You are here: Home > Medical Countermeasures Database > Sodium Bicarbonate
Sodium Bicarbonate - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Sodium Bicarbonate
2. Chemical Defense therapeutic area(s)
— including key possible usesSodium bicarbonate is a nonspecific antidote effective in the treatment of a variety of poisonings by means of a number of distinct mechanisms. Nebulized sodium bicarbonate serves as a useful adjunct in the treatment of patients with pulmonary injuries resulting from phosgene and chlorine gas inhalation exposures. Inhaled sodium bicarbonate neutralizes the hydrochloric acid that is formed when phosgene or chlorine gas react with the water in the respiratory tree.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
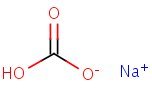
HSDB. Sodium Bicarbonate
Mechanism of action
-
Intravenous sodium bicarbonate therapy increases plasma bicarbonate, buffers excess hydrogen ion concentration, raises blood pH and reverses the clinical manifestations of acidosis. Sodium bicarbonate in water dissociates to provide sodium (Na+) and bicarbonate (HCO3-) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Bicarbonate (HCO3-) is a normal constituent of body fluids and the normal plasma level ranges from 24 to 31 mEq/liter. Plasma concentration is regulated by the kidney through acidification of the urine when there is a deficit or by alkalinization of the urine when there is an excess. Bicarbonate anion is considered "labile" since at a proper concentration of hydrogen ion (H+) it may be converted to carbonic acid (H2CO3) and thence to its volatile form, carbon dioxide (CO2) excreted by the lung.
Product label Sodium Bicarbonate injection, solution [Hospira, Inc.] Last revised: August 2012 [DailyMed]
-
Nebulized sodium bicarbonate may be another adjunctive treatment for chlorine pulmonary exposures. Theoretically, inhaled bicarbonate can neutralize hypochlorous and hydrochloric acids, decreasing severity of lung injury.
Jones R, Wills B, Kang C. Chlorine gas: an evolving hazardous material threat and unconventional weapon. West J Emerg Med 2010; 11 (2):151-6. [PubMed Citation]
Summary of clinical and non-clinical studies
Chlorine gas, a toxic pulmonary irritant, has been used as a chemical warfare agent by the Germans during World War I, and most recently, by insurgents during the Iraq War. Chlorine gas is more commonly released in industrial or residential settings, such as through swimming pool or home-cleaning preparations (Bosse, 1994). Symptoms of chlorine gas inhalation can occur very rapidly and range from dyspnea and nonproductive cough to pulmonary edema and acute respiratory distress syndrome (ARDS) (Chisholm et al., 1989; Vinsel, 1990). Although there is no specific antidote for treatment of chlorine gas exposure, nebulized sodium bicarbonate (NSB) has demonstrated efficacy and safety as an adjunctive treatment for chlorine gas-related pulmonary injuries. NSB neutralizes the hypochlorous and hydrochloric acids that form when chlorine gas comes into contact with water in the airways (Vinsel, 1990; Jones et al., 2010). In a sheep model, 4% inhaled NSB improved arterial blood gas values after chlorine gas inhalation without altering microscopic pathology (Chisholm et al., 1989). After brief exposure to chlorine, three patients who presented to the emergency department (ED) with mild symptoms were given 4 ml of a 3.75% NSB solution (Vinsel, 1990). The effects of NSB treatment were immediate and long-lasting, with complete relief of symptoms within 24 hours. No clinical deterioration to sodium bicarbonate therapy was observed. In a larger retrospective study (n=86) on the treatment of chlorine gas inhalation with 5% NSB, the clinical condition of 53 patients clearly improved upon release from the ED (Bosse, 1994). A small number of patients (n=17) were admitted to the hospital, receiving either a one-time dose of NSB or concomitant NSB therapy with inhaled beta-agonists and intravenous steroids. Gradual improvement occurred in all admitted patients with no observation of adverse effects. Similar therapeutic benefits from NSB administration have been observed in other studies (Douidar, 1997; Aslan et al., 2006; Howard et al., 2007; Cevik, 2009). Whether chlorine gas is released accidentally as a byproduct of a chemical reaction or intentionally as an unconventional weapon, adjunctive NSB therapy appears to be safe and effective for the treatment of potential pulmonary injuries.
B. Link to clinical studies
Studies involving multiple populations
-
...Although controlled clinical trials are lacking, in several published anecdotal case reports and case series, nebulized sodium bicarbonate solution has demonstrated safety and efficacy when administered for chlorine gas inhalation. Some patients were observed to experience rapid and dramatic relief of signs and symptoms. Treatment is based on the assumption that clinical benefit and cessation of tissue damage will occur with neutralization of the acidic byproducts created by inhaled chlorine gas. ... In one anecdotal case report, a markedly symptomatic 7-year-old exposed to chlorine gas experience dramatic relief from administration of nebulized sodium bicarbonate (Class IV).
Howard C, Ducre B, Burda AM, Kubic A. Management of chlorine gas exposure. J Emerg Nurs. 2007 Aug; 33 (4): 402-4. [PubMed Citation]
-
In this two year retrospective review, 86 cases of chlorine gas inhalation from 49 medical facilities were treated with nebulized sodium bicarbonate on the recommendation of the Kentucky Regional Poison Center. Typical manifestations included cough, chest discomfort, shortness of breath, and wheezing. No patients developed pulmonary edema or respiratory insufficiency requiring ventilatory support. Sixty-three cases (73.3%) were exposures to chlorine producing acid/hypochlorite mixtures. Six (7.0%) were exposed to chlorine gas in industrial settings. Twelve (14.0%) were exposed to chlorine gas in swimming pool settings. Sixty-nine (80.2%) were treated and released from the emergency department. In 53 patients, clinical condition was clearly improved on emergency department discharge. Seventeen (19.8%) were admitted to the hospital. All admitted patients gradually improved and had a mean hospital stay of 1.4 days (range 1 to 3 days). No patients in this study deteriorated clinically after nebulized sodium bicarbonate use. Nebulized sodium bicarbonate appears safe and merits prospective evaluation in the therapy of chlorine gas inhalation (Class IV).
Bosse GM. Nebulized sodium bicarbonate in the treatment of chlorine gas inhalation. J Toxicol Clin Toxicol. 1994;32(3):233-41. [PubMed Citation]
Adult
-
Chlorine gas is a potent pulmonary irritant that affects the mucous membranes and induces severe disturbances of pulmonary gas exchange within minutes of inhalation. The present study evaluated an extraordinary type of mass inhalational exposure. Clinical reports of 25 soldiers who were admitted to the emergency department of Maresal Cakmak Military Hospital, Erzurum were retrospectively evaluated. All patients were exposed to chlorine gas as a result of mixing sodium hypochlorite with hydrochloric acid during cleaning activities. All patients were male and the mean age of patients was 22.04+/-2.98 years. The main symptoms were coughing and dyspnea in 18 patients (72%). Forced expiratory volume in 1 second (FEV1) and FEV1/forced volume capacity (FVC) ratio were found to be normal in all patients but FVC and peak expiratory flow (PEF) were below the normal range (80%) in 9 patients (36%). All patients received warmed humidified oxygen combined with nebulized salbutamol. Inhaled budesonide and nebulized sodium bicarbonate were ordered additionally for 19 patients (76%). Thirteen patients (52%) were discharged from the emergency department and 12 patients (48%) were hospitalized. No mortality was observed. Chlorine gas is a potent pulmonary irritant that causes acute damage in both the upper and lower respiratory tract. We suggest that inhaled steroids combined with nebulized sodium bicarbonate could be a safe and effective alternative for the treatment of symptomatic patients. Education of the public about the dangers of mixing of hypochlorite bleach with acidic cleaning agents is also very important (Class IV).
Cevik Y, Onay M, Akmaz I, Sezigen S. Mass casualties from acute inhalation of chlorine gas. South Med J. 2009 Dec; 102 (12): 1209-13. [PubMed Citation]
-
Chlorine is one of the most common substances involved in toxic inhalation. As with all irritant gases, the airway injuries caused by chlorine gas may result in clinical manifestations similar to those of asthma. In this study, we investigated the effect of nebulized sodium bicarbonate (NSB) on the treatment and quality of life (QoL) of victims exposed to chlorine gas. Forty-four consecutive patients with reactive airways dysfunction syndrome (RADS) due to chlorine inhalation (40 females and 4 males, age range 17-56 yr) were included in this study. Patients were placed in control and treatment groups in a sequential odd-even fashion based on their order of presentation. Treatment of all patients included corticosteroids and nebulized short-acting beta2-agonists. Then the control group (n = 22) received nebulized placebo (NP), and the NSB group (n = 22) received NSB treatment (4 cm3 of 4.20% sodium bicarbonate solution). A quality of life (QoL) questionnaire and pulmonary function tests (PFTs) were performed before and after treatments in both groups. The most common symptoms were dyspnea (82%) and chest tightness (82%). Baseline characteristics of both groups were similar. Compared to the placebo group, the NSB group had significantly higher FEV1 values at 120 and 240 min (p < .05). Significantly more improvement in QoL questionnaire scores occurred in the NSB group compared to the NP group (p < .001). Thus, NSB is a clinically useful treatment, as tested by PFTs and QoL questionnaire, for patients with RADS caused by exposure to chlorine gas (Class III).
Aslan S, Kandiş H, Akgun M, Cakir Z, Inandi T, Gorquner M. The effect of nebulized NaHCO3 treatment on "RADS" due to chlorine gas inhalation. Inhal Toxicol. 2006 Oct; 18 (11): 895-900. [PubMed Citation]
-
Three male patients, 19 to 20 years old, were exposed to chlorine gas secondary to a leak in the chlorination system of an indoor pool. All of the patients were symptomatic with cough, chest pain, and shortness of breath. Physical examinations, arterial blood gases, and chest radiographs were normal. All patients were given a nebulized solution of 3.75% sodium bicarbonate which resulted in prompt relief of their symptoms. None of the patients suffered from prolonged symptomatology. This therapy appears to be useful in treating chlorine gas inhalation; however, it cannot be routinely recommended without prospective clinical studies evaluating its efficacy and safety (Class IV).
Vinsel PJ; Treatment of acute chlorine gas inhalation with nebulized sodium bicarbonate. J Emerg Med. 1990 May-Jun; 8 (3): 327-9. [PubMed Citation]
-
Mass exposure to chloramine gas has not been reported. We report two groups of 36 patients (72 total) suffering from acute inhalation of chloramine gas. Chloramine gas is produced from mixing common household cleaning agents containing sodium hypochlorite (bleach) and ammonia. The first mass casualty event occurred when 36 male soldiers were exposed during a "cleaning party" in their barracks. Ten days later, 36 female soldiers were exposed in a similar manner and presented to our emergency department. In each event, commonly available cleaning agents--liquid bleach and ammonia--were mixed together, liberating toxic chloramine gas. Nebulized sodium bicarbonate solution has been suggested for treatment of chlorine gas inhalation, but no report of nebulized sodium bicarbonate for treatment of chloramine gas inhalation injury exists. In our series, 22 patients exposed to chloramine gas were treated with a nebulized solution of 3.75% sodium bicarbonate. This treatment made no significant statistical or clinical difference in outcome. We present the largest case series of patients presenting to an emergency department for treatment of acute inhalation of chloramine gas (Class IV).
Pascuzzi TA, Storrow AB; Mass casualties from acute inhalation of chloramine gas. Mil Med. 1998 Feb; 163 (2):102-4. [PubMed Citation]
-
Clinical experience with five patients exposed to phosgene is described. The treatment of phosgene poisoning was focused upon the presenting problem, pulmonary edema. Arterial hypoxemia was treated with a face mask with 10 cm CPAP with the FiO2 adjusted as needed or with a volume ventilator with controlled ventilation. Ventilation was controlled to reduce the work of breathing. Metabolic acidosis was treated with NaCHO3 to produce a normal pH. A vigorous program of diuresis was used to treat the pulmonary edema. Lasix was administered to produce a negative fluid balance while maintaining a good urinary output. The negative fluid balance correlated well with reduced oxygen requirements (Class IV).
Wells BA; Phosgene: a practitioner's viewpoint. Toxicol Ind Health. 1985 Oct; 1 (2): 81-92. [PubMed Citation]
Pediatric studies
-
Nebulized sodium bicarbonate 3.75% overcame the symptoms caused by inhalation of chlorine gas by a 7-year-old girl. Fumes from a can of chlorine tablets used for a swimming pool caused coughing, vomiting, and respiratory distress with nasal flaring and intercostal and subcostal retraction. Her oxygen saturation was 85% on room air. With oxygen treatment, her oxygen saturation remained below 90% and her symptoms did not improve. She was then given 4.25 milliliters of sodium bicarbonate 3.75% by a hand-held nebulizer over 20 minutes. Her symptoms improved dramatically, and she was discharged from the hospital without symptoms or complications. It is thought that the sodium bicarbonate neutralizes the hydrochloric acid formed when chlorine gas comes into contact with water at the target tissues. The possibility of a harmful exothermic reaction with administration of sodium bicarbonate has prevented its widespread use for this purpose. There was no harmful exothermic reaction in this case, possibly because of the low concentration used (Class IV).
Douidar SM; Nebulized sodium bicarbonate in acute chlorine inhalation. Pediatr Emerg Care. 1997 Dec; 13 (6): 406-7. [PubMed Citation]
Clinical reviews
-
For the treatment of phosgene poisoning, glucocorticoids and positive pressure ventilation can be recommended as well as supporting measures such as physical rest, antitussives, buffers, sedatives, antibiotics, antispasmodics and possibly diuretics. Roentgenological evaluation of the lungs is advisable. A therapeutic strategy is presented which is based on the phosgene exposure intensity (Class IV).
Diller WF; Therapeutic strategy in phosgene poisoning. Toxicol Ind Health. 1985 Oct; 1 (2):93-9. [PubMed Citation]
-
Terrorism poses a clear and present danger to civilian populations. Although terrorist cells may gain access to traditional chemical weapons, there are literally thousands of other industrial chemicals to choose from. Common chemicals used on a daily basis in an industrialized society can be readily obtained from the local shopping center, rail yard, or from nearby industrial parks, and terrorists may choose to use these agents in an attack. The medical implications of a major incident involving the accidental or intentional release of a dangerous chemical are significant, and all healthcare facilities should have a plan in place to manage the casualties of such an event. This plan should include event recognition, crowd control, primary triage, emergency treatment, decontamination of injured and uninjured patients, and secondary triage. Emergency health care providers should be prepared to respond to classic chemical agents such as mustard, chlorine, and phosgene and must also work carefully with law enforcement and public health agencies to keep abreast of new threats. The ability to recognize an event promptly, triage patients, decontaminate casualties, administer antidotes when available, and provide best supportive care will minimize the adverse outcomes (Class IV).
Parrish JS, Bradshaw DA; Toxic inhalational injury: gas, vapor and vesicant exposure. Respir Care Clin N Am. 2004 Mar; 10 (1): 43-58. [PubMed Citation]
-
A literature search designed to collect information on therapy for phosgene poisoning has been conducted for the period 1920-1982. To achieve this goal, literature services were consulted, cross references were carefully followed and, whenever possible, unpublished reports (e.g., from Edgewood Arsenal and Porton Research Station) were evaluated. The various therapeutic agents and measures described in the literature are presented in alphabetical order. When available, detailed data are given for the phosgene dose, interval of time between exposure and institution of therapy, therapeutic effect and parameters used to assess efficacy. A final summary presents general recommendations for therapy and for further research (Class IV).
Diller WF, Zante R; A literature review: therapy for phosgene poisoning. Toxicol Ind Health. 1985 Oct; 1 (2): 117-28. [PubMed Citation]
-
Humans can come into contact with chlorine gas during short-term, high-level exposures due to traffic or rail accidents, spills, or other disasters. By contrast, workplace and public (swimming pools, etc.) exposures are more frequently long-term, low-level exposures, occasionally punctuated by unintentional transient increases. Acute exposures can result in symptoms of acute airway obstruction including wheezing, cough, chest tightness, and/or dyspnea. These findings are fairly nonspecific, and might be present after exposures to a number of inhaled chemical irritants. Clinical signs, including hypoxemia, wheezes, rales, and/or abnormal chest radiographs may be present. More severely affected individuals may suffer acute lung injury (ALI) and/or acute respiratory distress syndrome (ARDS). Up to 1% of exposed individuals die. Humidified oxygen and inhaled beta-adrenergic agents are appropriate therapies for victims with respiratory symptoms while assessments are underway. Inhaled bicarbonate and systemic or inhaled glucocorticoids also have been reported anecdotally to be beneficial. Chronic sequelae may include increased airways reactivity, which tends to diminish over time. Airways hyperreactivity may be more of a problem among those survivors that are older, have smoked, and/or have pre-existing chronic lung disease. Individuals suffering from irritant-induced asthma (IIA) due to workplace exposures to chlorine also tend to have similar characteristics, such as airways hyperresponsiveness to methacholine, and to be older and to have smoked. Other workplace studies, however, have indicated that workers exposed to chlorine dioxide/sulfur dioxide have tended to have increased risk for chronic bronchitis and/or recurrent wheezing attacks (one or more episodes) but not asthma, while those exposed to ozone have a greater incidence of asthma. Specific biomarkers for acute and chronic exposures to chlorine gas are currently lacking...(Class IV).
White CW, Martin JG; Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc. 2010 Jul; 7 (4): 257-63. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
Chlorine gas may cause inhalation injuries through exposures from industrial sources, home swimming pools, or admixtures of bleach and acidic cleaning solutions. Victims may rapidly present to the emergency department with dyspnea, chest pain, nonproductive cough, bronchospasm, tachypnea, and even pulmonary edema. Until recently, the Poisindex5 recommended 5% nebulized NaHCO3 as a treatment modality based on anecdotal reports, although the most recent edition states that "it cannot be routinely recommended." Our study was designed to examine the role of nebulized NaHCO3 in the treatment of chlorine gas inhalation injury using a sheep model. Twenty-one sheep had internal jugular and carotid artery catheters placed by direct visualization. After being anesthetized with pentobarbital and succinylcholine, each sheep was endotracheally intubated and exposed to chlorine gas (500 ppm) for four mintes by a closed system. The animals then were placed on a Bird Mark 7 ventilator on room air. At 30 minutes after exposure, the animals were divided into two groups to receive a five-minute nebulized treatment (8 mL of normal saline (Group A, ten) or 4% NaHCO3 (Group B, 11). Arterial blood gases were sampled serially at 5, 15, 30, 60, and 90 minutes and 24 hours after treatment. The animals then were euthanized and the organs taken for gross and microscopic examination. One-way ANOVA revealed no differences over time within groups with the exception of the GRA p02 (f = 6.57). t test for unequal groups revealed differences between groups with higher pCO2 (P < 0.001) and lower p02 (P < 0.05) values for the control group. There was no difference in mortality rates before 24 hours for either group (three) or in microscopic pathology in blinded comparisons. The use of a single inhalation treatment of bicarbonate does not appear to worsen arterial blood gases or alter pathology in this sheep model and may actually improve arterial blood gas values.
Chisholm CD, Singletary EM et al.; Inhaled Sodium Bicarbonate Therapy for Chlorine Inhalation Injuries. Ann Emerg Med 1989; 18 (4): 466.
-
We exposed 22 mongrel dogs to 94 ppm phosgene for 20 min from a non-rebreathing system. We expressed exposure to phosgene as ppm X VI X min X kg 1, i.e., the amount of gas containing a known phosgene concentration that was actually inhaled per min standardized to body weight, the "exposure index" (EI). In contrast, the conventional expression of exposure, i.e., ppm X min, fails to take volume inhaled (VI) and body weight into account. Five dogs received no intervention and served as controls. Fourteen dogs received basic therapy of oral cortisone (40 mg/kg) and NaHCO3 (3 mEq/kg) plus 100% O2 (FiO2 = 1.0) for 30 min after the exposure period. These animals were further divided according to the following selected additional therapies, which were started 30 min after exposure: Theophylline, 5 mg/kg iv for 20 min followed by 1 mg/kg/hr for 70 min (n = 5). Three dogs of this group were given a trial of 5 cm H2O expiratory resistance during the period of basic therapy. Because of the untoward response, expiratory resistance was discontinued and not used in other experiments. PGE2-hi, [1 microgram/kg/min] iv for 90 min (n = 3). PGE1-lo, [0.04 microgram/kg/min] iv for 90 min (n = 3). Atropine, 0.5 mg/kg iv at 30 and 50 min after exposure (n = 3). Three dogs [group 5] received oral cortisone and NaHCO3 plus inhaled supplementary surfactant, 2.7 mg/min ultrasonically nebulized (FiO2 = 0.5; phosphate buffer), for 30 min after exposure. All treated dogs, groups [1] through [4] and the surfactant group [5], received cortisone (40 mg/kg/hr iv), NaHCO3 to correct base deficit, and O2 to correct hypoxemia from 30 min to 120 min after exposure. Because of its clearly beneficial effect in group [1], theophylline was also given to all other treatment groups during this period. At the end of the study, all lungs were excised, examined and prepared for light microscopy. We found that EI, which varied among subjects because of spontaneous variations of VI during exposure, correlated significantly with the changes in base deficit induced by phosgene inhalation. We also found that the change in minute ventilation, delta VI X kg-1, correlated significantly with changes in lung compliance, peak flow and base deficit. Evaluation of the various therapeutic modalities revealed the following: Immediate therapy with O2 is vital and FiO2 of 0.4 to 0.5 is sufficient.
Mautone AJ, Katz Z, Scarpelli EM; Acute responses to phosgene inhalation and selected corrective measures (including surfactant). Toxicol Ind Health. 1985 Oct; 1 (2): 37-57. [PubMed Citation]
Non-clinical reviews
-
The inhalation of reactive gases and vapors can lead to severe damage of the airways and lung, compromising the function of the respiratory system. Exposures to oxidizing, electrophilic, acidic, or basic gases frequently occur in occupational and ambient environments. Corrosive gases and vapors such as chlorine, phosgene, and chloropicrin were used as warfare agents and in terrorist acts. Chemical airway exposures are detected by the olfactory, gustatory, and nociceptive sensory systems that initiate protective physiological and behavioral responses. This review focuses on the role of airway nociceptive sensory neurons in chemical sensing and discusses the recent discovery of neuronal receptors for reactive chemicals. Using physiological, imaging, and genetic approaches, Transient Receptor Potential (TRP) ion channels in sensory neurons were shown to respond to a wide range of noxious chemical stimuli, initiating pain, respiratory depression, cough, glandular secretions, and other protective responses. TRPA1, a TRP ion channel expressed in chemosensory C-fibers, is activated by almost all oxidizing and electrophilic chemicals, including chlorine, acrolein, tear gas agents, and methyl isocyanate, the highly noxious chemical released in the Bhopal disaster. Chemicals likely activate TRPA1 through covalent protein modification. Animal studies using TRPA1 antagonists or TRPA1-deficient mice confirmed the role of TRPA1 in chemically induced respiratory reflexes, pain, and inflammation in vivo. New research shows that sensory neurons are not merely passive sensors of chemical exposures. Sensory channels such as TRPA1 are essential for maintenance of airway inflammation in asthma and may contribute to the progression of airway injury following high-level chemical exposures.
Bessac BF, Jordt SE; Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010 Jul; 7 (4): 269-77. [PubMed Citation]
-
...Animal models for chlorine gas inhalation have demonstrated evidence of oxidative injury and inflammation. Early epithelial injury, airways hyperresponsiveness, and airway remodeling, likely diminishing over time, have been shown. As in humans, acute lung injury and/or acute respiratory distress syndrome (ALI/ARDS) can occur, becoming more likely when the upper airways are bypassed. Inhalation models of chlorine toxicity provide unique opportunities for testing potential pharmacologic rescue agents.
White CW, Martin JG; Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc. 2010 Jul; 7 (4): 257-63. [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Oral Bicarbonates, such as sodium bicarbonate, neutralizes gastric acid with the production of carbon dioxide. Bicarbonate not involved in that reaction is absorbed and in the absence of a deficit of bicarbonate in the plasma, bicarbonate ions are excreted in the urine, which is rendered alkaline, and there is accompanying dieresis.
Sweetman SC (ed.): Martindale: The Complete Drug Reference Thirty-sixth Edition. Royal Pharmaceutical Society of Great Britain, Pharmaceutical Press, London, UK , 2009 p. 1673-1674
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
-
Cardiopulmonary resuscitation: the guidelines on cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) state that IV sodium bicarbonate is not recommended for routine use in advanced cardiovascular life support (ACLS) during cardiopulmonary resuscitation and is not considered a first-line agent for the treatment of cardiac arrest; however, if the drug is used, an IV dose of 1 mEq/kg may be given initially to adults undergoing cardiac arrest.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Metabolic acidosis: a 2-5 mEq/kg dose of sodium bicarbonate may be administered to adults as a 4- to 8-hour IV infusion. Subsequent doses should be determined by the response of the patient and appropriate laboratory determinations. Sodium bicarbonate therapy should be planned in a stepwise manner, since the degree of response following a given dose is not always predictable. Generally, the dose and frequency of administration should be reduced after severe symptoms have improved.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Arrhythmias and hypotension associated with drug-induced cardiovascular emergencies (tricyclic antidepressant or sodium-channel blocking agent [e.g., procainamide, flecainide] toxicity): in adults, 1-2 mEq/kg of sodium bicarbonate has been administered by repeated direct IV (i.e., bolus) injections to maintain an arterial pH of 7.45-7.55; however, the optimal target pH with sodium bicarbonate therapy has not been established. Some experts recommend a maintenance infusion of 150 mEq/L of sodium bicarbonate plus 30 mEq of potassium chloride per liter in 5% dextrose injection. These experts also state that direct IV (i.e., bolus) injections of sodium bicarbonate may be used without prior determination of serum pH for acute decompensation, if QRS interval exceeds 100 msec or if hypotension develops.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Hyperkalemia associated with moderate potassium elevation (6-7 mEq/L) or severe potassium elevation (exceeding 7 mEq/L with toxic ECG changes): in adults, 50 mEq of sodium bicarbonate has been administered IV over 5 minutes.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Diabetic ketoacidosis: although the specific role of sodium bicarbonate therapy in the treatment of diabetic ketoacidosis has not been established, when IV sodium bicarbonate is administered, the acidosis should only partially be corrected, generally to an arterial pH of about 7.2, in order to avoid rebound alkalosis.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Alkalinization of urine: the usual oral dosage of sodium bicarbonate for alkalinization of urine in adults is 48 mEq (4 g) initially, followed by 12-24 mEq (1-2 g) every 4 hours. Dosages of 30-48 mEq (2.5-4 g) every 4 hours, up to 192 mEq (16 g) daily, may be required in some patients. Dosage should be individually titrated to maintain the desired urinary pH.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
Children (FDA)
-
Pediatric advanced life support: if sodium bicarbonate is used in children and infants, the guidelines for pediatric advanced life support (PALS) state that the initial pediatric dose of the drug is 1 mEq/kg (1 mL/kg of an 8.4%sodium bicarbonate solution), administered slowly by IV or intraosseous injection. Because of the potential association of intracranial hemorrhage and sodium bicarbonate infusion in premature infants, neonates should receive a 1:1 dilution of 7.5 or 8.4% sodium bicarbonate injection and 5% dextrose injection to avoid hypertonicity; alternatively, a commercially available 4.2% solution may be used.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Metabolic acidosis: a 2-5 mEq/kg dose of sodium bicarbonate may be administered to older children as a 4- to 8-hour IV infusion. Subsequent doses should be determined by the response of the patient and appropriate laboratory determinations. Sodium bicarbonate therapy should be planned in a stepwise manner, since the degree of response following a given dose is not always predictable. Generally, the dose and frequency of administration should be reduced after severe symptoms have improved.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Drug-induced cardiovascular emergencies (tricyclic antidepressant or other sodium-channel blocking agent toxicity): in pediatric patients, 1-2 mEq/kg of sodium bicarbonate have been administered by direct IV (i.e., bolus) injection until the arterial pH exceeded 7.45, then followed by an infusion of 150 mEq/L of sodium bicarbonate in 5% dextrose injection to maintain alkalosis; in severe intoxication, the pH has been increased to 7.50-7.55. For the treatment of ventricular arrhythmias associated with cocaine toxicity in pediatric patients, 1-2 mEq/kg of IV sodium bicarbonate has been administered.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Alkalinization of urine: in children, an oral dosage of 1-10 mEq (84-840 mg) per kg daily, adjusted according to response, has been suggested.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
Pregnancy (FDA)
FDA Pregnancy Risk Category:
-
C; RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk.
Product label Sodium Bicarbonate injection, solution [Hospira, Inc.] Last revised: August 2012 [DailyMed]
Nursing Mothers (FDA)
-
Sodium hydrogen carbonate is designated "compatible with breastfeeding"; Drugs are classified as compatible with breastfeeding if there are no known or theoretical contraindications for their use, and it is considered safe for the mother to take the drug and continue to breastfeed.
WHO/UNICEF. Breastfeeding and maternal medication: Recommendations for drugs in the eleventh WHO model list of essential drugs (2003)
Renal Impairment (FDA)
-
Acidosis associated with chronic renal failure: oral sodium bicarbonate therapy is generally initiated when plasma bicarbonate concentration is less than 15 mEq/L. Therapy is usually initiated in adults with an oral sodium bicarbonate dosage of 20-36 mEq daily, given in divided doses. Dosage is then titrated to provide a plasma bicarbonate concentration of about 18-20 mEq/L. Because of the sodium content of sodium bicarbonate, the fluid and electrolyte balance of the patient must be carefully monitored during therapy with the drug.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Renal failure and osteomalacia in patients with renal tubular acidosis: higher dosages of sodium bicarbonate are necessary. In adults with distal (type 1) renal tubular acidosis, an initial oral dosage of 0.5-2 mEq/kg daily, given in 4 or 5 divided doses, has been suggested. Dosage is titrated until hypercalciuria and acidosis are controlled, and according to the response and tolerance of the patient. Alternatively, an adult dosage of 48-72 mEq (about 4-6 g) daily has been suggested. Higher dosages are generally required in patients with proximal (type 2) renal tubular acidosis; oral dosages of 4-10 mEq/kg daily, given in divided doses, have been suggested.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
Emergency Use Authorization (FDA/CDC)
-
No Emergency Use Authorization for Sodium Bicarbonate has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (Public Law 108-276).
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
6. Current available formulations/shelf life
Formulation
-
Oral powder
-
Oral Tablets 325 mg*
-
Oral Tablets 650 mg*
-
Parenteral Injection 4.2% (0.5 mEq/mL) (2.5 or 5 mEq)*
-
Parenteral Injection 5% (0.595 mEq/mL) (297.5 mEq)*
-
Parenteral Injection 7.5% (0.892 mEq/mL) (8.92 or 44.6 mEq)*
-
Parenteral Injection 8.4% (1 mEq/mL) (10 or 50 mEq)*
-
Parenteral Injection, for preparation of IV ad mixtures 7.5% (0.892 mEq/mL) (178.4 mEq) pharmacy bulk package
-
Parenteral Solution, sterile, to adjust pH of injections 4% (0.48 mEq/mL) (2.4 mEq)
-
Parenteral Solution, sterile, to adjust pH of injections 4.2% (0.5 mEq/mL) (2.5 mEq)*
* Available from one or more manufacturer, distributor, and/or repackager by generic (nonproprietary) name
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
Shelf life
Shel life
-
Self life: Sodium Bicarbonate for Injection has a shelf life of 18 months.
Burda AM, Sigg T; Pharmacy preparedness for incidents involving weapons of mass destruction. Am J Health Syst Pharm. 2001; 58 (23):2274-84. [PubMed Citation]
Stability
-
Sodium bicarbonate is stable in dry air, but slowly decomposes into sodium carbonate, carbon dioxide, and water in moist air. When heated, sodium bicarbonate loses water and carbon dioxide and is converted into sodium carbonate. Solutions of sodium carbonate are much more alkaline than sodium bicarbonate; since sodium carbonate may be formed when the dry salt or its solutions are sterilized with heat, the pH of heat-sterilized solutions or of solutions prepared from heat-sterilized powder should be determined prior to use. When sodium bicarbonate is combined with acids in aqueous solutions, a vigorous evolution of carbon dioxide gas occurs; the liberated carbon dioxide bubbles through the solution resulting in effervescence. In the dry state, sodium bicarbonate and acids do not react.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
Storage
-
Sodium bicarbonate tablets and effervescent tablets should be stored in tightly closed containers at a temperature less than 40°C, preferably between 15-30°C.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Sodium bicarbonate injection should be stored at a temperature less than 40°C, preferably between 15-30°C; freezing should be avoided.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Prevention of contrast media nephrotoxicity: Sodium bicarbonate (154 mEq/L) was administered at 3 mL/kg/h for 1 h before contrast agent administration, followed by an infusion of 1 mL/kg/h for 6 h after the procedure.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.146-7
-
BACKGROUND: Volume supplementation by saline infusion combined with N-acetylcysteine (NAC) represents an effective strategy to prevent contrast agent-induced nephrotoxicity (CIN). Preliminary data support the concept that sodium bicarbonate and ascorbic acid also may be effective in preventing CIN. METHODS AND RESULTS: Three hundred twenty-six consecutive patients with chronic kidney disease, referred to our institutions for coronary and/or peripheral procedures, were randomly assigned to prophylactic administration of 0.9% saline infusion plus NAC (n=111), sodium bicarbonate infusion plus NAC (n=108), and 0.9% saline plus ascorbic acid plus NAC (n=107). All enrolled patients had serum creatinine > or = 2.0 mg/dL and/or estimated glomerular filtration rate < 40 mL x min(-1) x 1.73 m(-2). Contrast nephropathy risk score was calculated in each patient. In all cases, iodixanol (an iso-osmolar, nonionic contrast agent) was administered. The primary end point was an increase of > or = 25% in the creatinine concentration 48 hours after the procedure (CIN). The amount of contrast media administered (179+/-102, 169+/-92, and 169+/-94 mL, respectively; P=0.69) and risk scores (9.1+/-3.4, 9.5+/-3.6, and 9.3+/-3.6; P=0.21) were similar in the 3 groups. CIN occurred in 11 of 111 patients (9.9%) in the saline plus NAC group, in 2 of 108 (1.9%) in the bicarbonate plus NAC group (P=0.019 by Fisher exact test versus saline plus NAC group), and in 11 of 107 (10.3%) in the saline plus ascorbic acid plus NAC group (P=1.00 versus saline plus NAC group). CONCLUSIONS: The strategy of volume supplementation by sodium bicarbonate plus NAC seems to be superior to the combination of normal saline with NAC alone or with the addition of ascorbic acid in preventing CIN in patients at medium to high risk.
Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, Ricciardelli B, Colombo A; Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007 Mar 13; 115 (10): 1211-7. [PubMed Citation]
-
BACKGROUND: The combination of bicarbonate and mannitol (BIC/MAN) is commonly used to prevent renal failure (RF) in patients with rhabdomyolysis despite the absence of sufficient evidence validating its use. The purpose of this study was to determine whether BIC/ MAN is effective in preventing RF in patients with rhabdomyolysis caused by trauma. METHODS: This study was a review of all adult trauma intensive care unit (ICU) admissions over 5 years (January 1997-September 2002). Creatine kinase (CK) levels were checked daily (abnormal,>520 U/L). RF was defined as a creatinine greater than 2.0 mg/dL. Patients received BIC/MAN on the basis of the surgeon's discretion. RESULTS: Among 2,083 trauma ICU admissions, 85% had abnormal CK levels. Overall, RF occurred in 10% of trauma ICU patients. A CK level of 5,000 U/L was the lowest abnormal level associated with RF; 74 of 382 (19%) patients with CK greater than 5,000 U/L developed RF as compared with 143 of 1,701 (8%) patients with CK less than 5,000 U/L (p < 0.0001). Among patients with CK greater than 5,000 U/L, there was no difference in the rates of RF, dialysis, or mortality between those who received BIC/MAN and those who did not. Subanalysis of groups with various levels of CK still failed to show any benefit of BIC/MAN. CONCLUSION: Abnormal CK levels are common among critically injured patients, and a CK level greater than 5,000 U/L is associated with RF. BIC/MAN does not prevent RF, dialysis, or mortality in patients with creatine kinase levels greater than 5,000 U/L. The standard of administering BIC/MAN to patients with post-traumatic rhabdomyolysis should be reevaluated.
Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC; Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004 Jun; 56 (6): 1191-6 [PubMed Citation]
8. Route of Administration/Monitoring
-
Inhalation of three mL of 8.4% sodium bicarbonate mixed with 2 mL of normal saline to prepare a 5 mL of a 5% sodium bicarbonate solution.
Bosse GM. Nebulized sodium bicarbonate in the treatment of chlorine gas inhalation. J Toxicol Clin Toxicol. 1994;32(3):233-41. [PubMed Citation]
-
Dosage of sodium bicarbonate injection is determined by severity of the acidosis, appropriate laboratory determinations, and the patient's age, weight, and clinical condition. Frequent laboratory determinations and clinical evaluation of the patient are essential during therapy with sodium bicarbonate, especially during prolonged therapy, to monitor changes in fluid and electrolyte and acid-base balance.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Generally, full correction of bicarbonate deficit should not be attempted during the first 24 hours of sodium bicarbonate therapy, since this may result in precipitation of metabolic alkalosis because of delayed physiologic compensatory mechanisms. When total carbon dioxide content is returned to normal or beyond within the first day of therapy, substantially alkaline values for blood pH and subsequent adverse effects are likely to occur. When initial, rapid administration of the drug is considered necessary, it is generally recommended that no more than 33-50% of the calculated bicarbonate requirements be administered initially.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
9. Adverse effects
-
Gastric distention and flatulence may occur when sodium bicarbonate is administered orally.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Inadvertent extravasation of hypertonic solutions of sodium bicarbonate has reportedly caused chemical cellulitis because of their alkalinity, subsequently resulting in tissue necrosis, ulceration, and/or sloughing at the site of injection. One manufacturer recommends that extravasation be treated by elevating the affected area, applying warm compresses to the site, and locally injecting lidocaine or hyaluronidase.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Sodium bicarbonate, when given in large doses or to patients with renal insufficiency, may cause metabolic alkalosis. Metabolic alkalosis may be accompanied by hyperirritability or tetany; tetany is particularly likely to occur in patients with hypocalcemia, as may occur in uremia, since bicarbonate-induced increase in pH increases the binding of calcium to albumin. Metabolic alkalosis may impair the release of oxygen from peripheral tissues, possibly resulting in lactic acidosis. The manufacturers recommend that severe bicarbonate-induced alkalosis be treated with a parenteral calcium salt (e.g., calcium gluconate) and/or an acidifying agent (e.g., ammonium chloride). In patients with ketoacidosis, rapid alkalinization with sodium bicarbonate may reportedly result in clouding of consciousness, cerebral dysfunction, obtundation, seizures, and peripheral tissue hypoxia and lactic acidosis.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
-
Sodium and water retention and edema may occur during sodium bicarbonate therapy, especially when the drug is given in large doses or to patients with renal insufficiency, congestive heart failure, or those predisposed to sodium retention and edema. Sodium and water overload may result in hypernatremia and hyperosmolality. Severe hyperosmolal states may develop during cardiopulmonary resuscitation when excessive doses of sodium bicarbonate are administered. Serum potassium or calcium concentration may decrease during sodium bicarbonate therapy.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
10. Contraindication(s)
-
Sodium Bicarbonate Injection, USP is contraindicated in patients who are losing chloride by vomiting or from continuous gastrointestinal suction, and in patients receiving diuretics known to produce a hypochloremic alkalosis.
Product label Sodium Bicarbonate injection, solution [Hospira, Inc.] Last revised: August 2012 [DailyMed]
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations- No data available at this time.
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations- No data available at this time.
15. Study-related ethical concerns
— including review panel recommendationsEthical concerns include:
-
Informed consent for data collection in disaster/mass casualty situation
-
Central institutional review boards (IRBs)
-
How to conduct multicenter studies
Poison centers
Pediatric Emergency Care Applied Research Network
Hospital consortia.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
16. Global regulatory status
U.S.
-
Sodium Bicarbonate tablet
Relieves: acid indigestion; heartburn; sour stomach; upset stomach
Product label Sodium Bicarbonate tablet [Mirror Pharmaceuticals LLC] Last revised: April 2011 [DailyMed]
-
Sodium Bicarbonate solution
Sodium Bicarbonate Inj., 8.4% USP Neutralizing Additive Solution is indicated to hasten onset of analgesia and reduce injection pain, by adjusting commercial preparations of Lidocaine w/ Epinephrine anesthetic solution to a more physiologic pH.
Product label Sodium Bicarbonate solution [Onpharma, Inc.] Last revised: June 2012 [DailyMed]
-
Sodium Bicarbonate Injection
Sodium Bicarbonate Injection, USP is indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes, circulatory insufficiency due to shock or severe dehydration, extracorporeal circulation of blood, cardiac arrest and severe primary lactic acidosis. Sodium bicarbonate is further indicated in the treatment of certain drug intoxications, including barbiturates (where dissociation of the barbiturate-protein complex is desired), in poisoning by salicylates or methyl alcohol and in hemolytic reactions requiring alkalinization of the urine to diminish nephrotoxicity of blood pigments. Sodium bicarbonate also is indicated in severe diarrhea which is often accompanied by a significant loss of bicarbonate.
Treatment of metabolic acidosis should, if possible, be superimposed on measures designed to control the basic cause of the acidosis e.g., insulin in uncomplicated diabetes, blood volume restoration in shock. But since an appreciable time interval may elapse before all of the ancillary effects are brought about, bicarbonate therapy is indicated to minimize risks inherent to the acidosis itself.
Vigorous bicarbonate therapy is required in any form of metabolic acidosis where a rapid increase in plasma total CO2 content is crucial e.g., cardiac arrest, circulatory insufficiency due to shock or severe dehydration, and in severe primary lactic acidosis or severe diabetic acidosis.
Product label Sodium Bicarbonate injection, solution [Hospira, Inc.] Last revised: August 2012 [DailyMed]
U.K.
-
Sodium Bicarbonate is indicated for the relief of mild urinary-tract infections; alkalinization of urine.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.460
-
Sodium Bicarbonate is used to control severe metabolic acidosis (pH< 7.1) particularly that caused by loss of bicarbonate (as in renal tubular acidosis or from excessive gastro-intestinal losses).
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.532
-
Sodium Bicarbonate should no longer be prescribed alone for the relief of dyspepsia but it is present as an ingredient in many indigestion remedies.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.38
-
Sodium Bicarbonate ear drops are effective for the removal of ear wax but may cause dryness of the ear canal.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.611
17. Other potentially useful information
-
Sodium bicarbonate is a nonspecific antidote effective in the treatment of a variety of poisonings by means of a number of distinct mechanisms. Nebulized sodium bicarbonate serves as a useful adjunct in the treatment of patients with pulmonary injuries resulting from chlorine gas inhalation. Inhaled sodium bicarbonate neutralizes the hydrochloric acid that is formed when chlorine gas reacts with the water in the respiratory tree.
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 520-527
-
...Sodium bicarbonate should be administered by nebulization separately from the albuterol sulfate inhalation treatments because compatibility studies show that formation of a precipitate can occur when the solutions are mixed.
Howard C, Ducre B, Burda AM, Kubic A. Management of chlorine gas exposure. J Emerg Nurs. 2007 Aug; 33 (4): 402-4. [PubMed Citation]
-
Solubility in water = 6.4, 7.6, 8.7, 10.0, 11.3, 12.7, 14.2, 16.5, and 19.1 g/100g solution at 0, 10, 20, 30, 40, 50, 60, 80, and 100 deg C, respectively; Solubility in water = 6.9, 8.2, 9.6, 11.1, 12.7, 14.5, 16.5, 19.7, and 23.6 g/100g H2O at 0, 10, 20, 30, 40, 50, 60, 80, and 100 deg C, respectively; Solubility is lower in the presence of sodium carbonate
HSDB. Sodium bicarbonate
-
Log P(octanol-water) = -4.010 (est)
US NLM. ChemIDplus Lite. Sodium bicarbonate
18. Publications
Aslan S, Kandiş H, Akgun M, Cakir Z, Inandi T, Gorquner M. The effect of nebulized NaHCO3 treatment on "RADS" due to chlorine gas inhalation. Inhal Toxicol. 2006 Oct; 18 (11): 895-900. [PubMed Citation]
Bessac BF, Jordt SE; Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010 Jul; 7 (4): 269-77. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Bosse GM. Nebulized sodium bicarbonate in the treatment of chlorine gas inhalation. J Toxicol Clin Toxicol. 1994;32(3):233-41. [PubMed Citation]
Briguori C, Airoldi F, D'Andrea D, Bonizzoni E, Morici N, Focaccio A, Michev I, Montorfano M, Carlino M, Cosgrave J, Ricciardelli B, Colombo A; Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): a randomized comparison of 3 preventive strategies. Circulation. 2007 Mar 13; 115 (10): 1211-7. [PubMed Citation]
Brown CV, Rhee P, Chan L, Evans K, Demetriades D, Velmahos GC; Preventing renal failure in patients with rhabdomyolysis: do bicarbonate and mannitol make a difference? J Trauma. 2004 Jun; 56 (6): 1191-6 [PubMed Citation]
Burda AM, Sigg T; Pharmacy preparedness for incidents involving weapons of mass destruction. Am J Health Syst Pharm. 2001; 58 (23):2274-84. [PubMed Citation]
Cevik Y, Onay M, Akmaz I, Sezigen S. Mass casualties from acute inhalation of chlorine gas. South Med J. 2009 Dec; 102 (12): 1209-13. [PubMed Citation]
Chisholm CD, Singletary EM et al.; Inhaled Sodium Bicarbonate Therapy for Chlorine Inhalation Injuries. Ann Emerg Med 1989; 18 (4): 466.
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
Diller WF; Therapeutic strategy in phosgene poisoning. Toxicol Ind Health. 1985 Oct; 1 (2):93-9. [PubMed Citation]
Diller WF, Zante R; A literature review: therapy for phosgene poisoning. Toxicol Ind Health. 1985 Oct; 1 (2): 117-28. [PubMed Citation]
Douidar SM; Nebulized sodium bicarbonate in acute chlorine inhalation. Pediatr Emerg Care. 1997 Dec; 13 (6): 406-7. [PubMed Citation]
Howard C, Ducre B, Burda AM, Kubic A. Management of chlorine gas exposure. J Emerg Nurs. 2007 Aug; 33 (4): 402-4. [PubMed Citation]
HSDB. Sodium bicarbonate
Jones R, Wills B, Kang C. Chlorine gas: an evolving hazardous material threat and unconventional weapon. West J Emerg Med 2010; 11 (2):151-6. [PubMed Citation]
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.146-7
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.611
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.38
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.532
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p.460
Mautone AJ, Katz Z, Scarpelli EM; Acute responses to phosgene inhalation and selected corrective measures (including surfactant). Toxicol Ind Health. 1985 Oct; 1 (2): 37-57. [PubMed Citation]
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.2750-2754
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 520-527
Parrish JS, Bradshaw DA; Toxic inhalational injury: gas, vapor and vesicant exposure. Respir Care Clin N Am. 2004 Mar; 10 (1): 43-58. [PubMed Citation]
Pascuzzi TA, Storrow AB; Mass casualties from acute inhalation of chloramine gas. Mil Med. 1998 Feb; 163 (2):102-4. [PubMed Citation]
Product label Sodium Bicarbonate injection, solution [Hospira, Inc.] Last revised: August 2012 [DailyMed]
Product label Sodium Bicarbonate solution [Onpharma, Inc.] Last revised: June 2012 [DailyMed]
Product label Sodium Bicarbonate tablet [Mirror Pharmaceuticals LLC] Last revised: April 2011 [DailyMed]
Sweetman SC (ed.): Martindale: The Complete Drug Reference Thirty-sixth Edition. Royal Pharmaceutical Society of Great Britain, Pharmaceutical Press, London, UK , 2009 p. 1673-1674
US NLM. ChemIDplus Lite. Sodium bicarbonate
Vinsel PJ; Treatment of acute chlorine gas inhalation with nebulized sodium bicarbonate. J Emerg Med. 1990 May-Jun; 8 (3): 327-9. [PubMed Citation]
Wells BA; Phosgene: a practitioner's viewpoint. Toxicol Ind Health. 1985 Oct; 1 (2): 81-92. [PubMed Citation]
White CW, Martin JG; Chlorine gas inhalation: human clinical evidence of toxicity and experience in animal models. Proc Am Thorac Soc. 2010 Jul; 7 (4): 257-63. [PubMed Citation]
WHO/UNICEF. Breastfeeding and maternal medication: Recommendations for drugs in the eleventh WHO model list of essential drugs (2003)
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
