You are here: Home > Medical Countermeasures Database > Scopolamine
Scopolamine - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Scopolamine
2. Chemical Defense therapeutic area(s)
— including key possible usesScopolamine is an anticholinergic anticonvulsant that has been studied for use against organophosphate intoxication.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
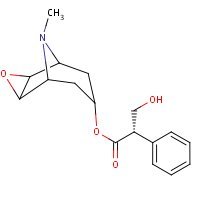
HSDB. Scopolamine
Mechanism of action
-
Scopolamine is a naturally occurring musarinic receptor antagonists and belladonna alkaloid. Scopolamine competes with acetylcholine (ACh) and other muscarinic agonists for a common binding site on the muscarinic receptor. Muscarinic receptor antagonists inhibit responses to postganglionic cholinergic nerve stimulation less effectively that they inhibit responses to injected choline esters. The difference may be explained by the fact that release of ACh by cholinergic nerve terminals occurs in close proximity to the receptors, resulting in very high concentrations of the transmitter at the receptors. Scopamine is able to permeate the blood brain barrier and is effective in preventing motion sickness, possibly by blocking neural pathways from the inner ear to the emetic center in the brainstem.
Brunton LL, Chabner BA, Knollmann BC (eds.) Goodman & Gilman's The Pharmacological Basis of Therapeutics (12th Ed.). McGraw-Hill Medical, New York, NY. (2011) p.226-230
Summary of clinical and non-clinical studies
Organophosphates (OP) are commonly used as pesticides and as military nerve agents; the latter include sarin, soman, tabun, and VX. OP intoxication is the result of irreversible inhibition of acetylcholinesterase (AChE) via phosphorylation of the active-site serine. As a result, acetylcholine accumulates at synapses, inducing convulsions, behavioral impairments, and eventually death, if untreated. Current therapy options for acute OP poisoning are atropine, atropine combined with an oxime. While this regimen is effective in preserving the life of the subject, it does not efficiently control the convulsions and behavioral deficits that may arise due to the initial spike in cholinergic activity. The classical anticholinergic anticonvulsant scopolamine, approved for human use against motion sickness, has been successfully used against OP intoxication in animal studies. In oxime-pretreated, soman-intoxicated (1.6×LD50) rats, scopolamine pretreatment was more effective than atropine pretreatment in preventing convulsions (Shih et al., 1991). A study of pretreatment scopolamine (or diazepam) combined with pretreatment pyridostigmine and posttreatment atropine and pralidoxime showed that scopolamine significantly improved survival (p<0.001) and reduced convulsions (p<0.05) in soman-exposed guinea pigs, compared to animals pretreated with diazepam (Anderson et al., 1994; Harris et al., 1994). Even when administered up to 40 minutes after 2×LD50 soman exposure (in pyridostigmine-pretreated, atropine/pralidoxime-posttreated guinea pigs), scopolamine reduced seizures, though it became less effective at the later time points (McDonough et al., 2000). When scopolamine and pyridostigmine were administered to rats via intramuscular injection prior to soman exposure (1×LD50), convulsions were completely abolished and impairments in learning and memory were partially restored (Raveh et al., 2002).
Nonhuman primate experiments have been similarly promising. Cynomolgus monkeys pretreated with physostigmine and scopolamine were able to completely survive 2×LD50 of soman exposure without convulsions or loss of consciousness; at a high soman dose (5×LD50), pretreated monkeys experienced a memory/behavioral recovery within 30 minutes, after a brief period of unconsciousness (von Bredow et al., 1991). Two weeks of pretreatment with scopolamine and physostigmine preserved memory and behavioral function in marmosets after injection of sublethal doses of sarin or soman (Muggleton et al., 2003).
More recent experiments with aerosolized scopolamine have yielded encouraging results. Soman-exposed (841 mg/m3) guinea pigs who received only aerosolized scopolamine (0.25 mg/kg) 30 seconds later showed improved lung function and histopathology compared to non-scopolamine (saline)-treated animals (Perkins et al., 2011). Scopolamine treatment resulted in normalization of respiratory flow; AChE levels, cell death, total cell count, and protein levels in broncheoalveolar fluid; epithelial/ subepithelial inflammation; and alveolar edema.
There is no available clinical data demonstrating efficacy of scopolamine against OP intoxication in humans.
B. Link to clinical studies
Pregnancy, breastfeeding studies
-
The drug is compatible with nursing and is considered to be nonteratogenic (Class IV).
Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005 Oct;27(5):655-65. [PubMed Citation]
-
During a clinical study among women undergoing cesarean section treated with Transderm Scōp in conjunction with epidural anesthesia and opiate analgesia, no evidence of CNS depression was found in the newborns. There are no other adequate and well-controlled studies in pregnant women. Other than in the adjunctive use for delivery by cesarean section, Transderm Scōp should be used in pregnancy only if the potential benefit justifies the potential risk to the fetus (Class IV).
Product label:
TRANSDERM SCOP (scopalamine) patch, extended release
[Baxter Healthcare Corporation] Last
revised: September 2012 [DailyMed]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
The protective efficacy of the antimuscarinic agent scopolamine was evaluated against soman (o-pinacolyl methylphosphonofluoridate [GD])-induced respiratory toxicity in guinea pigs. Anesthetized animals were exposed to GD (841 mg/m3) by microinstillation inhalation exposure and treated 30 seconds later with endotracheally aerosolized scopolamine (0.25 mg/kg) and allowed to recover for 24 hours. Treatment with scopolamine significantly increased survival and reduced clinical signs of toxicity and body weight loss in GD-exposed animals. Analysis of bronchoalveolar lavage (BAL) fluid showed normalization of GD-induced increased cell death, total cell count, and protein following scopolamine treatment. The BAL fluid acetylcholinesterase and butyrylcholinesterase levels were also increased by scopolamine treatment. Respiratory dynamics parameters were normalized at 4 and 24 hours post-GD exposure in scopolamine-treated animals. Lung histology showed that scopolamine treatment reduced bronchial epithelial and subepithelial inflammation and multifocal alveolar septal edema. These results suggest that aerosolized scopolamine considerably protects against GD-induced respiratory toxicity.
Perkins MW, Pierre Z, Rezk P, Song J, Oguntayo S, Morthole V, Sciuto AM, Doctor BP, Nambiar MP. Protective effects of aerosolized scopolamine against soman-induced acute respiratory toxicity in guinea pigs. Int J Toxicol. 2011 Dec;30(6):639-49. [PubMed Citation]
-
There is a requirement to ensure that UK armed forces are provided with the best possible medical countermeasures to prevent or mitigate the effects of exposure to nerve agents. When pretreatments are under consideration, it is of particular importance to ensure that they do not in themselves give rise to adverse effects and do not exacerbate the effects of agent exposure. The present study was designed to address these considerations for a combination of physostigmine and scopolamine as a potential pretreatment regimen. Common marmosets were trained to perform a two-choice discrimination serial reversal task, and baseline data were collected. Subjects received a dose of either soman or sarin after 2 weeks of pretreatment with either saline or physostigmine and scopolamine via miniosmotic pump. No effects of physostigmine and scopolamine were seen on task accuracy or response rates. Neither accuracy of reversal performance nor number of responses made were significantly changed by administration of either soman or sarin subsequent to pretreatment with physostigmine/scopolamine. In the groups pretreated with saline, performance of the behavioural task, in terms of responses made, was virtually abolished on the day the OP was administered, but a significant increase in accuracy of performance was seen over the 2- to 14-day period following administration. A combination of physostigmine and scopolamine, which is known to protect against nerve-agent lethality, offers protection against the effects of soman and sarin on behavioural performance, as measured by a discrimination reversal task. The improved performance observed following nerve agent requires further investigation.
Muggleton NG, Bowditch AP, Crofts HS, Scott EA, Pearce PC. Assessment of a combination of physostigmine and scopolamine as pretreatment against the behavioural effects of organophosphates in the common marmoset (Callithrix jacchus). Psychopharmacology (Berl). 2003 Mar;166(3):212-20. [PubMed Citation]
-
Exposure to soman, a toxic organophosphate nerve agent, causes severe adverse effects and long term changes in the peripheral and central nervous systems. The goal of this study was to evaluate the ability of prophylactic treatments to block the deleterious effects associated with soman poisoning. scopolamine, a classical anticholinergic agent, or caramiphen, an anticonvulsant anticholinergic drug with anti-glutamatergic properties, in conjunction with pyridostigmine, a reversible cholinesterase inhibitor, were administered prior to soman (1xLD50). Both caramiphen and scopolamine dramatically attenuated the process of cell death as assessed by the binding of [3H]RoS-4864 to peripheral benzodiazepine receptors (omega3 sites) on microglia and astrocytes. In addition, caramiphen but not scopolamine, blocked the soman-evoked down-regulation of [3H]AMPA binding to forebrain membrane preparations. Moreover, cognitive tests utilizing the Morris water maze, examining learning and memory processes as well as reversal learning, demonstrated that caramiphen abolished the effects of soman intoxication on learning as early as the first trial day, while scopolamine exerted its effect commencing at the second day of training. Whereas the former drug completely prevented memory deficits, the latter exhibited partial protection. Both agents equally blocked the impairment of reversal learning. In addition, there is a significant correlation between behavioral parameters and [3H]RoS-4864 binding to forebrain membrane preparations of rats, which participated in these tests (r(21) = 0.66, P < 0.001; r(21) = 0.66, P < 0.001, -0.62, P < 0.002). These results demonstrate the beneficial use of drugs exhibiting both anti-cholinergic and anti-glutamatergic properties for the protection against changes in cognitive parameters caused by nerve agent poisoning. Moreover, agents such as caramiphen may eliminate the need for multiple drug therapy in organophosphate intoxications.
Raveh L, Weissman BA, Cohen G, Alkalay D, Rabinovitz I, Sonego H, Brandeis R. Caramiphen and scopolamine prevent soman-induced brain damage and cognitive dysfunction. Neurotoxicology. 2002 May;23(1):7-17. [PubMed Citation]
-
A total of eight anticholinergic drugs (aprophen, atropine, azaprophen, benactyzine, biperiden, procyclidine, scopolamine, trihexyphenidyl) were tested in parallel with diazepam for the ability to terminate seizure activity induced by the nerve agent soman. Guinea pigs, implanted with electrodes to record cortical electroencephalographic (EEG) activity, were pretreated with pyridostigmine Br (0.026 mg:kg, i.m.) and 30 min later challenged with 2×LD50 soman (56 microg:kg, s.c.) followed 1 min later by treatment with atropine SO4 (2 mg:kg, i.m.) and pralidoxime chloride (2-PAM Cl; 25 mg:kg, i.m.). All guinea pigs developed sustained seizure activity following this treatment. Dose-effect curves were determined for the ability of each drug to terminate seizure activity when anticonvulsant treatment was given either 5 or 40 min after seizure onset. Body weight gain and recovery of behavioral performance of a previously trained one-way avoidance task were measured after exposure. With the exception of atropine, all anticholinergic drugs were effective at lower doses than diazepam in terminating seizures when given 5 min after seizure onset; benactyzine, procyclidine and aprophen terminated seizures most rapidly while scopolamine, trihexyphenidyl, biperiden, and diazepam were significantly slower. When given 40 min after seizure onset, diazepam was the most potent compound tested, followed by scopolamine, benactyzine and biperiden; atropine was not effective when tested 40 min after seizure onset. For diazepam, the time to terminate the seizure was the same whether it was given at the 5- or 40-min delay. In contrast, most anticholinergics were significantly slower in terminating seizure activity when given at the 40-min delay relative to when they were given at the 5-min delay. Successful control of seizure activity, regardless of the drug, was predictive of survival of the lethal effects of nerve agent exposure, a more rapid behavioral recovery (body weight, avoidance performance) and greater protection from neuropathology. In contrast, failure of a drug treatment to terminate seizure activity was closely associated with an increased probability of acute (<24 h) and delayed (10-day survival) lethality, a slower behavioral recovery in survivors, and an increased incidence and degree of neuropathology.
McDonough JH Jr, Zoeffel LD, McMonagle J, Copeland TL, Smith CD, Shih TM. Anticonvulsant treatment of nerve agent seizures: Anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000 Jan;38(1):1-14. [PubMed Citation]
-
Six FDA approved, injectable compounds [benztropine (BZT); biperiden (BIP); dicyclomine (DCL); l-hyoscyamine (HYO); orphenadrine (ORP); scopolamine (SCP)] were each compared to diazepam (DZ, the standard) in male guinea pigs against ongoing soman-induced convulsive or sub-CV (CV/sub-CV) activity. Three trained graders concurrently assigned CV/sub-CV scores to each animal based on signs of intoxication at various times post-soman. Animals received (im) pyridostigmine (26 micrograms/kg) 30 min before soman (56 micrograms/kg; 2 x LD50), atropine (2 mg/kg) admixed with 2-PAM (25 mg/kg) at one min after soman, and the candidate drug preparation at 5.67 min post soman, a time when CV activity was assured. BIP and SCP were effective over dosage ranges between 10 and 0.3, and 1.0 and 0.13 mg/kg, respectively, while the other preparations were less effective at their respective maximum dosages. At the most effective dosages of SCP (1.0 mg/kg) and BIP (10 mg/kg), the CV/sub-CV scores were significantly lower (p < 0.05) than those of DZ. Only 33% survival was observed at each of two doses of ORP and one dose of HYO; therefore, no further testing was done with these compounds. Using freshly prepared solutions, DCL (up to 40 mg/kg) and BZT (up to 96 mg/kg) were tested with mixed results; DCL lowered lethality while BZT increased lethality. CV/sub-CV scores for the most effective dose of DCL and BZT were, however, lower than those of DZ. SCP is an antimuscarinic drug devoid of antinicotinic activity, while BIP possesses antimuscarinic, antinicotinic, antispasmodic and anti-N-methyl-D-aspartate activity. Recent evidence suggests that, in late stages of intoxication by nerve agents, noncholinergic, excitatory amino acid receptors may become involved and necessitate the use of a multi-action drug like BIP. The findings herein suggest that SCP and BIP are superior to DZ, but further studies are needed to determine which drug or drug class should be pursued in more advanced testing.
Anderson DR, Harris LW, Bowersox SL, Lennox WJ, Anders JC. Efficacy of injectable anticholinergic drugs against soman-induced convulsive/subconvulsive activity. Drug Chem Toxicol. 1994;17(2):139-48. [PubMed Citation]
-
Diazepam (DZ) and scopolamine (SCP) are known to be beneficial when each is used in combination with atropine (AT) + oxime therapy against intoxication by soman, but the efficacy of each might be expected to vary with the dosage of AT. Thus the therapeutic efficacy of SCP (5 doses; 0-0.86 mg/kg) versus DZ (5 doses; 0-5 mg/kg), when used in conjunction with AT (3 doses; 0.5-8 mg/kg) + 2-PAM (25 mg/kg) therapy, was tested in groups of pyridostigmine pretreated guinea pigs exposed to 1.6, 2.0, 2.5 or 3.2 LD50s of soman. Response surface methodology was employed to describe the relationship between lethality and the AT/DZ or AT/SCP dosages. Results show that within the indicated dose ranges used, the efficacy of SCP is not dependent on the presence of AT, whereas AT is needed for DZ to maintain the lowest probability of death. These findings suggest that in guinea pigs SCP could supplement AT or replace DZ as therapy against nerve agent intoxication.
Harris LW, Gennings C, Carter WH, Anderson DR, Lennox WJ, Bowersox SL, Solana RP. Efficacy comparison of scopolamine (SCP) and diazepam (DZ) against soman-induced lethality in guinea pigs. Drug Chem Toxicol. 1994;17(1):35-50. [PubMed Citation]
-
Exposure to high doses of organophosphorus nerve agents such as soman, even with carbamate pretreatment, produces a variety of toxic cholinergic signs, including secretions, convulsions and death. Evidence suggests that soman-induced convulsions may be associated with postexposure brain neuropathology. The purpose of this study was to investigate the pharmacologic mechanism of action of soman-induced convulsions and of anticonvulsant drugs. Various classes of compounds were evaluated for their efficacy in preventing soman-induced convulsions in rats pretreated with the oxime HI-6 to increase survival time, along with various doses of the test compounds (IM) either in the absence or presence of atropine sulfate (16 mg/kg, IM) 30 minutes prior to a soman challenge dose (180 micrograms/kg, SC; equivalent to 1.6 x LD50) that produced 100% convulsions. Without atropine sulfate, only tertiary anticholinergics (scopolamine, trihexyphenidyl, biperiden, benactyzine, benztropine, azaprophen and aprophen), caramiphen, carbetapentane and MK-801 were effective anticonvulsants. In the presence of atropine sulfate, the benzodiazepines (diazepam, midazolam, clonazepam, loprazolam and alprazolam), mecamylamine, flunarizine, diphenylhydantoin, clonidine, CGS 19755 and Organon 6370 studied were effective. We have examined the possibility that diazepam may exert some of its anticonvulsant effects through cholinergic mechanisms and found that a reduced release of ACh into synapses after diazepam and atropine treatment may account for diazepam's anticonvulsant activity against soman. We also found that at anticonvulsant doses biperiden and trihexyphenidyl each significantly reversed the effects of soman on striatal levels of DOPAC and HVA, the metabolites of dopamine, and have concluded that in addition to actions on muscarinic receptors, the anticonvulsant effects of these anticholinergics in soman poisoning may be partially related to their actions on the striatal dopaminergic system. These findings allow us to postulate that central muscarinic cholinergic mechanisms are primarily involved in eliciting the convulsions following exposure to soman and that subsequent recruitment of other excitatory neurotransmitter systems and loss of inhibitory control may be responsible for sustaining the convulsions and for producing the subsequent brain damage. Future studies to confirm these neuropharmacological mechanisms are proposed.
Shih, T-M., T.A. Koviak, B.R. Capacio. Anticonvulsants for poisoning by the organophosphorus compound soman: Pharmacological mechanisms. Neurosci Biobehav Rev. 1991 Fall;15(3):349-62. [PubMed Citation]
-
Pretreatment of nonhuman primates with physostigmine (Phy) and scopolamine or physostigmine and trihexyphenidyl 25 min before exposure to 2 LD50 soman im resulted in complete survival without convulsions or loss of consciousness. When identically pretreated animals were challenged with 5 LD50s of soman followed by atropine and 2-PAM therapy 1 min later, all animals experienced a loss of consciousness for approximately 10 min followed by functional recovery within an additional 20 min. These findings indicated that a pretreatment regimen composed of Phy and cholinolytic is capable of protecting primates from an absolute lethal dose of soman with rapid recovery from incapacitation.
von Bredow J, Corcoran K, Maitland G, Kaminskis A, Adams N, Wade J. Efficacy evaluation of physostigmine and anticholinergic adjuncts as a pretreatment for nerve agent intoxication. Fundam Appl Toxicol. 1991 Nov;17(4):782-9. [PubMed Citation]
Pregnant animal studies
-
Teratogenic studies were performed in pregnant rats and rabbits with scopolamine hydrobromide administered by daily intravenous injection. No adverse effects were recorded in rats. Scopolamine hydrobromide has been shown to have a marginal embryotoxic effect in rabbits when administered by daily intravenous injection at doses producing plasma levels approximately 100 times the level achieved in humans using a transdermal system.
Product label:
TRANSDERM SCOP (scopalamine) patch, extended release
[Baxter Healthcare Corporation] Last
revised: September 2012 [DailyMed]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
The objective was to develop a microdialysis set-up to measure the concentration-time course of scopolamine in the interstitium of subcutaneous adipose tissue. Six healthy male volunteers were eligible for data analysis. Subjects received 0.5 mg scopolamine as a 15-minute intravenous infusion. Microdialysis samples from interstitial space fluid of subcutaneous adipose tissue and blood samples were taken at predefined intervals over a period of 360 minutes. Scopolamine concentrations were measured by liquid chromatography-tandem mass spectrometry (LC-MS-MS). High inter-individual variability was observed in all pharmacokinetic parameters. The mean peak serum concentration (C(max)) of 6.5 +/- 3.9 ng/ml (data in mean +/- SD) was attained after 15 +/- 3 minutes (t(max)), whereas in dialysate, a mean peak concentration of 2.7 +/- 1.7 ng/ml was measured after 27 +/- 8 minutes. The ratio of the area under the concentration versus time curve from 0-360 min for interstitium (AUC(interstitium 0-360 min0) to the AUC for serum (AUC(serum 0-360 min)) was 0.96 +/- 0.7. The elimination half-life of scopolamine was 121 +/- 85 minutes in serum and 166 +/- 117 minutes in dialysate. Values for total clearance and volume of distribution in serum were 99.1 +/- 35.0 1/h and 188 +/- 76 1, respectively. In the present study, we were able to define a microdialysis set-up, which allows for the measurement of scopolamine concentrations in target tissues. In addition, we demonstrated that the concentrations of scopolamine in subcutaneous adipose tissue resemble closely the concentration-time course in serum of healthy volunteers.
Stetina PM, Madai B, Kulemann V, Kirch W, Joukhadar C. Pharmacokinetics of scopolamine in serum and subcutaneous adipose tissue in healthy volunteers. Int J Clin Pharmacol Ther. 2005 Mar;43(3):134-9. [PubMed Citation]
-
The effects of subcutaneously administered scopolamine on quantitative electroencephalogram (qEEG) and cognitive performance were evaluated and correlated with pharmacokinetic parameters in a randomized, double-blind placebo-controlled crossover study of 10 healthy male volunteers. Changes in qEEG and cognition were determined for 8 hours after drug administration. Scopolamine produced dose- and time-dependent impairments of attention and memory and a time-dependent increase in delta power (1.25-4.50 Hz) and a decrease in fast alpha power (9.75-12.50 Hz) on qEEG compared with placebo. Maximum serum concentrations of scopolamine occurred 10 to 30 minutes after drug administration. Mean peak serum concentrations (free base) were 3.27, 8.99, and 18.81 ng/mL after administration of 0.4, 0.6 mg, and 0.8 mg scopolamine, respectively. Elimination half-life was approximately 220 minutes. The findings indicate temporary changes in qEEG and psychometric tests, and support the possible use of such a testing model for impaired cognitive functions such as age-related memory disturbances.
Ebert U, Siepmann M, Oertel R, Wesnes KA, Kirch W. Pharmacokinetics and pharmacodynamics of scopolamine after subcutaneous administration. J Clin Pharmacol. 1998 Aug;38(8):720-6. [PubMed Citation]
-
The alkaloid L-(-)-scopolamine [L-(-)-hyoscine] competitively inhibits muscarinic receptors for acetylcholine and acts as a nonselective muscarinic antagonist, producing both peripheral antimuscarinic properties and central sedative, antiemetic, and amnestic effects. The parasympatholytic scopolamine, structurally very similar to atropine (racemate of hyoscyamine), is used in conditions requiring decreased parasympathetic activity, primarily for its effect on the eye, gastrointestinal tract, heart, and salivary and bronchial secretion glands, and in special circumstances for a CNS action. Therefore, scopolamine is most suitable for premedication before anesthesia and for antiemetic effects. This alkaloid is the most effective single agent to prevent motion sickness. Scopolamine was the first drug to be made commercially available in a transdermal therapeutic system (TTS-patch) delivering alkaloid. Recently, pharmacokinetic data on scopolamine in different biologic matrices were obtained most efficiently using liquid chromatographic-tandem mass spectrometric (LC-MS/MS) or gas chromatography online coupled to mass spectrometry. Pharmacokinetic parameters are dependent on the dosage form (oral dose, tablets; parenteral application; IV infusion; SC and IM injection). Scopolamine has a limited bioavailability if orally administered. The maximum drug concentration occurs approximately 0.5 hours after oral administration. Because only 2.6% of nonmetabolized L-(-)-scopolamine is excreted in urine, a first-pass metabolism is suggested to occur after oral administration of scopolamine. Because of its short half-life in plasma and dose-dependent adverse effects (in particular hallucinations and the less serious reactions, eg, vertigo, dry mouth, drowsiness), the clinical use of scopolamine administered orally or parenterally is limited. To minimize the relatively high incidence of side effects, the transdermal dosage form has been developed. The commercially available TTS-patch contains a 1.5-mg drug reservoir and a priming dose (140 microg) to reach the steady-state concentration of scopolamine quickly. The patch releases 0.5 mg alkaloid over a period of 3 days (releasing rate 5 microg/h). Following the transdermal application of scopolamine, the plasma concentrations of the drug indicate major interindividual variations. Peak plasma concentrations (Cmax) of approximately 100 pg/mL (range 11-240 pg/mL) of the alkaloid are reached after about 8 hours and achieve steady state. During a period of 72 hours the plaster releases scopolamine, so constantly high plasma levels (concentration range 56-245 pg/mL) are obtained, followed by a plateau of urinary scopolamine excretion. Although scopolamine has been used in clinical practice for many years, data concerning its metabolism and the renal excretion in man are limited. After incubation with beta-glucuronidase and sulfatase, the recovery of scopolamine in human urine increased from 3% to approximately 30% of the drug dose (intravenously administered). According to these results from enzymatic hydrolysis of scopolamine metabolites, the glucuronide conjugation of scopolamine could be the relevant pathway in healthy volunteers. However, scopolamine metabolism in man has not been verified stringently. An elucidation of the chemical structures of the metabolites extracted from human urine is still lacking. Scopolamine has been shown to undergo an oxidative demethylation during incubation with CYP3A (cytochrome P-450 subfamily). To inhibit the CYP3A located in the intestinal mucosa, components of grapefruit juice are very suitable. When scopolamine was administered together with 150 mL grapefruit juice, the alkaloid concentrations continued to increase, resulting in an evident prolongation of tmax (59.5 +/- 25.0 minutes; P < 0.001). The AUC0-24h values of scopolamine were higher during the grapefruit juice period. They reached approximately 142% of the values associated with the control group (P < 0.005). Consequently, the related absolute bioavailabilities (range 6% to 37%) were significantly higher than the corresponding values of the drug orally administered together with water (range 3% to 27%). The effect of the alkaloid on quantitative electroencephalogram (qEEG) and cognitive performance correlated with pharmacokinetics was shown in studies with healthy volunteers. From pharmacokinetic-pharmacodynamic modeling techniques, a direct correlation between serum concentrations of scopolamine and changes in total power in alpha-frequency band (EEG) in healthy volunteers was provided. In conclusion, scopolamine is used for premedication in anesthesia and for the prevention of nausea and vomiting associated with motion sickness. Pharmacokinetics and pharmacodynamics of scopolamine depend on the dosage form. Effects on different cognitive functions have been extensively documented.
Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005 Oct;27(5):655-65. [PubMed Citation]
Pregnancy
-
The alkaloid readily crosses the placenta. Therefore, scopolamine should be administered to pregnant women only under observation.
Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005 Oct;27(5):655-65. [PubMed Citation]
Animal
-
A rapid and sensitive method is described for the determination of scopolamine and its metabolites in rat urine by combining liquid chromatography and tandem mass spectrometry (LC-MS/MS). Various extraction techniques (free fraction, acid hydrolyses and enzyme hydrolyses) and their comparison were carried out for investigation of the metabolism of scopolamine. After extraction procedure, the pretreated samples were injected into a reversed-phase C18 column with mobile phase of methanol/ ammonium acetate (2mM, adjusted to pH 3.5 with formic acid) (70:30, v/v) and detected by an on-line MS/MS system. Identification and structural elucidation of the metabolites were performed by comparing their changes in molecular masses (DeltaM), retention-times and full scan MS(n) spectra with those of the parent drug. The results revealed that at least 18 metabolites (norscopine, scopine, tropic acid, aponorscopolamine, aposcopolamine, norscopolamine, hydroxyscopolamine, hydroxyscopolamine N-oxide, p-hydroxy-m-methoxyscopolamine, trihydroxyscopolamine, dihydroxy-methoxyscopolamine, hydroxyl-dimethoxyscopolamine, glucuronide conjugates and sulfate conjugates of norscopolamine, hydroxyscopolamine and the parent drug) and the parent drug existed in urine after ingesting 55mg/kg scopolamine to healthy rats. Hydroxyscopolamine, p-hydroxy-m-methoxyscopolamine and the parent drug were detected in rat urine for up to 106 h after ingestion of scopolamine.
Chen H, Chen Y, Wang H, Du P, Han F, Zhang H. Analysis of scopolamine and its eighteen metabolites in rat urine by liquid chromatography-tandem mass spectrometry. Talanta. 2005 Oct 31;67(5):984-91. [PubMed Citation]
-
In vivo and in vitro metabolism of scopolamine is investigated using a highly specific and sensitive liquid chromatography-mass spectrometry (LC-MSn) method. Feces, urine, and plasma samples are collected individually after ingestion of 55 mg/kg scopolamine by healthy rats. Rat feces and urine samples are cleaned up by a liquid-liquid extraction and a solid-phase extraction procedure (C18 cartridges), respectively. Methanol is added to rat plasma samples to precipitate plasma proteins. Scopolamine is incubated with homogenized liver and intestinal flora of rats in vitro, respectively. The metabolites in the incubating solution are extracted with ethyl acetate. Then these pretreated samples are injected into a reversed-phase C18 column with mobile phase of methanol-ammonium acetate (2 mM, adjusted to pH 3.5 with formic acid) (70:30, v/v) and detected by an on-line MSn system. Identification and structural elucidation of the metabolites are performed by comparing their changes in molecular masses (DeltaM), retention-times and full scan MSn spectra with those of the parent drug. The results reveal that at least 8 metabolites (norscopine, scopine, tropic acid, aponorscopolamine, aposcopolamine, norscopolamine, hydroxyscopolamine, and hydroxyscopolamine N-oxide) and the parent drug exist in feces after administering 55 mg/kg scopolamine to healthy rats. Three new metabolites (tetrahydroxyscopolamine, trihydroxy-methoxyscopolamine, and dihydroxy-dimethoxyscopolamine) are identified in rat urine. Seven metabolites (norscopine, scopine, tropic acid, aponorscopolamine, aposcopolamine, norscopolamine, and hydroxyscopolamine) and the parent drug are detected in rat plasma. Only 1 hydrolyzed metabolite (scopine) is found in the rat intestinal flora incubation mixture, and 2 metabolites (aposcopolamine and norscopolamine) are identified in the homogenized liver incubation mixture.
Chen H, Chen Y, Du P, Han F. Liquid chromatography-electrospray ionization ion trap mass spectrometry for analysis of in vivo and in vitro metabolites of scopolamine in rats. J Chromatogr Sci. 2008 Jan;46(1):74-80. [PubMed Citation]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
Parenteral dose: the usual adult IM, IV, or subcutaneous dose of scopolamine hydrobromide is 0.3-0.65 mg; if necessary, these doses may be repeated 3 or 4 times daily. Alternatively, adult parenteral doses of scopolamine hydrobromide of 0.2-1 mg have been suggested for antiemetic effect, 0.2-0.6 mg for inhibition of salivation, 0.32-0.65 mg for amnestic effect, or 0.6 mg for sedation or tranquilization.
Motion sickness: the usual adult dose of scopolamine transdermal system is one system programmed to deliver approximately 1 mg of scopolamine over 72 hours.
Postoperative nausea and vomiting: the transdermal scopolamine system should be applied the evening before scheduled surgery. The transdermal system should remain in place for 24 hours following surgery, then removed and discarded.
The usual oral dose range of scopolamine hydrobromide soluble tablets is 0.4-0.8 mg. Motion sickness: 0.25-0.8 mg of the drug may be administered 1 hour before exposure to motion; subsequent doses of 0.25-0.8 mg may be given 3 times daily as needed and as tolerated.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
Children (FDA)
Parenteral dose: the usual pediatric IM, IV, or subcutaneous dose of scopolamine hydrobromide is 0.006 mg/kg (6 mcg/kg) or 0.2 mg/m2.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
The safety and effectiveness of Transderm Scōp in children has not been established. Children are particularly susceptible to the side effects of belladonna alkaloids. Transderm Scōp should not be used in children because it is not known whether this system will release an amount of scopolamine that could produce serious adverse effects in children.
Product label:
TRANSDERM SCOP (scopalamine) patch, extended release
[Baxter Healthcare Corporation] Last
revised: September 2012 [DailyMed]
Pregnancy (FDA)
Cesarean section: if the transdermal scopolamine system is used prophylactically in patients undergoing cesarean section, the system should be applied one hour prior to surgery to minimize exposure of the infant to the drug. The transdermal system should remain in place for 24 hours following surgery, then removed and discarded.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
Pregnancy category C
Scopolamine administered parenterally at higher doses than the dose delivered by Transderm Scōp does not increase the duration of labor, nor does it affect uterine contractions. Scopolamine does cross the placenta.
Product label:
TRANSDERM SCOP (scopalamine) patch, extended release
[Baxter Healthcare Corporation] Last
revised: September 2012 [DailyMed]
Nursing Mothers (FDA)
Because scopolamine is excreted in human milk, caution should be exercised when Transderm Scōp is administered to a nursing woman.
Product label:
TRANSDERM SCOP (scopalamine) patch, extended release
[Baxter Healthcare Corporation] Last
revised: September 2012 [DailyMed]
6. Current available formulations/shelf life
Formulation
Scopolamine topical transdermal system approximately 1 mg/72 hours (1.5 mg/2.5 cm2)
Scopolamine Hydrobromide tablets, soluble 0.4 mg
Scopolamine Hydrobromide bulk powder
Scopolamine Hydrobromide parenteral injection 0.4 mg/mL
Scopolamine Hydrobromide (EENT) ophthalmic solution 0.25%
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
Shelf life
Stability
-
When admixed in the same syringe, scopolamine hydrobromide injection is reported to be physically compatible for at least 15 minutes with the following injections: atropine sulfate, butorphanol tartrate, chlorpromazine hydrochloride, dimenhydrinate, diphenhydramine hydrochloride, droperidol, fentanyl citrate, glycopyrrolate, hydromorphone hydrochloride, hydroxyzine hydrochloride, meperidine hydrochloride, metoclopramide, morphine sulfate, concentrated opium alkaloids hydrochlorides, pentazocine lactate, pentobarbital sodium, perphenazine, prochlorperazine edisylate, promazine hydrochloride, promethazine hydrochloride, or thiopental sodium. Since the compatibility of these and other admixtures with scopolamine hydrobromide injection depends on several factors (e.g., concentration of the drugs, resulting pH, temperature), specialized references should be consulted for specific compatibility information. A haze may form within 1 hour when scopolamine hydrobromide injection is mixed with methohexital sodium solutions.
-
Scopolamine is readily racemized in the presence of dilute alkali. Scopolamine hydrobromide solutions are incompatible with alkalies.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
Storage
The commercially available transdermal system of scopolamine (Transderm Scop) should be stored at controlled room temperature between 20-25°C. Scopolamine hydrobromide should be stored in tight, light-resistant containers. Scopolamine hydrobromide injections should be stored in light-resistant, single-dose or multiple-dose containers, preferably of USP Type I glass, at 15-30°C; freezing of the injections should be avoided. Commercially available scopolamine hydrobromide soluble tablets should be stored at controlled room temperature (15-30°C).
Scopolamine hydrobromide ophthalmic solution should be stored in tight containers at a temperature less than 40°C, preferably between 15-30°C; freezing should be avoided.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
The effect of oral anticholinergic drugs has been limited in the treatment of drooling. Transdermal scopolamine (1.5 mg/2.5 cm2) offers advantages. One single application is considered to render a stable serum concentration for 3 days. A distinct reduction of basal salivation was demonstrated in an open trial of six healthy volunteers. Eighteen mentally retarded patients with a drooling problem were studied in a double-blind, placebo-controlled cross-over trial. The therapeutic effect of transdermal scopolamine was assessed by a visual analogue scale. Three patients dropped out due to loss of the system. In the remaining 15 patients, the active drug caused a reduction of drooling which was significant in the period from 24 to 72 h. There were few and slight objective signs of unwanted effects. Scopoderm may cause drowsiness and affect tooth health. The management of drooling should primarily be focused on the cause. Sensomotor training is often valuable in cerebral palsy. Factors such as nasal obstruction, mucosal irritation, and drug-induced parkinsonism should be given attention. Sometimes, however, a temporary symptomatic treatment is indicated, for example on special occasions or in order to cure perioral skin lesions. Transdermal scopolamine may offer this possibility.
Brodtkorb E, Wyzocka-Bakowska MM, Lillevold PE, Sandvik L, Saunte C, Hestnes A. Transdermal scopolamine in drooling. J Ment Defic Res. 1988 Jun;32 ( Pt 3):233-7. [PubMed Citation]
-
Scopoderm transdermal therapeutic system (TTS) is applied to prevent nausea and vomiting associated with motion sickness. Dry mouth is the most common side effect, appearing in up to two thirds of the patients treated. We have used this side effect to the benefit of patients with sialorrhea or with difficulties swallowing normally secreted amounts of saliva. More than 100 patients with tumors of the aerodigestive tract before and after surgery, and patients after parotidectomies, after tracheotomies, with peritonsillar abscesses, tonsillitis and pharyngitis, and neurologic disorders were thus treated. Reduced secretion of saliva was seen in 50% to 100% of the treatment groups. Other side effects were minimal and we recommend the use of scopoderm TTS for reduction of salivary flow.
Talmi YP, Finkelstein Y, Zohar Y. Reduction of salivary flow with transdermal scopolamine: a four-year experience. Otolaryngol Head Neck Surg. 1990 Oct;103(4):615-8. [PubMed Citation]
Children
-
Sialorrhea (drooling): 1.5 mg patch (scopolamine-transdermal) every 3 days.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1480-1
-
Transdermal scopolamine patches (1.5 mg) were used to control drooling in a two-year-old boy with severe spastic quadriparetic cerebral palsy and developmental delay. He responded well to the continuous scopolamine therapy, with a decrease in drooling, and a secondary decrease in respiratory distress and frequency of suctioning. No significant side-effects were noted.
Siegel LK, Klingbeil MA. Control of drooling with transdermal scopolamine in a child with cerebral palsy. Dev Med Child Neurol. 1991 Nov;33(11):1013-4. [PubMed Citation]
8. Route of Administration/Monitoring
-
Scopolamine hydrobromide is administered orally, by IM, direct IV, or as subcutaneous injection. Scopolamine is administered percutaneously by topical application of a transdermal system.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
9. Adverse effects
-
Although transdermal scopolamine has been associated with fewer adverse effects than orally administered scopolamine hydrobromide, adverse systemic effects have occurred following application to the skin of the transdermal system.
-
The most frequent adverse effect of transdermally administered scopolamine is dry mouth, occurring in about 67 or 29% of patients receiving the drug for prevention of motion sickness or postoperative nausea and vomiting, respectively.
-
Drowsiness was reported in about 17% of patients in clinical studies using the transdermal system for prevention of motion sickness. Dizziness was reported by about 12% of patients in clinical studies receiving the scopolamine transdermal system perioperatively for prevention of nausea and vomiting.
-
Transient impairment of ocular accommodation, including blurred vision and mydriasis, has also occurred following transdermal application of scopolamine.
-
Scopolamine-induced cycloplegia manifested as fixed, dilated pupils has occurred following transdermal application of the drug; cycloplegia may be bilateral or unilateral and has persisted for 48 hours or longer in some patients. It has been suggested that unilateral cycloplegia probably results from touching the eye or contact lens with scopolamine-contaminated fingers after handling the transdermal system. Since neurogenic mydriasis (e.g., caused by head injury, tumor) responds (pupillary constriction) to local instillation of a parasympathomimetic, failure of the pupillary sphincter to constrict within 30 minutes after instillation of several drops of a 0.5 or 1% ophthalmic solution of pilocarpine, in the absence of local ocular injury (traumatic iridoplegia) or increased intraocular pressure (angle-closure glaucoma), usually indicates chemically induced (e.g., scopolamine) blockade of the sphincter.
-
Transdermal application of scopolamine also has precipitated angle-closure glaucoma in several patients; at least one patient has required surgery.
-
Ocular dryness or pruritus or conjunctival injection of eyes has occasionally occurred following transdermal administration of the drug.
-
Less frequently, adverse CNS effects, including disorientation, memory disturbances, dizziness, restlessness, giddiness, hallucinations, delirium, and confusion, have occurred following transdermal administration of scopolamine.
-
Signs and symptoms of acute toxic psychosis, including confusion, agitation, rambling and pressured speech, visual hallucinations, paranoid behavior, and delusions, have occurred in a few patients receiving transdermal scopolamine; psychotic signs and symptoms resolved within 3 hours after IM administration of physostigmine in one patient and within 24-36 hours after removal of the transdermal system in several others.
-
Drug withdrawal symptoms, including nausea, vomiting, headache, dizziness, and disturbances of equilibrium, have been reported in some patients following discontinuance of the transdermal system. Such symptoms usually do not appear until 24 hours or more after the transdermal system has been removed. Some of these symptoms may be related to adaptation to a motion-free environment from an environment in motion.
-
More serious symptoms, including muscle weakness, bradycardia, and hypotension, also may occur following discontinuance of the transdermal system. It is necessary to distinguish the signs and symptoms of withdrawal following discontinuance of the transdermal scopolamine system from scopolamine overdosage. Although mental confusion and dizziness may be observed with both acute toxicity and withdrawal, patients with withdrawal symptoms typically exhibit signs and symptoms of bradycardia, headache, nausea, abdominal cramps, and sweating, while tachyarrhythmias, dry skin, and decreased bowel sounds are suggestive of anticholinergic toxicity.
-
Delayed allergic contact dermatitis, manifested as pruritus and erythema at the site of application, has occurred with transdermal administration of scopolamine.
-
The reaction developed 1.5-15 months after initiation of long-term transdermal therapy, subsided within 2 weeks after removal of the transdermal system, and was attributed to scopolamine rather than to components of the transdermal system.
-
Rash and erythema also have occurred occasionally following transdermal administration of the drug.
-
Difficulty in urinating and transient changes in heart rate have occurred occasionally following transdermal administration of scopolamine. However, a causal relationship to transdermal scopolamine has not been established.
-
Safety and efficacy of scopolamine hydrobromide soluble tablets or the scopolamine transdermal system in children have not been established. The manufacturer states that scopolamine transdermal system should not be used in children since it is not known whether the system might release an amount of drug that could cause serious adverse effects in the child. In addition, the manufacturer warns that children are particularly susceptible to adverse effects of scopolamine.
-
The manufacturer of transdermal scopolamine states that if symptoms of overdosage occur, an adequate airway should be established and cardiac and respiratory support should be instituted, followed by rapid removal of all transdermal systems from the skin and/or mouth. If there is evidence of ingestion of the transdermal system, gastric lavage, endoscopic removal of swallowed patches, or administration of activated charcoal should be considered depending on the clinical situation. In addition, appropriate symptomatic treatment should be initiated.
-
Scopolamine hydrobromide may cause increased intraocular pressure.
-
Prolonged administration of scopolamine hydrobromide to the eye may cause local irritation characterized by follicular conjunctivitis, vascular congestion and edema, exudate, and an eczematoid dermatitis.
-
Topical application of scopolamine hydrobromide to the eye may cause adverse systemic effects. Following topical application to the eye of several drops of 1% scopolamine hydrobromide, signs and symptoms of confusional psychosis, including restlessness, mental confusion, hallucinations, incoherence, violent behavior, amnesia, unconsciousness, spastic extremities, vomiting, and urinary incontinence, have occurred in several patients.
-
Somnolence has also been reported.
-
Systemic toxicity manifested as systemic antimuscarinic effects has also occurred in a patient following topical application to the eye of a 0.25% solution of the drug every 10 minutes for 2 hours prior to retinal detachment surgery.
-
Scopolamine hydrobromide ophthalmic solution is contraindicated in patients with known or suspected angle-closure glaucoma.
-
The possibility of undiagnosed glaucoma in geriatric patients should be considered.
-
Scopolamine hydrobromide should be used with extreme caution, if at all, in infants and small children.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
10. Contraindication(s)
-
Scopolamine generally is contraindicated in patients with glaucoma (i.e., angle-closure), pyloric obstruction, or urinary bladder neck obstruction.
-
Scopolamine generally also is contraindicated in patients with tachycardia secondary to cardiac insufficiency or thyrotoxicosis and in those with paralytic ileus.
-
The manufacturer states that scopolamine hydrobromide soluble tablets are contraindicated in patients with prostatic hypertrophy or impaired renal or hepatic function.
-
Scopolamine is contraindicated in patients who are hypersensitive to the drug, to any other belladonna alkaloid, or to any ingredient or component in the formulation or administration system.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904 Obtained online through https://about.medicinescomplete.com/#/browse/ahfs
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations-
Testing against atropine; scopolamine may have better CNS penetration with a lower profile of severe side effects, Atropine plus pralidoxime with scopolamine add-on
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
Developing country; find/join a research group, methodology to be identified
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
15. Study-related ethical concerns
— including review panel recommendations-
Resolve ethical issues.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
16. Global regulatory status
U.S.
-
Scopolamine is used principally for the prevention of nausea and vomiting induced by motion or recovery from anesthesia and surgery. The drug is also used as an adjunct to anesthesia. Scopolamine's usefulness in chronic forms of therapy (e.g., in the adjunctive treatment of peptic ulcer disease) is generally limited by its adverse effects, especially CNS effects.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
17. Other potentially useful information
-
Octanol/Water Partition Coefficient: log Kow = 0.98
HSDB. Scopolamine
18. Publications
Anderson DR, Harris LW, Bowersox SL, Lennox WJ, Anders JC. Efficacy of injectable anticholinergic drugs against soman-induced convulsive/subconvulsive activity. Drug Chem Toxicol. 1994;17(2):139-48. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Brodtkorb E, Wyzocka-Bakowska MM, Lillevold PE, Sandvik L, Saunte C, Hestnes A. Transdermal scopolamine in drooling. J Ment Defic Res. 1988 Jun;32 ( Pt 3):233-7. [PubMed Citation]
Brunton LL, Chabner BA, Knollmann BC (eds.) Goodman & Gilman's The Pharmacological Basis of Therapeutics (12th Ed.). McGraw-Hill Medical, New York, NY. (2011) p.226-230
Chen H, Chen Y, Wang H, Du P, Han F, Zhang H. Analysis of scopolamine and its eighteen metabolites in rat urine by liquid chromatography-tandem mass spectrometry. Talanta. 2005 Oct 31;67(5):984-91. [PubMed Citation]
Chen H, Chen Y, Du P, Han F. Liquid chromatography-electrospray ionization ion trap mass spectrometry for analysis of in vivo and in vitro metabolites of scopolamine in rats. J Chromatogr Sci. 2008 Jan;46(1):74-80. [PubMed Citation]
Ebert U, Siepmann M, Oertel R, Wesnes KA, Kirch W. Pharmacokinetics and pharmacodynamics of scopolamine after subcutaneous administration. J Clin Pharmacol. 1998 Aug;38(8):720-6. [PubMed Citation]
Harris LW, Gennings C, Carter WH, Anderson DR, Lennox WJ, Bowersox SL, Solana RP. Efficacy comparison of scopolamine (SCP) and diazepam (DZ) against soman-induced lethality in guinea pigs. Drug Chem Toxicol. 1994;17(1):35-50. [PubMed Citation]
HSDB. Scopolamine
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.1480-1
Marrs TC, Maynard RL, Sidell FR. (ed.s). Chemical warfare agents toxicology and treatment 2nd ed. John Wiley and Sons Ltd. (2007) p.258
McDonough JH Jr, Zoeffel LD, McMonagle J, Copeland TL, Smith CD, Shih TM. Anticonvulsant treatment of nerve agent seizures: Anticholinergics versus diazepam in soman-intoxicated guinea pigs. Epilepsy Res. 2000 Jan;38(1):1-14. [PubMed Citation]
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1299-1303, 2903-2904
Muggleton NG, Bowditch AP, Crofts HS, Scott EA, Pearce PC. Assessment of a combination of physostigmine and scopolamine as pretreatment against the behavioural effects of organophosphates in the common marmoset (Callithrix jacchus). Psychopharmacology (Berl). 2003 Mar;166(3):212-20. [PubMed Citation]
Perkins MW, Pierre Z, Rezk P, Song J, Oguntayo S, Morthole V, Sciuto AM, Doctor BP, Nambiar MP. Protective effects of aerosolized scopolamine against soman-induced acute respiratory toxicity in guinea pigs. Int J Toxicol. 2011 Dec;30(6):639-49. [PubMed Citation]
Product label:
TRANSDERM SCOP (scopalamine) patch, extended release
[Baxter Healthcare Corporation] Last
revised: September 2012 [DailyMed]
Raveh L, Weissman BA, Cohen G, Alkalay D, Rabinovitz I, Sonego H, Brandeis R. Caramiphen and scopolamine prevent soman-induced brain damage and cognitive dysfunction. Neurotoxicology. 2002 May;23(1):7-17. [PubMed Citation]
Renner UD, Oertel R, Kirch W. Pharmacokinetics and pharmacodynamics in clinical use of scopolamine. Ther Drug Monit. 2005 Oct;27(5):655-65. [PubMed Citation]
Shih, T-M., T.A. Koviak, B.R. Capacio. Anticonvulsants for poisoning by the organophosphorus compound soman: Pharmacological mechanisms. Neurosci Biobehav Rev. 1991 Fall;15(3):349-62. [PubMed Citation]
Siegel LK, Klingbeil MA. Control of drooling with transdermal scopolamine in a child with cerebral palsy. Dev Med Child Neurol. 1991 Nov;33(11):1013-4. [PubMed Citation]
Stetina PM, Madai B, Kulemann V, Kirch W, Joukhadar C. Pharmacokinetics of scopolamine in serum and subcutaneous adipose tissue in healthy volunteers. Int J Clin Pharmacol Ther. 2005 Mar;43(3):134-9. [PubMed Citation]
Talmi YP, Finkelstein Y, Zohar Y. Reduction of salivary flow with transdermal scopolamine: a four-year experience. Otolaryngol Head Neck Surg. 1990 Oct;103(4):615-8. [PubMed Citation]
von Bredow J, Corcoran K, Maitland G, Kaminskis A, Adams N, Wade J. Efficacy evaluation of physostigmine and anticholinergic adjuncts as a pretreatment for nerve agent intoxication. Fundam Appl Toxicol. 1991 Nov;17(4):782-9. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
