You are here: Home > Medical Countermeasures Database > Sodium Nitrite
Sodium Nitrite - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Sodium nitrite
2. Chemical Defense therapeutic area(s)
— including key possible usesSodium nitrite can be used for treatment of acute cyanide poisoning in combination with sodium thiosulfate.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
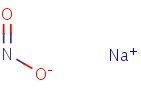
HSDB. Sodium Nitrite
Mechanism of action
-
Exposure to a high dose of cyanide can result in death within minutes due to the inhibition of cytochrome oxidase resulting in arrest of cellular respiration. Specifically, cyanide binds rapidly with cytochrome a3, a component of the cytochrome c oxidase complex in mitochondria. Inhibition of cytochrome a3 prevents the cell from using oxygen and forces anaerobic metabolism, resulting in lactate production, cellular hypoxia and metabolic acidosis. In massive acute cyanide poisoning, the mechanism of toxicity may involve other enzyme systems as well. The synergy resulting from treatment of cyanide poisoning with the combination of sodium nitrite and sodium thiosulfate is the result of differences in their primary mechanisms of action as antidotes for cyanide poisoning.
-
Sodium nitrite is thought to exert its therapeutic effect by reacting with hemoglobin to form methemoglobin, an oxidized form of hemoglobin incapable of oxygen transport but with high affinity for cyanide. Cyanide preferentially binds to methemoglobin over cytochrome a3, forming the nontoxic cyanomethemoglobin. Methemoglobin displaces cyanide from cytochrome oxidase, allowing resumption of aerobic metabolism. The chemical reaction is as follows:
NaNO2 + Hemoglobin → Methemoglobin
HCN + Methemoglobin → Cyanomethemoglobin
Vasodilation has also been cited to account for at least part of the therapeutic effect of sodium nitrite. It has been suggested that sodium nitrite-induced methemoglobinemia may be more efficacious against cyanide poisoning than comparable levels of methemoglobinemia induced by other oxidants. Also, sodium nitrite appears to retain some efficacy even when the formation of methemoglobin is inhibited by methylene blue.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
-
The principal mechanism of nitrite toxicity is the oxidation of the ferrous iron (Fe2+) in deoxyhemoglobin to the ferric (Fe3+) valence state, producing methemoglobin. Methemoglobin cannot reversibly bind or transport circulating oxygen. Depending on the percentage of total methemoglobin in oxidized form, the clinical picture is one of oxygen deprivation with cyanosis, cardiac dysrhythmias and circulatory failure, and progressive central nervous system (CNS) effects. CNS effects can range from mild dizziness and lethargy to coma and convulsions. ... A chocolate-brown or slate-gray central cyanosis (involving the trunk and proximal portions of the limbs, as well as the distal extremities, mucous membranes, and lips) is one of the hallmarks of methemoglobinemia. This cyanosis is due to the dark chocolate-brown color of methemoglobin itself and can become noticeable at a concentration of 10% to 15% of total hemoglobin
ATSDR; Case Studies in Environmental Medicine. NITRATE/NITRITE TOXICITY. p 9-11. Course: SS3054. Revision Date: January 2001 Original Date: October 1991 Expiration Date: January 2007.
-
Cyanide inhibits aerobic metabolism by binding to the binuclear heme center of cytochrome c oxidase (CcOX). Amyl nitrite and sodium nitrite (NaNO(2)) antagonize cyanide toxicity in part by oxidizing hemoglobin to methemoglobin (mHb), which then scavenges cyanide. mHb generation is thought to be a primary mechanism by which the NO(2)(-) ion antagonizes cyanide. On the other hand, NO(2)(-) can undergo biotransformation to generate nitric oxide (NO), which may then directly antagonize cyanide inhibition of CcOX. In this study, nitrite-mediated antagonism of cyanide inhibition of oxidative phosphorylation was examined in rat dopaminergic N27 cells. NaNO(2) produced a time- and concentration-dependent increase in whole-cell and mitochondrial levels of NO. The NO scavenger 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxy 3-oxide (PTIO) reversed this increase in cellular and mitochondrial NO. NO generated from NaNO(2) decreased cellular oxygen consumption and inhibited CcOX activity. PTIO reversed the NO-mediated inhibition, thus providing strong evidence that NO mediates the action of NaNO(2). Under similar conditions, KCN (20muM) inhibited cellular state-3 oxygen consumption and CcOX activity. Pretreatment with NaNO(2) reversed KCN-mediated inhibition of both oxygen consumption and CcOX activity. The NaNO(2) antagonism of cyanide was blocked by pretreatment with the NO scavenger PTIO. It was concluded that NaNO(2) antagonizes cyanide inhibition of CcOX by generating of NO, which then interacts directly with the binding of KCN x CcOX to reverse the toxicity. In vivo antagonism of cyanide by NO(2)(-) appears to be due to both generation of mHb and direct displacement of cyanide from CcOX by NO.
Leavesley HB, Li L, Mukhopadhyay S, Borowitz JL, Isom GE. Nitrite-mediated antagonism of cyanide inhibition of cytochrome c oxidase in dopamine neurons. Toxicol Sci. 2010 Jun; 115(2):569-76. [PubMed Citation]
-
Cyanide quickly and reversibly binds to the ferric iron in cytochrome oxidase, inhibiting effective energy production throughout the body. The ferric iron in methemoglobin preferentially combines with cyanide, producing cyanomethemoglobin. This drives the reaction toward cyanomethemoglobin and liberates cyanide from cytochrome oxidase. Strom-free methemoglobin is effective against four minimum lethal doses of cyanide in rats. Nitrites oxidize the iron in hemoglobin to produce methemoglobin. Because nitrites are accepted antidotes for cyanide poisoning, for many years methemoglobin formation was assumed to be their sole antidotal mechanism of action. Other faster methemoglobin induces, such as 4-dimethyaminophenol and hydroxylamine, also are affective as cyanide antidotes. The production of methemoglobin by nitrite is slow, but when methylene blue is administered to prevent methemoglobin formation, nitrite still is an effective antidote. Reasoning that nitrite-induced vasodilation might be a part of the mechanism of action, investigators considered the antidotal actions of other vasodialators. Only the alpha-adrenergic antagonists and ganglionic blockers demonstrate antidotal activity, and only when administered with sodium thiosulfate. It is possible that the benefits of nitrites given shortly after cyanide result from reversal of cyanide-induced circulatory effects rather than reversal of the effects of cyanide on cytochrome oxidase. Experimental evidence in organ damage induced by hypoxia or hypotension suggest that the benefits of nitrite may be related to its ability to be converted to nitric oxide, a potent vasodilator. The conversion to nitric oxide appears to occur only in tissues or blood with the lowest oxygen concentrations.
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1689-91
Summary of clinical and nonclinical studies
Cyanide is a potent chemical that can cause death within minutes (van Heijst et al., 1987). With several attributes that make it an ideal terrorist weapon, including ease of use as a weapon, versatility in means of delivery to intended victims, and widespread availability, cyanide poses a significant threat to individuals and targeted populations as a terrorist weapon. Sodium nitrite has been a global go-to antidote for cyanide poisoning (van Heijst et al., 1987). If administered intravenously, sodium nitrite is able to convert hemoglobin to cyanide-binding methemoglobin in approximately 12 minutes. It is often, but not always, administered with other agents (amyl nitrite and/or sodium thiosulfate) in a cyanide antidote kit and with other supportive measures such as oxygen. However, the reports on the safety and efficacy of sodium nitrite in treating acute cyanide exposure are contradictory. Several clinical case reports have noted a therapeutic benefit from the use of sodium nitrite combined with other components of the cyanide antidote kit or with other supportive measures (Lasch et al., 1981; Hall et al., 1987; Johnson et al., 1989). Even when administered alone in mice, sodium nitrite has demonstrated effectiveness in treating acute and sublethal cyanide poisoning (Cambal et al., 2011). For mice that were treated with sodium nitrite (3-16 mg/kg), administered IP 2 minutes after receiving a 5.0 mg/kg injection of cyanide, mortality was only 18%, compared to 34% for untreated mice. Based on righting recovery time, the effectiveness of sodium nitrite treatment was time- and dose-dependent: A 4 mg/kg dose of sodium nitrite 4 min after cyanide administration was ineffective, whereas 12 mg/kg of sodium nitrite was the effective minimum antidotal dose when given beyond 2 min after cyanide administration. Although there were no adverse effects after administration of sodium nitrite alone, standard nitrite-thiosulfate combination therapy may result in additive inhibition of cytochrome c oxidase. Also, large doses of sodium nitrite/sodium thiosulfate or even nitrite alone may result in overproduction of methemoglobin (methemoglobinemia), which can reduce the oxygen-carrying capacity of blood (Berlin, 1970; Ten Eyck et al., 1985-1986; van Heijst et al., 1987). Other studies have found the combination of sodium nitrite and sodium thiosulfate to be minimally effective, or less effective than other agents, in treating cyanide poisoning. In a rat model, stroma-free methemoglobin solution (SFMS) or sodium nitrite/sodium thiosulfate was administered 30 seconds after a 36 μmol/kg (one times the lethal dose [1 x LD90]) cyanide injection (Ten Eyck et al., 1985-1986). There was a significant difference (p < 0.002) in mortality between the group given SFMS and the group given nitrite/thiosulfate (20% and 70%, respectively). Nitrite/thiosulfate was only effective against 2 x LD90 of cyanide if animals were pretreated with the combination regimen, whereas posttreatment with SFMS was effective against 4 x LD90 of cyanide. Using a swine model, the effectiveness of the nitrite-thiosulfate combination regimen in treating acute cyanide toxicity was also surpassed by a hydroxocobalamin-thiosulfate regimen (Bebarta et al., 2010). Twenty-four swine were continuously monitored for arterial and cardiac output and received continuous infusion of cyanide (0.2 mg/kg per minute) until severe hypotension. The animals were then randomized to receive either hydroxocobalamin (150 mg/kg, IV)/sodium thiosulfate (413 mg/kg) or sodium nitrite (10 mg/kg)/sodium thiosulfate (413 mg/kg) over the course of 10 minutes. Although there was no statistically significant difference between the antidote combinations in mortality, serum acidosis, or serum lactate levels, the hydroxocobalamin-thiosulfate combination therapy yielded a significantly faster return to baseline mean arterial pressure (p < 0.05). Thus, sodium nitrite appears to offer some efficacy in treating cyanide poisoning, but its efficacy is hampered by its dose-limiting toxicities. Other therapies, such as hydroxocobalamin/sodium thiosulfate, may offer more favorable risk-benefit profiles for the treatment of acute cyanide toxicity
B. Link to clinical studies
Adult
-
A 24-year-old woman ingested an unknown amount of potassium cyanide in a suicide attempt. Coma and metabolic acidosis developed. Administration of the Lilly Cyanide Antidote kit (Eli Lilly and Co, Indianapolis) resulted in prompt resolution of symptoms and full recovery. Whole blood cyanide level was 13 micrograms/mL approximately one hour after ingestion. The highest measured methemoglobin level after sodium nitrite administration was 9.2%, demonstrating that attaining a "therapeutic methemoglobin level" of 25% is unnecessary to insure a satisfactory clinical outcome. Because severe hypotension or excessive methemoglobinemia can be caused by the sodium nitrite component of the Lilly kit, only enough to produce an acceptable clinical response should be administered. (Class IV)
Johnson WS, Hall AH, Rumack BH. Cyanide poisoning successfully treated without 'therapeutic methemoglobin levels'. Am J Emerg Med. 1989 Jul;7(4):437-40. [PubMed Citation]
-
A 34 year old, 73 kg man ingested a 1 gram potassium cyanide pellet in a suicide attempt. Within one hour, coma, apnea, metabolic acidosis, and seizures developed. Sodium nitrite and sodium thiosulfate were administered. Dramatic improvement in the clinical condition occurred by the completion of antidote infusion. Methemoglobin level was 2% immediately after nitrite administration. Serial whole blood cyanide levels were obtained, documenting a highest measured level of 15.68 mcg /mL. Estimations of toxicokinetic parameters including terminal half-life (t 1/2) (19 hours), clearance (163 mL/minute), and volume of distribution (Vd) (0.41 L/kg) were calculated. The nitrite/thiosulfate combination was clinically efficacious in this case and resulted in complete recovery. (Class IV)
Hall AH, Doutre WH, Ludden T, Kulig KW, Rumack BH. Nitrite/thiosulfate treated acute cyanide poisoning: Estimated kinetics after antidote. Clin Toxicol. 1987; 25(1-2):121-133. [PubMed Citation]
-
In three patients with severe acute cyanide poisoning, a cyanosis was observed instead of the bright pink skin coloration often mentioned as a sign in textbooks. Treatment of cardiopulmonary insufficiency is as essential as antidotal therapy and the use of sodium nitrite... is not without risk as, in practice, the methemoglobin-level induced is difficult to control. (Class IV)
van Heijst JM, Douze RG, van Kesteren RG, van Bergen JE, van Dijk A. Therapeutic problems in cyanide poisoning. J Toxicol Clin Toxicol 1987; 25(5):383-398. [PubMed Citation]
-
Two cases of acute poisoning by the inhalation of hydrogen cyanide are described. The first patient regained consciousness 40 minutes after his collapse; in the interval he was treated with inhalations of amyl nitrite and intravenous injections of 0.3 gm. of sodium nitrite and 12.5 gm. of sodium thiosulfate. The second patient remained stuporous during the five hours that followed her collapse; during that interval she received inhalations of oxygen and carbon dioxide and of amyl nitrite as well as an intravenous injection of 0.6 gm. of sodium thiosulfate. Much improvement followed the intravenous injection of 50 cc. of a 1% solution of methylene blue. No dramatic effects were obtained from alternating injections of sodium nitrite and sodium thiosulfate in her case. Both patients made complete recoveries. (Class IV)
Chen KK, Rose CL. Treatment of acute cyanide poisoning. J Am Med Assoc. 1956;162(12):1154-5
-
A 39-year-old woman ingested 59 mL of Super Nail Nail Off (American International Industries, Hollywood, CA) (containing 99% acetonitrile) in a suicide attempt. Following a latent period of approximately 12 hours, the patient developed cyanide poisoning with severe metabolic acidosis, seizures, and shallow respirations. She responded to the administration of sodium nitrite and sodium thiosulfate, although the administration of nitrite produced bradycardia and hypotension. She developed several relapses over the course of her hospitalization and each time responded to sodium thiosulfate administration. The patient developed hypernatremia from the sodium load given to her; hemodialysis and charcoal hemoperfusion were initiated to correct the hypernatremia and to attempt to remove cyanide, thiocyanate, and acetonitrile. On the fifth hospital day, the patient was fully recovered and was discharged. (Class IV)
Turchen SG, Manoguerra AS, Whitney C. Severe cyanide poisoning from the ingestion of an acetonitrile-containing cosmetic. Am J Emerg Med. 1991 May;9(3):264-7. [PubMed Citation]
Pediatric Studies
-
Two episodes of cyanide poisoning occurred in children after ingestion of apricot kernels. The first episode involved eight children who exhibited typical signs and symptoms of cyanide poisoning two hours after having ingested a large amount of apricot kernels. Seven children recovered. One died soon after admission. The second episode involved 16 children who had eaten a sweet prepared from such kernels. The symptoms and signs were identical with those in the first group but appeared one-half hour after the ingestion and were much more severe. Thirteen children recovered, two died shortly after admission, and a third child died two hours later. Apricot kernels contain a cyanogenetic substance called amygdalin, which after hydrolysis, liberates hydrocyanic acid. This activation usually occurs only after ingestion. In the second instance hydrolysis probably occurred during the preparation of the sweet, explaining the short interval between the ingestion and the appearance of the signs of poisoning. /The study describes two cases of the sodium nitrite use to treat cyanide poisoning in children./(Class IV)
Lasch EE and Shawa RE. Multiple cases of cyanide poisoning by apricot kernels in children from Gaza. Pediatrics. 1981; 68(1):5-7. [PubMed Citation]
-
The widely published schedule for the treatment of cyanide poisoning by the nitrite thiosulfate method of Chen is based on adult work and is potentially lethal for children under 25 kg in weight. Unless a dose of sodium nitrite appropriate for the amount of hemoglobin in the child is chosen, lethal methemoglobinemia may result. The dose of NaNO2 for children with a hemoglobin of 12 gm/100 ml is 10 mg/kg per body weight immediately and 5 mg/kg repeated within 30 minutes if necessary. (Class IV)
Berlin CM The treatment of cyanide poisoning in children. The treatment of cyanide poisoning in children. Pediatrics. 1970; 46(5):793-796. [PubMed Citation]
-
Two patients, a 4-year-old girl and her brother 1 1/2 year-old, with cyanide poisoning are reported. They vomited and became comatose 9 hours after ingestion of boiled cassava. At a community hospital, they were intubated and given ventilatory support. The girl was transferred to Ramathibodi Intensive Care Unit. At 19 hours after ingestion, sodium nitrite and sodium thiosulfate were given as well as other supportive treatment. She recovered with normal breathing on the next day. The boy was referred to Ramathibodi 4 hours later. On arrival, he appeared normal except for the bitter almond breathe. Only supportive treatment was given. Their blood cyanide levels on arrival were 0.56 and 0.32 microgram/ml (normal value < 0.3 microgram/ml) respectively confirming the diagnosis of cyanide poisoning. Other abnormal laboratory findings included metabolic acidosis and lactic acidemia. The pathogenesis and management of cyanide poisoning are reviewed. (Class IV)
Ruangkanchanasetr S, Wananukul V, Suwanjutha S. Cyanide poisoning, 2 cases report and treatment review. J Med Assoc Thai. 1999 Nov;82 Suppl 1:S162-7. [PubMed Citation]
-
The case of a 4-yr-old child in whom cyanide poisoning developed with extremely high blood cyanide levels after ingestion of oral laetrile (I) tablet, 12 tablets of 500 mg, successfully treated with amyl nitrate perles by intermittent inhalation followed by 5 ml of 3% sodium nitrite, and 25 ml of 25% sodium thiosulfate, given as intravenous injection, Lilly Cyanide Antidote Package, is reported. Treatment with the Lilly cyanide antidote kit resulted in rapid, complete recovery. It was concluded that although the necessity for using the Lilly cyanide antidote kit in serious cyanide poisoning has been questioned, the case demonstrates the benefit from antidotal treatment. (Class IV)
Hall AH , Linden CH, Kulig KW, Rumack BH; Cyanide poisoning from laetrile ingestion: role of nitrite therapy. Pediatrics. 1986 Aug;78:269-272
Pregnancy, breastfeeding studies
-
Sodium nitrite produces methemoglobin. Fetal hemoglobin is oxidized to methemoglobin more easily than adult hemoglobin. In addition, the fetus has lower levels of methemoglobin reductase than adults. Collectively, these data suggest that the human fetus would show greater sensitivity to methemoglobin resulting in nitrite-induced prenatal hypoxia leading to retarded development of certain neurotransmitter systems in the brain and long lasting dysfunction (Class IV).
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
Geriatric
-
An 80-year-old diabetic patient was admitted to the hospital because of sudden unconsciousness and severe metabolic acidosis. His son reported the possibility of cyanide poisoning. Clinical data and the detection of cyanide in blood and gastric material confirmed this possibility. Supportive therapy and the following antidotes--sodium nitrite two doses 300 mg i.v., sodium thiosulfate 3 g i.v., and hydroxocobalamin 4 g in 24 hours--were administered immediately and the patient completely recovered in 48 hours. Our observations suggest that timely and appropriate use of antidotes for cyanide intoxication may prevent death, even in aged diabetic patients. (Class IV)
Mannaioni G, Vannacci A, Marzocca C, Zorn AM, Peruzzi S, Moroni F. Acute cyanide intoxication treated with a combination of hydroxycobalamin, sodium nitrite, and sodium thiosulfate. J Toxicol Clin Toxicol. 2002;40(2):181-3. [PubMed Citation]
Clinical reviews
-
Severe, acute cyanide poisoning is uncommon and can be very difficult to diagnose if a history of exposure is unavailable. Victims of smoke inhalation may have significant cyanide poisoning as well as carbon monoxide toxicity. The Lilly Cyanide Antidote Kit currently available in America unfortunately has its own inherent toxicity. An efficacious antidote lacking toxicity is desirable, especially in cases where the diagnosis of cyanide poisoning cannot be made with certainty. Hydroxycobalamin/ sodium thiosulfate has been used in France since 1970. Both components have been shown to be safe and efficacious in animal studies. Case reports of human cyanide poisoning treated with hydroxycobalamin/sodium thiosulfate have been published only in French. Animal and human data on the use of this antidotal combination are reviewed. Hydroxycobalamin/sodium thiosulfate is an efficacious cyanide antidote with little inherent toxicity (Class IV)
Hall AH, Rumack BH. Hydroxycobalamin/sodium thiosulfate as a cyanide antidote. J Emerg Med. 1987;5(2):115-21. [PubMed Citation]
-
Confirmed cases of childhood exposure to cyanide are rare despite multiple potential sources including inhalation of fire smoke, ingestion of toxic household and workplace substances, and ingestion of cyanogenic foods. Because of its infrequent occurrence, medical professionals may have difficulty recognizing cyanide poisoning, confirming its presence, and treating it in pediatric patients. The sources and manifestations of acute cyanide poisoning seem to be qualitatively similar between children and adults, but children may be more vulnerable than adults to poisoning from some sources. The only currently available antidote in the United States (the cyanide antidote kit) has been used successfully in children but has particular risks associated with its use in pediatric patients. Because hemoglobin kinetics vary with age, methemoglobinemia associated with nitrite-based antidotes may be excessive at standard adult dosing in children. A cyanide antidote with a better risk/benefit ratio than the current agent available in the United States is desirable. The vitamin B12 precursor hydroxocobalamin, which has been used in Europe, may prove to be an attractive alternative to the cyanide antidote kit for pediatric patients. In this article we review the available data on the sources, manifestations, and treatment of acute cyanide poisoning in children and discuss unmet needs in the management of pediatric cyanide poisoning (Class IV).
Geller RJ, Barthold C, Saiers JA, Hall AH. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006 Nov;118(5):2146-58. [PubMed Citation]
-
Cyanide poisoning is uncommon, but generates interest because of the presumed utility of an antidote immediately available in those areas with a high risk of cyanide exposure. As part of its regular review of guidelines, the Australian Resuscitation Council conducted a systematic review of the human evidence for the use of various proposed cyanide antidotes, and a narrative review of the relevant pharmacological and animal studies. There have been no relevant comparative or placebo-controlled human trials. Nine case series were identified. Treatment with hydroxocobalamin was reported in a total of 361 cases. No serious adverse effects of hydroxocobalamin were reported, and many patients with otherwise presumably fatal poisoning survived. Sodium thiosulphate use was reported in two case series, similarly with no adverse effects. Treatment with a combination of sodium nitrite, amyl nitrite and sodium thiosulphate was reported in 74 patients, with results indistinguishable from those of hydroxocobalamin and sodium thiosulphate. No case series using dicobalt edetate or 4-dimethylaminophenol were identified, but successful use in single cases has been reported. Hydroxocobalamin and sodium thiosulphate differ from alternatives in having negligible adverse effects, and on the basis of current evidence are the antidotes of choice. The indications for the use of an antidote, the requirements for supportive care and a recommended approach for workplaces where there is a risk of cyanide poisoning are presented (Class IV).
Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Review article: management of cyanide poisoning. Emerg Med Australas. 2012 Jun;24(3):225-38. [PubMed Citation]
-
The combination of sodium thiosulfate and sodium nitrite has been used in the United States since the 1930s as the primary antidote for cyanide intoxication. Although this combination was shown to exhibit much greater efficacy than either ingredient alone, the two compounds could not be used prophylactically because each exhibits a number of side effects. This review discusses the pharmacodynamics, pharmacokinetics, and toxicology of the individual agents, and their combination (Class IV).
Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992 Apr;32(4):368-75. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
Study objective: Cyanide can cause severe hypotension with acute toxicity. To /the authors/ knowledge, no study has directly compared hydroxocobalamin and sodium nitrite with sodium thiosulfate in an acute cyanide toxicity model. /Their/ objective is to compare the return to baseline of mean arterial blood pressure between 2 groups of swine with acute cyanide toxicity and treated with hydroxocobalamin with sodium thiosulfate or sodium nitrite with sodium thiosulfate. Methods: Twenty-four swine were intubated, anesthetized, and instrumented (continuous arterial and cardiac output monitoring) and then intoxicated with a continuous cyanide infusion until severe hypotension. The animals were divided into 2 arms of 12 each and then randomly assigned to intravenous hydroxocobalamin (150 mg/kg) sodium thiosulfate (413 mg/kg) or sodium nitrite (10 mg/kg) sodium thiosulfate (413 mg/kg) and monitored for 40 minutes after start of antidotal infusion. Twenty animals were needed for 80% power to detect a significant difference in outcomes (< 0.05). Repeated measures of analysis of covariance and post hoc t test were used for determining significance. Results: Baseline mean weights, time to hypotension (31 minutes 3 seconds versus 28 minutes 6 seconds), and cyanide dose at hypotension (5.6 versus 5.9 mg/kg) were similar. One animal in the hydroxocobalamin group and 2 animals in the sodium nitrite group died during antidote infusion and were excluded from analysis. Hydroxocobalamin resulted in a faster return to baseline mean arterial pressure, with improvement beginning at 5 minutes and lasting through the conclusion of the study (P<.05). No statistically significant difference was detected between groups for cardiac output, pulse rate, systemic vascular resistance, or mortality at 40 minutes postintoxication. Mean cyanide blood levels (4.03 versus 4.05 microg/mL) and lactate levels (peak 7.9 versus 8.1 mmol/L) at hypotension were similar. Lactate levels (5.1 versus 4.48 mmol/L), pH (7.40 versus 7.37), and base excess (-0.75 versus 1.27) at 40 minutes were also similar. Conclusion: Hydroxocobalamin with sodium thiosulfate led to a faster return to baseline mean arterial pressure compared with sodium nitrite with sodium thiosulfate; however, there was no difference between the antidote combinations in mortality, serum acidosis, or serum lactate.
Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier S, Bush A. Hydroxocobalamin and Sodium Thiosulfate Versus Sodium Nitrite and Sodium Thiosulfate in the Treatment of Acute Cyanide Toxicity in a Swine (Sus scrofa) Model. Ann Emerg Med. 2010 Apr;55:345-351. [PubMed Citation]
-
The standard nitrite/thiosulfate regimen for cyanide poisoning was tested in /a/rat model. By modifying the treatment regimen and the nitrite solution an effective antidote against an LD of cyanide could be produced. However, this treatment was effective against two times the LD only when administered ten minutes prior to cyanide injection. These results are marked contrast to ... results with stroma-free methemoglobin solutions (SFMS) which showed SFMS to be highly effective antidote against four times the LD when administered 30 seconds after an intravenous injection of cyanide SFMS proved to be an effective antidote for two times the LD when administered up to sixty seconds after the cessation of respiration.
Ten Eyck RP, Schaerdel AD, Ottinger WE. Comparison of nitrite treatment and stroma-free methemoglobin solution as antidotes for cyanide poisoning in a rat model. J Toxicol Clin Toxicol. 1985-1986;23(7-8):477-87. [PubMed Citation]
-
Sodium nitrite alone is shown to ameliorate sublethal cyanide toxicity in mice when given from about 1 h before until 20 min after the toxic dose as demonstrated by the recovery of righting ability. An optimum dose (12 mg/kg) was determined to significantly relieve cyanide toxicity (5.0 mg/kg) when administered to mice intraperitoneally. Nitrite so administered was shown to rapidly produce NO in the bloodsteam as judged by the dose-dependent appearance of EPR (Electron Paramagnetic Resonance) signals attributable to nitrosylhemoglobin and methemoglobin. It is argued that antagonism of cyanide inhibition of cytochrome c oxidase by NO is the crucial antidotal activity rather than the methemoglobin-forming action of nitrite. Concomitant addition of sodium thiosulfate to nitrite-treated blood resulted in the detection of sulfidomethemoglobin by EPR spectroscopy. Sulfide is a product of thiosulfate hydrolysis and, like cyanide, is known to be a potent inhibitor of cytochrome c oxidase, the effects of the two inhibitors being essentially additive under standard assay conditions rather than dominated by either one. The findings afford a plausible explanation for an observed detrimental effect in mice associated with the use of the standard nitrite-thiosulfate combination therapy at sublethal levels of cyanide intoxication.
Cambal LK, Swanson MR, Yuan Q, Weitz AC, Li HH, Pitt BR, Pearce LL, Peterson J. Acute, sublethal cyanide poisoning in mice is ameliorated by nitrite alone: complications arising from concomitant administration of nitrite and thiosulfate as an antidotal combination. Chem Res Toxicol. 2011 Jul 18;24(7):1104-12. [PubMed Citation]
-
The objective of this study was to evaluate the effectiveness of five regimens in treating cyanide poisoning. A series of anesthetized adult beagle dogs were instrumented to record hemodynamic and respiratory function and given 2.5 mg/kg sodium cyanide intravenously. The 10 control animals given only cyanide died at from 5 to 7 minutes. Therapy, as described below, was given to other groups at from 2 to 3 minutes following the cyanide administration. Artificial respiration did not alter the lethal effects of cyanide nor prolong survival time in any of the 10 animals. Amyl nitrite given by inhalation or by the intravenous route allowed survival of all 15 animals. Sodium nitrite (20 mg/kg), dimethylaminophenol (DMAP) (5 mg/kg), and hydroxylamine hydrochloride (5 mg/kg) given intravenously with no artificial ventilation also allowed for 100% survival (15 animals). Amyl nitrite, sodium nitrite, and sodium thiosulfate were ineffective when given intramuscularly (I.M.) (0 of 12 dogs); however, I.M. DMAP (5 mg/kg) and I.M. hydroxylamine hydrochloride (50 mg/kg) increased heart rate and blood pressure and restored spontaneous breathing. All 15 animals treated with I.M. doses of either of these drugs survived the lethal dose of cyanide. Results of these studies indicate that intravenous sodium nitrite, DMAP, and hydroxylamine hydrochloride, and amyl nitrite by inhalation, are all effective in reversing the lethal effects of cyanide poisoning. Only DMAP and hydroxylamine hydrochloride are effective when given by the intramuscular route. These results provide data to support an approach to therapy that is more practical and applicable where expert medical care may not be available following cyanide exposure.
Vick JA, Froehlich H. Treatment of cyanide poisoning. Mil Med. 1991 Jul;156(7):330-9. [PubMed Citation]
-
An estimated 35% of all fire victims in the United States have toxicologically significant blood levels of CO and CN. However, the treatment of concurrent CO/CN intoxication has been paid scant attention. The suggestion has been made that these victims should be treated for CN poisoning. The current therapeutic management of CN poisoning in this country includes the utilization of two methemoglobin formers: amyl nitrite and sodium nitrite. This study was undertaken to determine if the administration of methemoglobin formers is advisable, as the victim is already suffering from O2 deprivation due to the presence of carboxyhemoglobin. Groups of 28 male ICR mice (22-24 g) were injected i.p. with 5.0 mg/kg of KCN and then were exposed immediately to 0.35% CO for 8.5 min in a dynamic inhalation chamber. Half of the animals were marked randomly for antidotal intervention, the other 14 animals acted as controls. Treatment of survivors with amyl nitrite (12 mg/l of chamber air) for 1 min increased mortality 43%, whereas treatment for 2 min resulted in a 59% increase in mortality. A 25% increase in mortality was noted among those animals treated with sodium nitrite (80 mg/kg i.p.), as compared to the nontreated control survivors. Treatment with dimethylaminophenol (49 mg/kg i.p.) did not statistically affect mortality.
Moore SJ, Norris JC, Walsh DA, Hume AS. Antidotal use of methemoglobin forming cyanide antagonists in concurrent carbon monoxide/cyanide intoxication. J Pharmacol Exp Ther. 1987 Jul;242(1):70-3. [PubMed Citation]
-
To test the efficacies of various antidotes to cyanide (CN) poisoning, the lethal dose of cyanide in dogs was estimated during constant infusion of potassium cyanide at a rate of 0.1 mg/kg/min. Additionally, arterial blood pressure, right ventricular pressure, heart rate, electrocardiogram, blood-gas and pH values, and whole blood and tissue CN concentrations were measured. The lethal dose in animals whose lungs were ventilated with room air was 2.4 +/- 0.2 mg/kg (mean +/- SE), while the whole-blood CN concentration was 438 +/- 40 micrograms/dl and the gracilis muscle concentration was 2.0 +/- 0.3 micrograms/100 g. A low dose of vitamin B12a (100 mg/kg), an infusion of thiosulfate (12 mg/kg/h), or ventilation with 100 per cent O2 increased the amount of CN needed to cause death. A bolus injection of nitrite (5 mg/kg), thiosulfate (150 mg/kg), or cysteine (450 mg/kg) increased the protection from lethality even further. Protection against CN administration for the total 150-min period of observation was provided by a bolus injection plus a constant infusion of nitrite (5 mg/kg bolus plus 5 mg/kg/h). thiosulfate (30 mg/kg bolus plus 60 mg/kg/h), or vitamin B12a (50 mg/kg bolus plus 100 mg/kg/h). However, nitrite infusion produced high levels of methemoglobin 7.2 +/- 1.1 g/dl, while vitamin B12a infusion and cysteine injection, at the stated doses, did not prevent cyanide-induced circulatory failure. Therefore, thiosulfate appears to be the most effective and safest prophylactic agent against cyanide toxicity in dogs.
Ivankovich AD, Braverman B, Kanuru RP, Heyman HJ, Paulissian R. Cyanide antidotes and methods of their administration in dogs: a comparative study. Anesthesiology. 1980 Mar;52(3):210-6. [PubMed Citation]
Pregnant animal studies
-
Sodium nitrite has caused fetal death in humans as well as animals. There are no studies in humans that have directly evaluated the potential reproductive toxicity of sodium nitrite. The potential reproductive toxicity of sodium nitrite exposure restricted to the prenatal period has been reported in guinea pigs, mice, and rats. There was no evidence of teratogenicity in guinea pigs, mice, or rats. However, sodium nitrite treatment of pregnant guinea pigs with 60 or 70 mg/kg/day resulted in abortion of the litters within 1-4 days of treatment. All animals treated subcutaneously with 70 mg/kg, sodium nitrite died within 60 minutes of treatment. Further studies demonstrated that a dose of 60 mg/kg resulted in measurable blood levels of methemoglobin in the dams and their fetuses for up to 6 hours post treatment. Maternal methemoglobin levels were higher than the levels in the offspring at all times measured. Based on a body surface area comparison, a 60 mg/kg dose in the guinea pig that resulted in death was only 1.7 times higher than the highest clinical dose of sodium nitrite that would be used to treat cyanide poisoning (based on a body surface area comparison).
-
Studies testing prenatal and postnatal exposure have been reported in mice and rats. Treatment of pregnant rats via drinking water with sodium nitrite at concentrations of either 2000 or 3000 mg/L resulted in a dose-related increased mortality postpartum. This exposure regimen in the rat model would result in dosing of approximately 220 and 300 mg/kg/day (43 and 65 times the highest clinical dose of sodium nitrite that would be used to treat cyanide poisoning, based on a body surface area comparison).
-
Behavioral and neurodevelopmental studies in rats suggest persistent effects of prenatal exposure to sodium nitrite that were detectable postnatally. Specifically, animals that were exposed prenatally to sodium nitrite demonstrated impaired discrimination learning behavior (both auditory and visual) and reduced long-term retention of the passive-avoidance response compared to control animals. Additional studies demonstrated a delay in the development of AchE and 5-HT positive fiber ingrowth into the hippocampal dentate gyrus and parietal neocortex during the first week of life of prenatal nitrite treated pups. These changes have been attributed to prenatal hypoxia following nitrite exposure.
-
In studies conducted with Long-Evans rats, sodium nitrite administered in drinking water during pregnancy and lactation resulted in severe anemia, reduced growth and increased mortality in the offspring.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Sodium nitrite is a strong oxidant, and reacts rapidly with hemoglobin to form methemoglobin. The pharmacokinetics of free sodium nitrite in humans have not been well studied. It has been reported that approximately 40% of sodium nitrite is excreted unchanged in the urine while the remaining 60% is metabolized to ammonia and related small molecules.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
Cyanide poisoning:
- Sodium Nitrite - 10 mL of a 3% solution (300 mg) of sodium nitrite at the rate of 2.5 to 5 mL/minute
- Sodium Thiosulfate - 50 mL of a 25% solution (12.5 g) of a sodium thiosulfate solution immediately following administration of sodium nitrite.
Note: If signs of poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate.
In adult and pediatric patients with known anemia, it is recommended that the dosage of sodium nitrite should be reduced proportionately to the hemoglobin concentration.
All parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
Children (FDA)
Cyanide poisoning:
- Sodium Nitrite - 0.2 mL/kg of a 3% solution (6 mg/kg or 6-8 mL/m2 BSA) of sodium nitrite at the rate of 2.5 to 5 mL/minute not to exceed 10 mL (300 mg).
- Sodium Thiosulfate - 1 mL/kg of body weight using a 25% solution (250 mg/kg or approximately 30-40 mL/m2 of BSA) not to exceed 50 mL (12.5 g) total dose immediately following administration of sodium nitrite.
NOTE: If signs of poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate.
In adult and pediatric patients with known anemia, it is recommended that the dosage of sodium nitrite should be reduced proportionately to the hemoglobin concentration.
All parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
Pregnancy (FDA)
Both sodium nitrite and sodium thiosulfate are Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women. NITHIODOTE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Because fetal hemoglobin is more readily oxidized to methemoglobin and lower levels of methemoglobin appear to be fatal to the fetus compared to the adult, sodium nitrite should be used during labor and delivery only if the potential benefit justifies the potential risk to the fetus.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
Nursing Mothers (FDA)
It is not known whether sodium nitrite or sodium thiosulfate is excreted in human milk. Because NITHIODOTE may be administered in life-threatening situations, breast-feeding is not a contraindication to its use. Because many drugs are excreted in human milk, caution should be exercised following NITHIODOTE administration to a nursing woman. There are no data to determine when breastfeeding may be safely restarted following administration of sodium nitrite and sodium thiosulfate. In studies conducted with Long-Evans rats, sodium nitrite administered in drinking water during pregnancy and lactation resulted in severe anemia, reduced growth and increased mortality in the offspring.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
Geriatric (FDA)
Sodium nitrite and sodium thiosulfate are known to be substantially excreted by the kidney, and the risk of adverse reactions to these drugs may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
Renal Impairment (FDA)
Sodium nitrite and sodium thiosulfate are known to be substantially excreted by the kidney, and the risk of toxic reactions to these drugs may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Sodium Nitrite has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Formulation
Parenteral Injection 30 mg per mL*
* NITHIODOTE Injection consists of:one vial of sodium nitrite injection, USP 300 mg/10 mL (30 mg/mL) and one vial of sodium thiosulfate injection USP 12.5 grams/50 mL (250 mg/mL). Administration of one vial of each medication constitutes a single dose.
Shelf life
Shelf life
-
The New Drug Application (NDA) for Nithiodote (NDA 201444) only permits the marketing of the two drug products, sodium nitrite and sodium thiosulfate, as components of one package. Since the drug products have different expiry dates, this has necessitated that product users, such as the military and hospitals, replace the entire package when only one of the components has reached its expiry date. By submitting separate applications for the two products, the Applicant intends to market the components separately, thus increasing the flexibility for product users as they manage their supply stocks.
Center for Drug Evaluation and Research: Summary Review for Regulatory Action. February 14, 2012 (FDA)
Storage
-
Store at controlled room temperature between 20°C and 25°C (68°F - 77°F); excursions permitted to 15-30°C (59 to 86°F). Protect from direct light. Do not freeze.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Cyanide Antidote Package (Amyl Nitrite Inhalants: 0.3 mL [12 ampoules], Sodium Nitrite: 200 mg/10 mL [2 ampoules], Sodium Thiosulfate: 12.5 mg/50 mL [2 viles]). Cyanide poisoning (adult dosage): apply 1 ampoule of Amyl Nitrite to a handkerchief and hold in front of patient's mouth for 15 seconds followed by a rest for 15 seconds. Then reapply until Sodium Nitrite can be administered. Discontinue Amyl Nitrite and give Sodium Nitrite IV 300 mg at a rate of 2.5 to 5 mL/minute. Immediately after inject 12.5 g of Sodium Thiosulfate.
Bartlett JG, Greenberg MI, eds. PDR Guide to Terrorism Response. Montvale, NJ: Thomson PDR, 2005 p.321-41
-
Hydrogen sulfide poisoning
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.682-3
Children
-
Cyanide Antidote Package (Amyl Nitrite Inhalants: 0.3 mL [12 ampoules], Sodium Nitrite: 200 mg/10 mL [2 ampoules], Sodium Thiosulfate: 12.5 mg/50 mL [2 viles]). Cyanide poisoning (children dosage): apply 1 ampoule of Amyl Nitrite to a handkerchief and hold in front of patient's mouth for 15 seconds followed by a rest for 15 seconds. Then reapply until Sodium Nitrite can be administered. Discontinue Amyl Nitrite and give 6-8 mL/sq m of Sodium Nitrite IV, max of 12.5 g. Immediately after inject 7 g/sq m of Sodium Thiosulfate, max of 12.5 g.
Bartlett JG, Greenberg MI, eds. PDR Guide to Terrorism Response. Montvale, NJ: Thomson PDR, 2005 p.321-41
8. Route of Administration/Monitoring
-
Sodium nitrite injection and sodium thiosulfate injection are administered by slow intravenous injection. They should be given as early as possible after a diagnosis of acute life-threatening cyanide poisoning has been established. Sodium nitrite should be administered first, followed immediately by sodium thiosulfate. Blood pressure must be monitored during infusion in both adults and children. The rate of infusion should be decreased if significant hypotension is noted.
-
Patients should be monitored for at least 24-48 hours after NITHIODOTE administration for adequacy of oxygenation and perfusion and for recurrent signs and symptoms of cyanide toxicity. When possible, hemoglobin/hematocrit should be obtained when treatment is initiated. Measurements of oxygen saturation using standard pulse oximetry and calculated oxygen saturation values are unreliable in the presence of methemoglobinemia. Administrations of sodium nitrite solely to achieve an arbitrary level of methemoglobinemia may be unnecessary and potentially hazardous. The therapeutic effects of sodium nitrite do not appear to be mediated by methemoglobin formation alone and clinical responses to sodium nitrite administration have been reported in association with methemoglobin levels of less than 10%. Administration of sodium nitrite beyond the initial dose should be guided primarily by clinical response to treatment (a second dose should be considered only if there is inadequate clinical response to the first dose). It is generally recommended that methemoglobin concentrations be closely monitored and kept below 30%. Serum methemoglobin levels should be monitored during treatment using co-oximetry, and administration of sodium nitrite should generally be discontinued when methemoglobin levels exceed 30%. Intravenous methylene blue and exchange transfusion have been reported in the literature as treatments for life-threatening methemoglobinemia.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
9. Adverse effects
-
Warning: life threatening hypotension and methemoglobin formation
Sodium nitrite can cause serious adverse reactions and death in humans, even at doses less than twice the recommended therapeutic dose. Sodium nitrite causes hypotension and methemoglobin formation, which diminishes oxygen carrying capacity. Hypotension and methemoglobin formation can occur concurrently or separately. Because of these risks, sodium nitrite should be used to treat acute life-threatening cyanide poisoning and be used with caution in patients where the diagnosis of cyanide poisoning is uncertain. Patients should be closely monitored to ensure adequate perfusion and oxygenation during treatment with sodium nitrite.
Alternative therapeutic approaches should be considered in patients known to have diminished oxygen or cardiovascular reserve (e.g., smoke inhalation victims, pre-existing anemia, cardiac or respiratory compromise), and those at higher risk of developing methemoglobinemia (e.g., congenital methemoglobin reductase deficiency) as they are at greater risk for potentially life-threatening adverse events related to the use of sodium nitrite.
-
There have been no controlled clinical trials conducted to systematically assess the adverse events profile of sodium nitrite or sodium thiosulfate.
-
The medical literature has reported the following adverse events in association with sodium nitrite or sodium thiosulfate administration. These adverse events were not reported in the context of controlled trials or with consistent monitoring and reporting methodologies for adverse events. Therefore, frequency of occurrence of these adverse events cannot be assessed.
-
Cardiovascular system: syncope, hypotension, tachycardia, methemoglobinemia, palpitations, dysrhythmia.
-
Hematological: methemoglobinemia
-
Central nervous system: headache, dizziness, blurred vision, seizures, confusion, coma
-
Gastrointestinal system: nausea, vomiting, abdominal pain
-
Respiratory system: tachypnea, dyspnea
-
Body as a Whole: anxiety, diaphoresis, lightheadedness, injection site tingling, cyanosis, acidosis, fatigue, weakness, urticaria, generalized numbness and tingling
-
Severe hypotension, methemoglobinemia, cardiac dysrhythmias, coma and death have been reported in patients without life-threatening cyanide poisoning but who were treated with injection of sodium nitrite at doses less than twice those recommended for the treatment of cyanide poisoning.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
10. Contraindication(s)
-
None, but sodium nitrite should be used with caution in the presence of other drugs that may cause methemoglobinemia such as procaine and nitroprusside. Sodium nitrite should be used with caution in patients who may be particularly susceptible to injury from vasodilation and its related hemodynamic sequelae. Sodium nitrite should be used with caution in patients with known anemia. Sodium nitrite should be used with caution in persons with smoke inhalation injury or carbon monoxide poisoning because of the potential for worsening hypoxia due to methemoglobin formation. Because patients with G6PD deficiency are at increased risk of a hemolytic crisis with sodium nitrite administration, alternative therapeutic approaches should be considered in these patients. Sodium nitrite should be used with caution in the presence of concomitant antihypertensive medications, diuretics or volume depletion due to diuretics, or drugs known to increase vascular nitric oxide, such as PDE5 inhibitors.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues-
Title: Safety, Tolerability, and PK Parameters of Sodium Nitrite Inhalation Solution in Healthy Subjects.
Conditions: Pulmonary Hypertension; Pulmonary Arterial Hypertension
Interventions: Drug: 15 mg sodium nitrite inhalation solution; Drug: 90 mg sodium nitrite inhalation solution; Drug: 45 mg sodium nitrite inhalation solution; Drug: 120 mg sodium nitrite inhalation solution; Drug: 25% MTD sodium nitrite inhalation solution
ClinicalTrials.gov.Sodium Nitrite
-
Title: Development of a field-deployable device to rapidly measure blood cyanide levels
The objective of this proposal is to assess the feasibility of a fully integrated device for the rapid and early diagnosis of cyanide poisoning in whole blood using the spectral shift of the vitamin B12 precursor cobinamide upon binding with cyanide as an indicator. Cyanide is an extremely potent and rapid acting poison with as little as 50 mg fatal to humans. Currently there are no portable rapid tests for the detection of cyanide in whole blood available. The primary goal of this proposal is to demonstrate feasibility of the cobinamide-cyanide chemistry in a rapid test using a whole blood sample from a finger-stick. The total assay time from the sample collection to a valid result will be less than 5 minutes. The innovation in this proposal is to incorporate the cobinamide chemistry with blood separation technology, fluid path designs, and a detection area, all integrated in a compact, disposable device which can be interpreted with a handheld visualization system. Feasibility work of this novel rapid assay for detecting blood cyanide through cobinamide will initially focus on several technical approaches for the device design. Parallel paths to pursue a wet chemistry design, a design where the reagents are dried in the device, and a combination thereof will be considered, compared and evaluated. In all of the proposed methods the whole blood sample will be added to the device, followed by an integrated whole blood processing step to yield a plasma sample. Either prior to or immediately after the whole blood separation and lysis, the sample will be mixed with the on-board reagent, dried or wet, and possibly buffers. The processed sample-reagent mixture then moves through the device through various fluid paths to a detection area. To allow for sufficient mixing time between the sample and the reagents, different fluid paths, mixing chambers, polymeric time-gates, and dissolvable films will be evaluated with the goal of maximizing the interaction time. The spectral shift of cobinamide upon binding of cyanide to cobinamide will be measured in the detection area using a handheld or small, portable reader instrument. Upon successful completion of this Phase 1 project we intend to further develop our concept/prototype assay, complete the device design and integrate the finished test with a reader instrument through a Phase 2 proposal. Specific aims for a Phase 2 of this project include the validation of the cobinamide-based device for measuring cyanide in blood. Results will be compared to two established methods for measuring cyanide in biological samples. The sensitivity, specificity, accuracy, precision, and reproducibility of the devices will be determined. Additional studies will be designed to obtain regulatory approval and subsequently we will commercialize the test. If successful, this test will provide the medical community and first responders with a fast, reliable and economic alternative to determine cyanide poisoning. PUBLIC HEALTH RELEVANCE: Cyanide poisoning has been recognized as a threat from smoke inhalation and potentially through weapons of mass destruction, but there are currently no methods available to rapidly detect cyanide in blood from at-risk patients. The objective of the proposed work is to assess the feasibility of a fully integrated method for the rapid and early detection and diagnosis of cyanide poisoning in whole blood using the spectral shift of the vitamin B12 precursor cobinamide upon binding with cyanide as an indicator. Successful accomplishment of the goals of this project will be the basis for the development of a test to detect and measure cyanide blood poisoning which will allow healthcare providers to treat patients quickly and effectively.
RePORTER. NIH. Development of a field-deployable device to rapidly measure blood cyanide levels.
-
Title: Countermeasures against chemical threats: countermeasures against cyanide
Sources for potential exposure to cyanide of both civilians and military personnel include combustion of nitrogenous materials, commercial accidents and the deliberate release of a cyanogenic chemical. The relative ease in obtaining and releasing cyanide means that the risk of cyanide use in a terrorist attack resulting in a mass casualty situation should not be ignored. Rapid intervention is critical for effective medical intervention in cases of cyanide exposure: treatments require a "three minute solution."
The availability of a rapid, IM injectable antidote should meet this requirement. However, there are no such available treatments - the current cyanide antidotes are administered intravenously. Because intravenous administration is time consuming and requires well trained medical personnel mass exposure to cyanide would likely leave many victims untreated. Because IM administration can be performed via an autoinjector and can therefore be done rapidly by minimally trained personnel there is a critical need for a rapid-acting, IM-injectable antidote for the treatment of mass cyanide casualties. In order to address this need /the authors/ are advancing /a/preclinical lead, sulfanegen to clinical development. /They/ have previously demonstrated efficacy of sulfanegen in murine, swine and rabbit models of cyanide toxicity. In these models sulfanegen is effective in reversal of cyanide toxicity by IM injection and therefore should meet the three minute solution. /The authors/ have recently held a pre-IND meeting with the FDA and /their/ goal is to advance this cyanide antagonist to the clinic by validation of animal models, demonstrate efficacy of sulfanegen in these animal models and perform the required GLP pharmacokinetic and safety evaluation required for clinical trials. /The authors/ will then commence a Phase 1 human safety study while simultaneously performing the required GLP studies for NDA submission under the animal rule. Successful completion of the aims of this project should lead to a clinical countermeasure for cyanide toxicity that will be applicable in all cases of cyanide exposure from individuals to mass casualty settings. PUBLIC HEALTH RELEVANCE: /The authors/ have discovered a novel cyanide antidote that /they/ have named sulfanegen and demonstrated that it is more effective than existing antidotes in models of cyanide toxicity. /They/will perform the necessary studies to translate this antidote from the bench to the bedside. Successful completion of the goals of this project will therefore result in a clinical antidote that could be useful in the case of a terrorist attack involving cyanide.
RePORTER. NIH. Countermeasures against chemical threats: countermeasures against cyanide.
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations-
A better understanding of the mechanism of action of cyanide at the molecular level is needed.
-
The use of antidotes to cyanide poisoning in patients exposed to other toxic hazards such as smoke or industrial chemicals needs further evaluation.
-
Models should be developed to characterize the long-term effects of acute exposure to cyanide and identify how cyanide-containing compounds are metabolized.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendationsStudies of pediatric fire victims
-
Hydroxycobalamin versus cyanide antidote kit
-
Need more sensitive measure of blood cyanide
-
Consider three-arm trial (one group receives all)
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
A better understanding of the mechanism of action of cyanide at the molecular level is needed.
-
The use of antidotes to cyanide poisoning in patients exposed to other toxic hazards such as smoke or industrial chemicals needs further evaluation.
-
Models should be developed to characterize the long-term effects of acute exposure to cyanide and identify how cyanide-containing compounds are metabolized.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
15. Study-related ethical concerns
— including review panel recommendations- No data available at this time.
16. Global regulatory status
U.S.
NITHIODOTE is indicated for the treatment of acute cyanide poisoning that is judged to be life-threatening. When the diagnosis of cyanide poisoning is uncertain, the potentially life-threatening risks associated with NITHIODOTE should be carefully weighed against the potential benefits, especially if the patient is not in extremis.
Caution should be exercised when administering other cyanide antidotes simultaneously with NITHIODOTE, as the safety of co-administration has not been established. If a decision is made to administer another cyanide antidote with NITHIODOTE, these drugs should not be administered concurrently in the same IV line.
Comprehensive treatment of acute cyanide intoxication requires support of vital functions. Administration of sodium nitrite and sodium thiosulfate should be considered adjunctive to appropriate supportive therapies. Airway, ventilatory and circulatory support, and oxygen administration should not be delayed to administer sodium nitrite and sodium thiosulfate.
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
E.U.
-
For cyanide intoxication: Sodium nitrite 3% or 30 mg/ml in 10 ml vials indicated as a second choice when complexation agents (hydroxocobalamin, dicobalt edentate) are not available.
Adults: 10 ml = 300 mg dose IV at an infusion rate of 2.5 ml/min. Additional half-sized dose, if no response in 30 to 60 min.
Children: 0.3 ml/kg = 10 mg/kg at an infusion rate of 2.5 ml/min to max of 10 ml. Additional half-sized dose, if no response in 30 to 60 min.
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
U.K.
-
Poisoning with cyanides (used in conjuction with Sodium thiosulphate).
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 34
Other
17. Other potentially useful information
-
Traditional treatment of cyanide poisoning involves a two-step process: administration of amyl or sodium nitrite followed by administration of sodium thiosulfate. In a mass casualty scenario, there are issues with administering the traditional antidotes amyl nitrite and sodium nitrite: (1) the degree of methemoglobinemia can be exceeded, which is critical in pediatric treatment, and (2) because the nitrites are vasodilators, hypotension may be induced. A new antidote- hydroxycobalamin (HC)- does not have these issues. HC is used extensively in Europe and by emergency medical services (EMS) in the United States. It is approved by FDA as an antidote for cyanide toxicity.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Solubility: 84.8 g/100 g of water at 25 deg C
HSDB. Sodium Nitrite
18. Publications
ATSDR; Case Studies in Environmental Medicine. NITRATE/NITRITE TOXICITY. p 9-11. Course: SS3054. Revision Date: January 2001 Original Date: October 1991 Expiration Date: January 2007.
Bartlett JG, Greenberg MI, eds. PDR Guide to Terrorism Response. Montvale, NJ: Thomson PDR, 2005 p.321-41
Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992 Apr;32(4):368-75. [PubMed Citation]
Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier S, Bush A. Hydroxocobalamin and Sodium Thiosulfate Versus Sodium Nitrite and Sodium Thiosulfate in the Treatment of Acute Cyanide Toxicity in a Swine (Sus scrofa) Model. Ann Emerg Med. 2010 Apr;55:345-351. [PubMed Citation]
Berlin CM. The treatment of cyanide poisoning in children. The treatment of cyanide poisoning in children. Pediatrics. 1970; 46(5):793-796. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Cambal LK, Swanson MR, Yuan Q, Weitz AC, Li HH, Pitt BR, Pearce LL, Peterson J. Acute, sublethal cyanide poisoning in mice is ameliorated by nitrite alone: complications arising from concomitant administration of nitrite and thiosulfate as an antidotal combination. Chem Res Toxicol. 2011 Jul 18;24(7):1104-12. [PubMed Citation]
Center for Drug Evaluation and Research: Summary Review for Regulatory Action. February 14, 2012 (FDA)
Chen KK, Rose CL. Treatment of acute cyanide poisoning. J Am Med Assoc. 1956;162(12):1154-5
ClinicalTrials.gov.Sodium Nitrite
DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
Geller RJ, Barthold C, Saiers JA, Hall AH. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006 Nov;118(5):2146-58. [PubMed Citation]
Hall AH, Rumack BH. Hydroxycobalamin/sodium thiosulfate as a cyanide antidote. J Emerg Med. 1987;5(2):115-21. [PubMed Citation]
Hall AH, Doutre WH, Ludden T, Kulig KW, Rumack BH. Nitrite/thiosulfate treated acute cyanide poisoning: Estimated kinetics after antidote. Clin Toxicol. 1987; 25(1-2):121-133. [PubMed Citation]
Hall AH, Linden CH, Kulig KW, Rumack BH; Cyanide poisoning from laetrile ingestion: role of nitrite therapy. Pediatrics. 1986 Aug;78:269-272
HSDB. Sodium Nitrite
Ivankovich AD, Braverman B, Kanuru RP, Heyman HJ, Paulissian R. Cyanide antidotes and methods of their administration in dogs: a comparative study. Anesthesiology. 1980 Mar;52(3):210-6. [PubMed Citation]
Johnson WS, Hall AH, Rumack BH. Cyanide poisoning successfully treated without 'therapeutic methemoglobin levels'. Am J Emerg Med. 1989 Jul;7(4):437-40. [PubMed Citation]
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.682-3
Lasch EE, Douze RG and Shawa RE. Multiple cases of cyanide poisoning by apricot kernels in children from Gaza. Pediatrics. 1981; 68(1):5-7. [PubMed Citation]
Leavesley HB, Li L, Mukhopadhyay S, Borowitz JL, Isom GE. Nitrite-mediated antagonism of cyanide inhibition of cytochrome c oxidase in dopamine neurons. Toxicol Sci. 2010 Jun; 115(2):569-76. [PubMed Citation]
Mannaioni G, Vannacci A, Marzocca C, Zorn AM, Peruzzi S, Moroni F. Acute cyanide intoxication treated with a combination of hydroxycobalamin, sodium nitrite, and sodium thiosulfate. J Toxicol Clin Toxicol. 2002;40(2):181-3. [PubMed Citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 34
Moore SJ, Norris JC, Walsh DA, Hume AS. Antidotal use of methemoglobin forming cyanide antagonists in concurrent carbon monoxide/cyanide intoxication. J Pharmacol Exp Ther. 1987 Jul;242(1):70-3. [PubMed Citation]
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1689-91
Product label:
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit
[Hope Pharmaceuticals] Last revised Jan
2011 [DailyMed]
Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Review article: management of cyanide poisoning. Emerg Med Australas. 2012 Jun;24(3):225-38. [PubMed Citation]
RePORTER. NIH. Countermeasures against chemical threats: countermeasures against cyanide.
RePORTER. NIH. Development of a field-deployable device to rapidly measure blood cyanide levels.
Ruangkanchanasetr S, Wananukul V, Suwanjutha S. Cyanide poisoning, 2 cases report and treatment review. J Med Assoc Thai. 1999 Nov;82 Suppl 1:S162-7. [PubMed Citation]
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
Ten Eyck RP, Schaerdel AD, Ottinger WE. Comparison of nitrite treatment and stroma-free methemoglobin solution as antidotes for cyanide poisoning in a rat model. J Toxicol Clin Toxicol. 1985-1986;23(7-8):477-87. [PubMed Citation]
Turchen SG, Manoguerra AS, Whitney C. Severe cyanide poisoning from the ingestion of an acetonitrile-containing cosmetic. Am J Emerg Med. 1991 May;9(3):264-7. [PubMed Citation]
van Heijst JM, Douze RG, van Kesteren RG, van Bergen JE, van Dijk A. Therapeutic problems in cyanide poisoning. J Toxicol Clin Toxicol. 1987; 25(5):383-398. [PubMed Citation]
Vick JA, Froehlich H. Treatment of cyanide poisoning. Mil Med. 1991 Jul;156(7):330-9. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
