMed Citation]
Kuca K, Cabal J, Jun D, Kassa J, Bartosova L, Kunesova G. In vitro reactivation potency of some acetylcholinesterase reactivators against sarin- and cyclosarin-induced inhibitions. J. Appl. Toxicol. 2005 Jul-Aug;25(4):296-300. [PubMed Citation]
Kuca K, Cabal J, Kassa J, Jun D, Hrabinova M. In vitro potency of H oximes (HI-6, HLo-7), the oxime BI-6, and currently used oximes (pralidoxime, obidoxime, trimedoxime) to reactivate nerve agent-inhibited rat brain acetylcholinesterase. Journal of Toxicology and Environmental Health, Part A 2006 Aug;69(15):1431-40. [PubMed Citation]
Kuca K, Jun D, Bajgar J. Currently used cholinesterase reactivators against nerve agent intoxication: comparison of their effectivity in vitro. Drug and Chemical Toxicology 2007;30(1):31-40. [PubMed Citation]
Kuca K, Jun D, Cabal J, Hrabinova M, Bartosova L, Opletalova V. Russian VX: inhibition and reactivation of acetylcholinesterase compared with VX agent. Basic & Clinical Pharmacology & Toxicology 2006 Apr;98(4):389-94. [PubMed Citation]
Kuca K, Kassa J. Oximes-induced reactivation of rat brain acetylcholinesterase inhibited by VX agent. Human & Experimental Toxicology 2004;23:167-71. [PubMed Citation]
Luo C, Chambers C, Pattabiraman N, Tong M, Tipparaju P, Saxena A. Y124 at the peripheral anionic site is important for the reactivation of nerve agent-inhibited acetylcholinesterase by H oximes. Biochemical Pharmacology 2010 Nov;80(9): 1427-36. [PubMed Citation]
Luo C, Tong M, Chilukuri N, Brecht K, Maxwell DM, Saxena A. An in vitro comparative study on the reactivation of nerve agent-inhibited guinea pig and human acetylcholinesterases by oximes. Biochemistry 2007 Oct;46(42):11771-9. [PubMed Citation]
Lundy PM, Hansen AS, Hand BT, Boulet CA. Comparison of several oximes against poisoning by soman, tabun and GF. Toxicology 1992;72:99-105. [PubMed Citation]
Luo C, Tong M, Maxwell DM, Saxena A. Comparison of oxime reactivation and aging of nerve agent-inhibited monkey and human acetylcholinesterases. Chemico-Biological Interactions 2008 Sep;175(1-3):261-6. [PubMed Citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 36
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p. 3628-30
Moore DH, Hayward IJ, Tucker FS, Lukey B. HI-6 and 2-PAM in sheep: pharmacokinetics and effects on muscle tissue following intramuscular injection. Biopharmaceutics & Drug Disposition 1991 Apr;12(3): 223-32. [PubMed Citation]
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1450-66
Product label:
PRALIDOXIME CHLORIDE injection
[Meridian Medical Technologies, Inc.] Last revised: June 2008
[DailyMed]
Product label: PROTOPAM CHLORIDE (pralidoxime chloride) injection, powder, lyophilized, for solution [Baxter Healthcare Corporation] Last Revised: December 2010 [DailyMed]
Quail MT, Shannon MW. Pralidoxime safety and toxicity in children. Prehosp Emerg Care. 2007 Jan-Mar;11(1):36-41. [PubMed Citation]
Reddy VK, Deshpande SS, Cintra WM, Scoble GT, Albuquerque EX. Effectiveness of oximes 2-PAM and HI-6 in recovery of muscle function depressed by organophosphate agents in the rat hemidiaphragm: an in vitro study. Fundamental and Applied Toxicology 1991 Nov;17(4):746-60. [PubMed Citation]
RePORTER. NIH. Next generation oxime therapeutic for chemical agent inhibited CNS (brain) ChEs.
RePORTER. NIH. Optimization of nonpyridinium oximes for BChE hydrolysis of OPs in plasma.
RePORTER. NIH. Oxime-assisted catalysis of organophosphates and reactivation of AChE.
RePORTER. NIH. Rapid development of in vivo models for countermeasure discovery: organophosphate.
RePORTER. NIH. Synthesis of methylating ligands that reactivate aged acetylcholinesterase.
Rotenberg JS, Newmark J. Nerve agent attacks on children: diagnosis and management. Pediatrics 2003 Sep;112(3 Pt 1):648-58 [PubMed Citation]
Sakurada K, Matsubara K, Shimizu K, Shiono H, Seto Y, Tsuge K, Yoshino M, Sakai I, Mukoyama H, Takatori T. Pralidoxime iodide (2-PAM) penetrates across the blood-brain barrier. Neurochemical Research 2003 Sep;28(9):1401-7. [PubMed Citation]
Shih T-M, Guarisco JA, Myers TM, Kan RK, McDonough JH. The oxime pro-2-PAM provides minimal protection against the CNS effects of the nerve agents sarin, cyclosarin, and VX in guinea pigs. Toxicol Mech Methods 2011 Jan; 21(1):53-62. [PubMed Citation].
Shih T-M, Skovira JW, O'Donnell JC, McDonough. JH. In vivo reactivation by oximes of inhibited blood, brain and peripheral tissue cholinesterase activity following exposure to nerve agents in guinea pigs. Chemico-Biological Interactions 2010 Sep;187(1-3):207-14. [PubMed Citation]
Shrot S , Markel G, Dushnitsky T, Krivoy A. The possible use of oximes as antidotal therapy in organophosphate-induced brain damage. NeuroToxicology 2009 Mar;30(2):167-73. [PubMed Citation]
Singh G, Avasthi G, Khurana D, Whig J, Mahajan R. Neurophysiological monitoring of pharmacological manipulation in acute organophosphate (OP) poisoning. The effects of pralidoxime, magnesium sulphate and pancuronium. Electroencephalography and clinical Neurophysiology 1998 Aug;107(2):140-8. [PubMed Citation]
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
Sweetman SC (ed). Martindale, The Complete Drug Reference. London: Pharmaceutical Press (2009) p.1460
Tokuda Y, Kikuchib M, Takahashib O, Stein GH. Prehospital management of sarin nerve gas terrorism in urban settings: 10 years of progress after the Tokyo subway sarin attack. Resuscitation 2006;68:193-202. [PubMed Citation]
US NLM. ChemIDplus Lite. Pralidoxime
Worek F, Aurbek N, Wetherell J, Pearce P, T. Mann T, Thiermann H. Inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds: pig versus mini pig acetylcholinesterase. Toxicology 2008 Feb;244:35-41. [PubMed Citation]
Worek F, Aurbek N, Wille T, Eyer P, Thiermann H. Kinetic analysis of interactions of paraoxon and oximes with human, Rhesus monkey, swine, rabbit, rat and guinea pig acetylcholinesterase. Toxicology Letters 2011 Jan;200(1-2):19-23. [PubMed Citation]
Worek F, Szinicz L, Eyer P, Thiermann H. Evaluation of oxime efficacy in nerve agent poisoning: Development of a kinetic-based dynamic model. Toxicology and Applied Pharmacology 2005 Dec;209(3):193-202. [PubMed Citation]
Worek F, Thiermann H. Strategies for the development of effective broad-spectrum oximes. In defense against the effects of chemical hazards: toxicology, diagnosis and medical countermeasures 2007 p. 30-1-30-6
Yanagisawa N, Morita H, Nakajima T. Sarin experiences in Japan: Acute toxicity and long-term effects. Journal of the Neurological Sciences 2006 Nov;249(1):76-85. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
