You are here: Home > Medical Countermeasures Database > Sodium Thiosulfate
Sodium Thiosulfate - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Sodium thiosulfate
2. Chemical Defense therapeutic area(s)
— including key possible usesSodium thiosulfate can be used for treatment of acute cyanide poisoning in combination with sodium nitrite.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
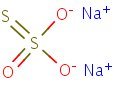
HSDB. Sodium thiosulfate
Mechanism of action
-
Although acute acrylonitrile (ACN) toxicity is very profound, the mechanism of its toxicity and immediate lethality is unclear. Many have suggested that ACN or its reactive metabolite acts directly at the target tissues, while others have implicated the release of CN ions from the parent compound as the toxic moiety. Since sodium thiosulfate (STS) is both an effective cyanide antidote and neutralizing agent capable of binding to reactive chemicals or metabolites, its antidotal role was investigated in mice exposed to 60 mg/kg intraperitoneal (IP) ACN injection. Treatment with an IP injection of 400 mg/kg of STS from 10 to 30 minutes before ACN administration protected animals from ACN-induced lethality. All mice appeared normal after prophylactic treatment with STS and showed no ill effects from ACN exposure. Similar data was observed when STS was administered 10 and 30 minutes after ACN administration. Non-protein sulfhydryl (NPSH) concentration was determined in the brain, kidneys, and liver of the mice exposed to a single or multiple doses of STS and ACN. The levels of NPSH were significantly lowered by ACN in the liver (45% of the control), and kidneys (51% of the control), whereas in the brain NPSH levels were least affected and decreased modestly (85% of the control) following either acute or chronic administration of acrylonitrile. The data indicate a marked protective effect of STS either before or after ACN exposure and this STS-induced antidotal response does not involve GSH in the brain.
Mehta C. Antidotal effect of sodium thiosulfate in mice exposed to acrylonitrile. Res Commun Mol Pathol Pharmacol. 1995 Feb;87(2):155-65. [PubMed Citation]
-
The in vivo effects of sodium cyanide and its antidotes, sodium nitrite, sodium thiosulfate and 4-dimethylaminophenol (DMAP), as well as the alpha-adrenergic blocking agent phentolamine, on rat brain cytochrome oxidase were studied. The course of inhibition was time-dependent and a peak of 40% was attained between 15 and 20 min after the s.c. injection of 1.3 LD50 (12 mg/kg) of cyanide. Pronounced dose-dependence was observed in the inhibition of the enzyme, at this relatively low, but lethal dose. Further observation was impossible because of rapidly lethal effects of cyanide. In animals artificially ventilated with room air, observation was possible up to 60 min. However, maximum inhibition was also 40%. When antidotes were applied 30 min after 20 mg/kg of cyanide, marked reactivation of cytochrome oxidase activity was observed with all antidotes (particularly with thiosulfate) except for phentolamine which had no effect. Prevention of methemoglobin forming with toluidine blue did not affect the reactivating ability of nitrite or DMAP, thus suggesting more complex protective mechanisms then simple methemoglobin formation. The high efficacy of thiosulfate may be attributed to its rhodanese catalyzed, direct binding to free blood cyanide, leading thus to its dissociation from cytochrome oxidase. The theory that cytochrome oxidase inhibition is a basic mechanism of cyanide toxicity could not be disproved.
Tadić V. The in vivo effects of cyanide and its antidotes on rat brain cytochrome oxidase activity. Toxicology. 1992 Nov 22;76(1):59-67. [PubMed Citation]
-
The primary route of endogenous cyanide detoxification is by enzymatic transulfuration to thiocyanate (SCN-), which is relatively nontoxic and readily excreted in the urine. Sodium thiosulfate is thought to serve as a sulfur donor in the reaction catalyzed by the enzyme rhodanese, thus enhancing the endogenous detoxification of cyanide in the following chemical reaction:
- Rhodanese
- Na2S2O3 + CN- → SCN- + Na2SO3.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
-
One method to treat cyanide poisoning involves the administration of a combination of sodium thiosulfate and sodium nitrite. Sodium thiosulfate is believed to exert its antidotal effect by serving as a sulfur donor, thereby increasing the rate of rhodanese catalyzed biotransformation of cyanide to thiocyanate. To gain insight into the mechanism of action of thiosulfate on cyanide toxicity, a pharmacokinetic analysis of cyanide distribution and metabolism with and without sodium thiosulfate was conducted in mongrel dogs. A compartmental model for thiocyanate, the major metabolite of cyanide, was developed from plasma concentrations determined at various times after iv administration of thiocyanate; sodium thiosulfate did not alter thiocyanate model parameters. The model for thiocyanate was coupled to a model for cyanide, and model based equations were fitted to the blood levels of both cyanide and thiocyanate that were measured after iv administration of cyanide. This kinetic analysis showed that thiosulfate increased the rate of conversion of-cyanide to thiocyanate over 30-fold. The mechanism of thiosulfate protection appeared to be due to extremely rapid formation of thiocyanate in the central compartment, which thereby limited the amount of cyanide distribution to sites of toxicity.
Sylvester DM, Hayton WL, Morgan RL, Way JL. Effects of thiosulfate on cyanide pharmacokinetics in dogs. Toxicol Appl Pharmacol. 1983 Jun 30;69(2):265-71. [PubMed Citation]
-
The sulfur provided by sodium thiosulfate binds to cyanide with the help of rhodanese (cyanide sulfur transferase) and mercaptopyruvate sulfur transferase. This sulfur, a sulfane sulfur (a divalent sulfur bound to one other sulfur), is the only type of sulfur that reacts with cyanide to produce thiocyanate, which is minimally toxic and renally eliminated. In many different animal models, sodium thiosulfate protects against several minimum lethal doses of cyanide. The addition of rhodanese increases the efficacy of sodium thiosulfate, but the use of rhodanese is impractical in the clinical setting. The cationic site on rhodanese is crucial to cleaving the sulfur-sulfur bond of thiosulfate and forming a sulfur-rhodanese complex that readily rects to cyanide. Rhodanese is probably not solely responsible form sulfur-sulfur bond cleavage, as rhodanese is largely a mitochondrial enzyme found in the liver and skeletal muscle, and sodium thiosulfate is a divalent ion that poorly crosses membranes. An additional theory proposes the both mercaptopyruvate sulfurtransferase and rhodanese are involved in the formation of sulfane sulfur in the liver from sodium thiosulfate, and that serum albumin then carries the sulfane sulfur from the liver to other organs. When cyanide is present, albumin delivers this sulfur to cyanide, forming thiocyanate.
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1692-94
Summary of clinical and non-clinical studies
Cyanide can be obtained from a variety of sources, including industrial, medical, and even common household products. It has been used as a poison and contaminant in the past and poses a serious threat as a terrorist agent because of the wide availability and ease of use. There have been recent thwarted terrorist attempts at dispersing cyanide, and historically hydrogen cyanide and cyanogen chloride have been used as warfare agents. The effects of cyanide exposure are rapid and include tachycardia, profound hypotension, death, and the prevalance of shock and cardiac arrest in 50% of cyanide-exposed patients. There are two antidotal treatments for cyanide poisoning in the United States (U.S.): 1) sodium nitrite combined with amyl nitrite and sodium thiosulfate (Cyanide Antidote Kit) and 2) hydroxocobalamin (Cyanokit) which is also commonly combined with sodium thiosulfate. Most experimental studies have compared sodium thiosulfate alone to its combination with hydroxocobalamin or sodium nitrite. In swine exposed to acute cyanide toxicity, hydroxocobalamin combined with sodium thiosulfate was significantly faster (p<0.05) in returning mean arterial pressure (a measure of hypotension) to baseline than sodium nitrite combined with sodium thiosulfate and produced a significant reduction (p<0.0001) in blood cyanide (Bebarta et al., 2010). There was, however, no difference in the mortality, serum acidosis, or serum lactate between the two combinations. Contrary to hydroxocobalmin alone which reversed shock induced by cyanide in the swine model, sodium thiosulfate as a single agent, lacked efficacy and did not improve the efficacy of hydroxocobalamin when tested in combination (Bebarta et al., 2012). In mice, a sulfide product from thiosulfate hydrolysis, sulfidomethemoglobin, is detected in the blood by EPR spectroscopy following treatment with sodium nitrite in combination with sodium thiosulfate (Cambal et al., 2011). Sulfidomethemoglobin, like cyanide, is a potent inhibitor of cytochrome c oxidase and could explain the detrimental effect observed in mice following the use of sodium nitrite-thiosulfate combination therapy at sublethal levels of cyanide intoxication. Although sodium thiosulfate has been used in the U.S., there are no clinical trials to assess efficacy due to ethical concerns, thus results from the use effectiveness of cyanide treatment with sodium thiosulfate in humans is presented in a review of several successful case studies (Hall et al., 2007). Most patients recovered, although a few had persistent neurological symptoms. The authors conclude that the onset of antidotal action of sodium thiosulfate may be too slow for it to be used as a single agent following cyanide toxicity; however, they warn that there is concern of chemical interaction when it is administered simultaneously with hydroxocobalamin. Additionally, in a patient with cyanide poisoning who was admitted in a coma with seizures, the administration of amyl nitrite and sodium thiosulfate led to a rapid improvement in oxygen metabolism and the plasma level of cyanide (Nakatani et al., 1993).
B. Link to clinical studies
Adult
-
A 34 year old, 73 kg man ingested a 1 gram potassium cyanide pellet in a suicide attempt. Within one hour, coma, apnea, metabolic acidosis, and seizures developed. Sodium nitrite and sodium thiosulfate were administered. Dramatic improvement in the clinical condition occurred by the completion of antidote infusion. Methemoglobin level was 2% immediately after nitrite administration. Serial whole blood cyanide levels were obtained, documenting a highest measured level of 15.68 mcg/mL. Estimations of toxicokinetic parameters including terminal half-life (t 1/2) (19 hours), clearance (163 mL/minute), and volume of distribution (Vd) (0.41 L/kg) were calculated. The nitrite/thiosulfate combination was clinically efficacious in this case and resulted in complete recovery. (Class IV)
Hall AH, Doutre WH, Ludden T, Kulig KW, Rumack BH. Nitrite/thiosulfate treated acute cyanide poisoning: Estimated kinetics after antidote. Clin Toxicol. 1987; 25(1-2):121-133. [PubMed Citation]
-
A 24-year-old woman ingested an unknown amount of potassium cyanide in a suicide attempt. Coma and metabolic acidosis developed. Administration of the Lilly Cyanide Antidote kit (Eli Lilly and Co, Indianapolis) resulted in prompt resolution of symptoms and full recovery. Whole blood cyanide level was 13 micrograms/mL approximately one hour after ingestion. The highest measured methemoglobin level after sodium nitrite administration was 9.2%, demonstrating that attaining a "therapeutic methemoglobin level" of 25% is unnecessary to insure a satisfactory clinical outcome. Because severe hypotension or excessive methemoglobinemia can be caused by the sodium nitrite component of the Lilly kit, only enough to produce an acceptable clinical response should be administered. (Class IV)
Johnson WS, Hall AH, Rumack BH. Cyanide poisoning successfully treated without 'therapeutic methemoglobin levels'. Am J Emerg Med. 1989 Jul;7(4):437-40. [PubMed Citation]
-
A patient presented without symptoms 30 minutes after ingesting acetonitrile, also known as methylacyanide. He had prompt gastric lavage and activated charcoal administration. Hours later, the onset of clinical toxicity was heralded by mental status abnormalities and vomiting prior to a generalized seizure. Following administration of sodium thiosulfate, the patient made an uneventful recovery. A blood cyanide level drawn shortly after presentation, but reported after the patient had been discharged, documented significant exposure. During hospitalization, cyanide toxicity was inferred from the history of ingestion of acetonitrile, plus a significant absence of venous blood hemoglobin desaturation. Because even small amounts can be harmful and toxicity is delayed, all acetonitrile ingestions should be presumed dangerous. Patients should be observed and repeatedly evaluated for at least 24 hours. In the absence of cyanide level determinations, lethargy, vomiting, seizures, and the lack of normal venous blood hemoglobin desaturation are clues to cyanide toxicity. (Class IV)
Geller RJ, Ekins BR, Iknoian RC. Cyanide toxicity from acetonitrile-containing false nail remover. Am J Emerg Med. 1991 May;9(3):268-70. [PubMed Citation]
-
A 23-year-old man survived a suicide attempt in which he had swallowed 1500 mg potassium cyanide. Initially there were cerebral seizure and marked lactacidosis. Six hours after hospital admission the cyanide blood level was 6 mg/l. The poisoning having at first not been recognized he at that time merely received supportive treatment to counteract the acidosis, as well as assisted ventilation with hyperventilation and hyperoxigenation. Afterwards, sodium thiosulphate was given additionally for 24 h at a dosage of 1 g per hour. The clinical course underlines the great importance of supportive measures in the treatment of cyanide poisoning. In the individual case the balance between risk and value of an intrinsically toxic antidote administration must be critically assessed. (Class IV)
Heintz B, Bock TA, Kierdorf H, Sieberth HG. Cyanide poisoning: treatment with hyperoxygenation and sodium thiosulphate. Dtsch Med Wochenschr. 1990 Jul 13;115(28-29):1100-3. [PubMed Citation]
-
There is limited information about the effects of large doses of sodium thiosulfate in humans. Oral administration of 3 g sodium thiosulfate per day for 1-2 weeks in humans resulted in reductions in room air arterial oxygen saturation to as low as 75%, which was due to a rightward shift in the oxygen hemoglobin dissociation curve. The subjects returned to baseline oxygen saturations 1 week after discontinuation of sodium thiosulfate. A single intravenous administration of 20 mL of 10% sodium thiosulfate reportedly did not change oxygen saturations. (Class IV)
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
-
Two cases of acute poisoning by the inhalation of hydrogen cyanide are described. The first patient regained consciousness 40 minutes after his collapse; in the interval he was treated with inhalations of amyl nitrite and intravenous injections of 0.3 gm. of sodium nitrite and 12.5 gm. of sodium thiosulfate. The second patient remained stuporous during the five hours that followed her collapse; during that interval she received inhalations of oxygen and carbon dioxide and of amyl nitrite as well as an intravenous injection of 0.6 gm. of sodium thiosulfate. Much improvement followed the intravenous injection of 50 cc. of a 1% solution of methylene blue. No dramatic effects were obtained from alternating injections of sodium nitrite and sodium thiosulfate in her case. Both patients made complete recoveries. (Class IV)
Chen KK, Rose CL. Treatment of acute cyanide poisoning. J Am Med Assoc. 1956;162(12):1154-5.
-
A 39-year-old woman ingested 59 mL of Super Nail Nail Off (American International Industries, Hollywood, CA) (containing 99% acetonitrile) in a suicide attempt. Following a latent period of approximately 12 hours, the patient developed cyanide poisoning with severe metabolic acidosis, seizures, and shallow respirations. She responded to the administration of sodium nitrite and sodium thiosulfate, although the administration of nitrite produced bradycardia and hypotension. She developed several relapses over the course of her hospitalization and each time responded to sodium thiosulfate administration. The patient developed hypernatremia from the sodium load given to her; hemodialysis and charcoal hemoperfusion were initiated to correct the hypernatremia and to attempt to remove cyanide, thiocyanate, and acetonitrile. On the fifth hospital day, the patient was fully recovered and was discharged. (Class IV)
Turchen SG, Manoguerra AS, Whitney C. Severe cyanide poisoning from the ingestion of an acetonitrile-containing cosmetic. Am J Emerg Med. 1991 May;9(3):264-7. [PubMed Citation]
-
Poisoning with potassium cyanide is usually fatal because of the inhibition of cytochrome oxidase. The parameters of oxygen metabolism in a patient with cyanide poisoning who was admitted in a coma with seizures was monitored. The administration of amyl nitrite and sodium thiosulfate led to a rapid improvement: the parameters reflecting oxygen metabolism improved and the plasma level of cyanide decreased. The patient revived 1 1/2 hours after treatment. The arterial ketone body ratio (AKBR), which is the ratio of acetoacetate to beta-hydroxybutyrate in arterial blood and which reflects the redox state in liver mitochondria, improved dramatically following treatment. Because the AKBR changes in relation to electron transport in liver mitochondria, it seems to be a logical parameter for evaluating the effect of potassium cyanide poisoning on electron transport. The AKBR also reflects the efficacy of treatment for cyanide poisoning. (Class IV)
Nakatani T, Kosugi Y, Mori A, Tajimi K, Kobayashi K; Changes in the parameters of oxygen metabolism in a clinical course recovering from potassium cyanide. Am J Emerg Med. 1993 May; 213-7. [PubMed Citation]
Pediatric studies
-
Two patients, a 4-year-old girl and her brother 1 1/2 year-old, with cyanide poisoning are reported. They vomited and became comatose 9 hours after ingestion of boiled cassava. At a community hospital, they were intubated and given ventilatory support. The girl was transferred to Ramathibodi Intensive Care Unit. At 19 hours after ingestion, sodium nitrite and sodium thiosulfate were given as well as other supportive treatment. She recovered with normal breathing on the next day. The boy was referred to Ramathibodi 4 hours later. On arrival, he appeared normal except for the bitter almond breathe. Only supportive treatment was given. Their blood cyanide levels on arrival were 0.56 and 0.32 microgram/ml (normal value < 0.3 microgram/ml) respectively confirming the diagnosis of cyanide poisoning. Other abnormal laboratory findings included metabolic acidosis and lactic acidemia. The pathogenesis and management of cyanide poisoning are reviewed. (Class IV)
Ruangkanchanasetr S, Wananukul V, Suwanjutha S. Cyanide poisoning, 2 cases report and treatment review. J Med Assoc Thai. 1999 Nov;82 Suppl 1:S162-7. [PubMed Citation]
-
The case of a 4-yr-old child in whom cyanide poisoning developed with extremely high blood cyanide levels after ingestion of oral laetrile (I) tablet, 12 tablets of 500 mg, successfully treated with amyl nitrate perles by intermittent inhalation followed by 5 ml of 3% sodium nitrite, and 25 ml of 25% sodium thiosulfate, given as intravenous injection, Lilly Cyanide Antidote Package, is reported. Treatment with the Lilly cyanide antidote kit resulted in rapid, complete recovery. It was concluded that although the necessity for using the Lilly cyanide antidote kit in serious cyanide poisoning has been questioned, the case demonstrates the benefit from antidotal treatment. (Class IV)
Hall AH , Linden CH, Kulig KW, Rumack BH; Cyanide poisoning from laetrile ingestion: role of nitrite therapy. Pediatrics. 1986 Aug;78:269-272
-
The widely published schedule for the treatment of cyanide poisoning by the nitrite thiosulfate method of Chen is based on adult work and is potentially lethal for children under 25 kg in weight. Unless a dose of sodium nitrite appropriate for the amount of hemoglobin in the child is chosen, lethal methemoglobinemia may result. The dose of NaNO2 for children with a hemoglobin of 12 gm/100 ml is 10 mg/kg per body weight immediately and 5 mg/kg repeated within 30 minutes if necessary. (Class IV)
Berlin CM The treatment of cyanide poisoning in children. The treatment of cyanide poisoning in children. Pediatrics. 1970; 46(5):793-796. [PubMed Citation]
-
Two episodes of cyanide poisoning occurred in children after ingestion of apricot kernels. The first episode involved eight children who exhibited typical signs and symptoms of cyanide poisoning two hours after having ingested a large amount of apricot kernels. Seven children recovered. One died soon after admission. The second episode involved 16 children who had eaten a sweet prepared from such kernels. The symptoms and signs were identical with those in the first group but appeared one-half hour after the ingestion and were much more severe. Thirteen children recovered, two died shortly after admission, and a third child died two hours later. Cyanide poisoning was diagnosed and appropriate treatment was initiated. This included100% oxygen when indicated, gastric lavage, inhalations of amyl nitrite, and intravenous administration of 3% sodium nitrite followed by a 25% solution of sodium thiosulfate. (Class IV)
Lasch EE and Shawa RE. Multiple cases of cyanide poisoning by apricot kernels in children from Gaza. Pediatrics. 1981; 68(1):5-7.
Pregnancy, breastfeeding studies
-
There are no reported epidemiological studies of congenital anomalies in infants born to women treated with sodium thiosulfate during pregnancy (Class IV).
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
Geriatic
-
An 80-year-old diabetic patient was admitted to the hospital because of sudden unconsciousness and severe metabolic acidosis. His son reported the possibility of cyanide poisoning. Clinical data and the detection of cyanide in blood and gastric material confirmed this possibility. Supportive therapy and the following antidotes--sodium nitrite two doses 300 mg i.v., sodium thiosulfate 3 g i.v., and hydroxocobalamin 4 g in 24 hours--were administered immediately and the patient completely recovered in 48 hours. Our observations suggest that timely and appropriate use of antidotes for cyanide intoxication may prevent death, even in aged diabetic patients. (Class IV)
Mannaioni G, Vannacci A, Marzocca C, Zorn AM, Peruzzi S, Moroni F. Acute cyanide intoxication treated with a combination of hydroxycobalamin, sodium nitrite, and sodium thiosulfate. J Toxicol Clin Toxicol. 2002;40(2):181-3. [PubMed Citation]
Clinical reviews
-
The authors reviewed the clinical manifestations, complications, and the prognosis affected by Lilly Cyanide Antidote in 21 victims of acute cyanide poisoning over a 10-year period. The clinical signs and symptoms in cyanide poisoning are variable. Among 21 cases, loss of consciousness (15), metabolic acidosis (14), and cardiopulmonary failure (9) were the three leading manifestations of cyanide intoxication. Anoxic encephalopathy (6) was not uncommon in the severely intoxicated victims. Diabetes insipidus (1) or clinical signs and symptoms mimicking diabetes insipidus (3) may be an ominous sign to encephalopathy victims. The major cause of fatal cyanide poisoning is the intentional ingestion of cyanide compounds as part of a suicide attempt. Decrease of arteriovenous difference of O2 partial pressure may be a clue for the suspicion of cyanide intoxication. Although the authors cannot show a statistically significant difference (P = .47) for the Lilly cyanide antidote kit in terms of improving the survival rate for victims of cyanide poisoning, the antidote kit was always mandatory in our study in the cases of severely intoxicated victims who survived. Early diagnosis, prompt, intensive therapy with antidote, and supportive care are still the golden rules for the treatment of acute cyanide poisoning, whether in the Emergency Department or on the scene. (Class III)
Yen D, Tsai J, Wang LM, Kao WF, Hu SC, Lee CH, Deng JF. The clinical experience of acute cyanide poisoning. Am J Emerg Med. 1995 Sep;13(5):524-8. [PubMed Citation]
-
Cyanide poisoning must be seriously considered in victims of smoke inhalation from enclosed space fires; it is also a credible terrorism threat agent. The treatment of cyanide poisoning is empiric because laboratory confirmation can take hours or days. Empiric treatment requires a safe and effective antidote that can be rapidly administered by either out-of-hospital or emergency department personnel. Among several cyanide antidotes available, sodium thiosulfate and hydroxocobalamin have been proposed for use in these circumstances. The evidence available to assess either sodium thiosulfate or hydroxocobalamin is incomplete. According to recent safety and efficacy studies in animals and human safety and uncontrolled efficacy studies, hydroxocobalamin seems to be an appropriate antidote for empiric treatment of smoke inhalation and other suspected cyanide poisoning victims in the out-of-hospital setting. Sodium thiosulfate can also be administered in the out-of-hospital setting. The efficacy of sodium thiosulfate is based on individual case studies, and there are contradictory conclusions about efficacy in animal models. The onset of antidotal action of sodium thiosulfate may be too slow for it to be the only cyanide antidote for emergency use. Hydroxocobalamin is being developed for potential introduction in the United States and may represent a new option for emergency personnel in cases of suspected or confirmed cyanide poisoning in the out-of-hospital setting. (Class IV)
Hall AH, Dart R, Bogdan G. Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning? Ann Emerg Med. 2007 Jun;49(6):806-13. [PubMed Citation]
-
Confirmed cases of childhood exposure to cyanide are rare despite multiple potential sources including inhalation of fire smoke, ingestion of toxic household and workplace substances, and ingestion of cyanogenic foods. Because of its infrequent occurrence, medical professionals may have difficulty recognizing cyanide poisoning, confirming its presence, and treating it in pediatric patients. The sources and manifestations of acute cyanide poisoning seem to be qualitatively similar between children and adults, but children may be more vulnerable than adults to poisoning from some sources. The only currently available antidote in the United States (the cyanide antidote kit) has been used successfully in children but has particular risks associated with its use in pediatric patients. Because hemoglobin kinetics vary with age, methemoglobinemia associated with nitrite-based antidotes may be excessive at standard adult dosing in children. A cyanide antidote with a better risk/benefit ratio than the current agent available in the United States is desirable. The vitamin B12 precursor hydroxocobalamin, which has been used in Europe, may prove to be an attractive alternative to the cyanide antidote kit for pediatric patients. In this article we review the available data on the sources, manifestations, and treatment of acute cyanide poisoning in children and discuss unmet needs in the management of pediatric cyanide poisoning (Class IV).
Geller RJ, Barthold C, Saiers JA, Hall AH. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006 Nov;118(5):2146-58. [PubMed Citation]
-
Cyanide poisoning is uncommon, but generates interest because of the presumed utility of an antidote immediately available in those areas with a high risk of cyanide exposure. As part of its regular review of guidelines, the Australian Resuscitation Council conducted a systematic review of the human evidence for the use of various proposed cyanide antidotes, and a narrative review of the relevant pharmacological and animal studies. There have been no relevant comparative or placebo-controlled human trials. Nine case series were identified. Treatment with hydroxocobalamin was reported in a total of 361 cases. No serious adverse effects of hydroxocobalamin were reported, and many patients with otherwise presumably fatal poisoning survived. Sodium thiosulphate use was reported in two case series, similarly with no adverse effects. Treatment with a combination of sodium nitrite, amyl nitrite and sodium thiosulphate was reported in 74 patients, with results indistinguishable from those of hydroxocobalamin and sodium thiosulphate. No case series using dicobalt edetate or 4-dimethylaminophenol were identified, but successful use in single cases has been reported. Hydroxocobalamin and sodium thiosulphate differ from alternatives in having negligible adverse effects, and on the basis of current evidence are the antidotes of choice. The indications for the use of an antidote, the requirements for supportive care and a recommended approach for workplaces where there is a risk of cyanide poisoning are presented (Class IV).
Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Review article: management of cyanide poisoning. Emerg Med Australas. 2012 Jun;24(3):225-38. [PubMed Citation]
-
Cyanide causes intracellular hypoxia by reversibly binding to mitochondrial cytochrome oxidase a3. Signs and symptoms of cyanide poisoning usually occur less than 1 minute after inhalation and within a few minutes after ingestion. Early manifestations include anxiety, headache, giddiness, inability to focus the eyes, and mydriasis. As hypoxia progresses, progressively lower levels of consciousness, seizures, and coma can occur. Skin may look normal or slightly ashen, and arterial oxygen saturation may be normal. Early respiratory signs include transient rapid and deep respirations. As poisoning progresses, hemodynamic status may become unstable. The key treatment is early administration of 1 of the 2 antidotes currently available in the United States: the well-known cyanide antidote kit and hydroxocobalamin. Hydroxocobalamin detoxifies cyanide by binding with it to form the renally excreted, nontoxic cyanocobalamin. Because it binds with cyanide without forming methemoglobin, hydroxocobalamin can be used to treat patients without compromising the oxygen carrying capacity of hemoglobin (Class IV).
Hamel J; A Review of acute cyanide poisoning with a treatment update. Critical Care Nurse. 2011 Feb;31(1):72-82. [PubMed Citation]
-
Cyanide has several antidotes, with differing mechanisms of action and diverse toxicological, clinical, and risk-benefit profiles. The international medical community lacks consensus about the antidote or antidotes with the best risk-benefit ratio. Critical assessment of cyanide antidotes is needed to aid in therapeutic and administrative decisions that will improve care for victims of cyanide poisoning (particularly poisoning from enclosed-space fire-smoke inhalation), and enhance readiness for cyanide toxic terrorism and other mass-casualty incidents. This paper reviews preclinical and clinical data on available cyanide antidotes and considers the profiles of these antidotes relative to properties of a hypothetical ideal cyanide antidote. Each of the antidotes shows evidence of efficacy in animal studies and clinical experience. The data available to date do not suggest obvious differences in efficacy among antidotes, with the exception of a slower onset of action of sodium thiosulfate (administered alone) than of the other antidotes. The potential for serious toxicity limits or prevents the use of the Cyanide Antidote Kit, dicobalt edetate, and 4-dimethylaminophenol in prehospital empiric treatment of suspected cyanide poisoning. Hydroxocobalamin differs from these antidotes in that it has not been associated with clinically significant toxicity in antidotal doses. Hydroxocobalamin is an antidote that seems to have many of the characteristics of the ideal cyanide antidote: rapid onset of action, neutralizes cyanide without interfering with cellular oxygen use, tolerability and safety profiles conducive to prehospital use, safe for use with smoke-inhalation victims, not harmful when administered to non-poisoned patients, easy to administer (Class IV).
Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol 2009;39(7):541-52. [PubMed Citation]
-
The potential for domestic or international terrorism involving cyanide has not diminished and in fact may have increased in recent years. This paper discusses cyanide as a terrorist weapon and the current state of readiness for a cyanide attack in the United States. Many of the factors that render cyanide appealing to terrorists are difficult to modify sufficiently to decrease the probability of a cyanide attack. For example, the relative ease with which cyanide can be used as a weapon without special training, its versatile means of delivery to intended victims, and to a large degree, its ready availability cannot be significantly modified through preparedness efforts. On the other hand, the impact of an attack can be mitigated through preparedness measures designed to minimize the physical, psychological, and social consequences of cyanide exposure. Although the nation remains ill-equipped to manage a cyanide disaster, significant progress is being realized in some aspects of preparedness. Hydroxocobalamin-a cyanide antidote that may be appropriate for use in the prehospital setting for presumptive cases of cyanide poisoning-currently is under development for potential introduction in the US. If it becomes available in the US, hydroxocobalamin could enhance the role of the prehospital emergency responder in providing care to victims of a cyanide disaster. Additional progress is required in the areas of ensuring local and regional availability of antidotal treatment and supportive interventions, educating emergency healthcare providers about cyanide poisoning and its management, and raising public awareness of the potential for a cyanide attack and how to respond (Class IV).
Keim ME. Terrorism involving cyanide: the prospect of improving preparedness in the prehospital setting. Prehosp Disaster Med. 2006 Mar-Apr;21(2):s56-60. [PubMed Citation]
-
Cyanide is both widely available and easily accessible throughout the world. Although the compound is not frequently encountered, it has been used as a poison and contaminant in the past and is a potential terrorist agent. Cyanide has the ability to cause significant social disruption and demands special attention to public health preparedness. It can be obtained from a variety of sources, including industrial, medical, and even common household products. Another frequently encountered source of cyanide exposure is residential fires. Exposure to high concentrations of the chemical can result in death within seconds to minutes. Long-term effects from cyanide exposure can cause significant morbidity. The only treatment for cyanide toxicity approved for use in the United States is a kit consisting of amyl nitrite, sodium nitrite, and sodium thiosulfate. Future research aims to find a faster-acting, more effective, and better tolerated treatment for cyanide toxicity (Class IV).
Garcia R, Sherpherd G; Cyanide poisoning and its treatment. Pharmacotherapy. 2004 Oct;24(10):1358-65. [PubMed Citation]
-
Cyanide poisoning may result from different exposures: residential fires, industrial accidents, drug and plant intoxication. Clinical features include coma, respiratory arrest and cardiovascular collapse. The biological hallmark is lactic acidosis. A plasma lactate concentration > or = 10 mmol/L in fire victims without severe burns and > or = 8 mmol/L in pure cyanide poisoned patients is a sensitive and specific indicator of cyanide intoxication. Many antidotes are available and efficient. However, therapeutic strategies are still debated. Our objective was to compare conventional treatments to hydroxocobalamin. This article reviews the literature on cyanide poisoning treatment. Conventional treatment of cyanide poisoning includes decontamination, supportive and specific treatment. Decontamination should be adapted to the route of poisoning and never postpone supportive treatment. Basic life support includes immediate administration of high flow of oxygen, airway protection and cardiopulmonary resuscitation. Advanced life support includes mechanical ventilation, catecholamine and sodium bicarbonate infusion. Supportive treatment is efficient but does not modify the time course or the body burden of cyanide. Numerous antidotes are available. Oxygen counteracts efficiently cyanide action at the mitochondrial level. Sodium thiosulfate, methemoglobin forming agents and cobalt compounds act efficiently by complexing or transforming cyanide into non-toxic stable derivatives. However, regarding the main clinical condition of cyanide poisoning, i.e. smoke inhalation, we should take into account not only the efficiency of antidotes but also their safety. Sodium thiosulfate is both efficient and safe, but acts with delay. Methemoglobin-forming agents are potent, but due to the transformation of hemoglobin into methemoglobin, they impair tissue delivery of oxygen. Experimental data showed increased mortality in carbon monoxide- and cyanide-poisoned rats treated with these agents. Cobalt EDTA and hydroxocobalamin are efficient and act immediately. Cobalt EDTA is more potent on a molar basis; however, numerous side effects limit its use to evidenced cyanide poisoning. In a prospective study, hydroxocobalamin appeared safe in fire victims with or without cyanide poisoning. The only reported side effect was a red coloration of skin and urine. In conclusion, antidotes are beneficial in cyanide poisoning. In suspected cyanide-poisoned patients, we recommend the use of hydroxocobalamin as first-line antidote, owing to its safety. In massive cyanide poisoning, due to the limited potency of hydroxocobalamin, continuous infusion of sodium thiosulfate should be associated (Class IV).
Mégarbane B, Delahaye A, Goldgran-Tolédano D, Baud FJ. Antidotal treatment of cyanide poisoning. J Chin Med Assoc. 2003 Apr;66(4):193-203. [PubMed Citation]
-
The combination of sodium thiosulfate and sodium nitrite has been used in the United States since the 1930s as the primary antidote for cyanide intoxication. Although this combination was shown to exhibit much greater efficacy than either ingredient alone, the two compounds could not be used prophylactically because each exhibits a number of side effects. This review discusses the pharmacodynamics, pharmacokinetics, and toxicology of the individual agents, and their combination (Class IV).
Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992 Apr;32(4):368-75. [PubMed Citation]
C. Link to non-clinical (e.g., animal) studies
Adult animal studies
-
STUDY OBJECTIVE: We compare the efficacy of hydroxocobalamin to sodium thiosulfate to reverse the depressive effects on mean arterial pressure in a swine model of acute cyanide toxicity and gain a better understanding of the mechanism of action of the hydroxocobalamin in reversal of the toxicity. METHODS: Swine were intubated, anesthetized, and instrumented with central arterial and venous lines and a pulmonary artery catheter. Animals (n=36) were randomly assigned to one of 3 groups: hydroxocobalamin alone (150 mg/kg), sodium thiosulfate alone (413 mg/kg), or hydroxocobalamin (150 mg/kg)+sodium thiosulfate (413 mg/kg) and monitored for 60 minutes after the start of antidotal infusion. Cyanide was infused until severe hypotension developed, defined as blood pressure 50% of baseline mean arterial pressure. Repeated-measures ANOVA was used to determine statistically significant changes between groups over time. RESULTS: Time to hypotension (25, 28, and 33 minutes), cyanide dose at hypotension (4.7, 5.0, and 5.6 mg/kg), and mean cyanide blood levels (3.2, 3.7, and 3.8 μg/mL) and lactate levels (7, 8.2, 8.3 and mmol/L) were similar. All 12 animals in the sodium thiosulfate group died compared with 2 of 12 in the hydroxocobalamin/sodium thiosulfate group and 1 of 12 in hydroxocobalamin group. No statistically significant differences were detected between the hydroxocobalamin and hydroxocobalamin/sodium thiosulfate groups for carbon monoxide, mean arterial pressure, cyanide levels, or mortality at 60 minutes. Lactate level (2.6 versus 2.1 mmol/L), pH (7.44 versus 7.42), and bicarbonate level (25 versus 26 mEq/L) at 60 minutes were also similar between groups. CONCLUSION: Sodium thiosulfate failed to reverse cyanide-induced shock in swine model of severe cyanide toxicity. Further, sodium thiosulfate was not found to be effective when added to hydroxocobalamin in the treatment of cyanide-induced shock. Hydroxocobalamin alone was again found to be effective for severe cyanide toxicity.
Bebarta VS, Pitotti RL, Dixon P, Lairet JR, Bush A, Tanen DA. Hydroxocobalamin versus sodium thiosulfate for the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. 2012; Jun;59(6):532-9. [PubMed Citation]
-
STUDY OBJECTIVES: Cyanide can cause severe hypotension with acute toxicity. To our knowledge, no study has directly compared hydroxocobalamin and sodium nitrite with sodium thiosulfate in an acute cyanide toxicity model. The authors objective is to compare the return to baseline of mean arterial blood pressure between 2 groups of swine with acute cyanide toxicity and treated with hydroxocobalamin with sodium thiosulfate or sodium nitrite with sodium thiosulfate. METHODS: Twenty-four swine were intubated, anesthetized, and instrumented (continuous arterial and cardiac output monitoring) and then intoxicated with a continuous cyanide infusion until severe hypotension. The animals were divided into 2 arms of 12 each and then randomly assigned to intravenous hydroxocobalamin (150 mg/kg)/sodium thiosulfate (413 mg/kg) or sodium nitrite (10 mg/kg)/sodium thiosulfate (413 mg/kg) and monitored for 40 minutes after start of antidotal infusion. Twenty animals were needed for 80% power to detect a significant difference in outcomes (alpha 0.05). Repeated measures of analysis of covariance and post hoc t test were used for determining significance. RESULTS: Baseline mean weights, time to hypotension (31 minutes 3 seconds versus 28 minutes 6 seconds), and cyanide dose at hypotension (5.6 versus 5.9 mg/kg) were similar. One animal in the hydroxocobalamin group and 2 animals in the sodium nitrite group died during antidote infusion and were excluded from analysis. Hydroxocobalamin resulted in a faster return to baseline mean arterial pressure, with improvement beginning at 5 minutes and lasting through the conclusion of the study (P < 0.05). No statistically significant difference was detected between groups for cardiac output, pulse rate, systemic vascular resistance, or mortality at 40 minutes post intoxication. Mean cyanide blood levels (4.03 versus 4.05 ug/mL) and lactate levels (peak 7.9 versus 8.1 mmol/L) at hypotension were similar. Lactate levels (5.1 versus 4.48 mmol/L), pH (7.40 versus 7.37), and base excess (-0.75 versus 1.27) at 40 minutes were also similar. CONCLUSION: Hydroxocobalamin with sodium thiosulfate led to a faster return to baseline mean arterial pressure compared with sodium nitrite with sodium thiosulfate; however, there was no difference between the antidote combinations in mortality, serum acidosis, or serum lactate.
Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier S, Bush A. Hydroxocobalamin and Sodium Thiosulfate Versus Sodium Nitrite and Sodium Thiosulfate in the Treatment of Acute Cyanide Toxicity in a Swine (Sus scrofa) Model. Ann Emerg Med. 2010 Apr;55:345-351. [PubMed Citation]
-
Sodium nitrite alone is shown to ameliorate sublethal cyanide toxicity in mice when given from 1 h before until 20 min after the toxic dose as demonstrated by the recovery of righting ability. An optimum dose (12 mg/kg) was determined to significantly relieve cyanide toxicity (5.0 mg/kg) when administered to mice intraperitoneally. Nitrite so administered was shown to rapidly produce NO in the bloodstream as judged by the dose-dependent appearance of EPR signals attributable to nitrosylhemoglobin and methemoglobin. It is argued that antagonism of cyanide inhibition of cytochrome c oxidase by NO is the crucial antidotal activity rather than the methemoglobin-forming action of nitrite. Concomitant addition of sodium thiosulfate to nitrite-treated blood resulted in the detection of sulfidomethemoglobin by EPR spectroscopy. Sulfide is a product of thiosulfate hydrolysis and, like cyanide, is known to be a potent inhibitor of cytochrome c oxidase, the effects of the two inhibitors being essentially additive under standard assay conditions rather than dominated by either one. The findings afford a plausible explanation for an observed detrimental effect in mice associated with the use of the standard nitrite_thiosulfate combination therapy at sublethal levels of cyanide intoxication.
Cambal LK, Swanson MR, Yuan Q, Weitz AC, Li H, Pitt BR, Pearce LL, and Jim Peterson J. Acute, sublethal cyanide poisoning in mice is ameliorated by nitrite alone: complications arising from concomitant administration of nitrite and thiosulfate as an antidotal combination. Chem. Res. Toxicol. 2011;Jul 24(7):1104-12. [PubMed Citation]
-
Unlike practices in the United States where it is associated with other antidotes, sodium thiosulfate is not used for emergency therapy for cyanide poisoning in France. The purpose of this study was to develop a rat model using intraperitoneal injections of sodium thiosulfate at a dose of 225 mg/kg to test its therapeutic efficacy for acute cyanide poisoning. Efficacy was assessed directly by quantifying arterial blood cyanide and indirectly using markers of hypoxia: serum lactate and arteriolization of venous blood gases. Cyanide poisoning induced intense biological anomalies which were persistent (serum lactate) or transient (blood gases). Sodium thiosulfate was found to be an effective antidote in the rat enabling rapid normalization of hypoxia markers and clearing of cyanide from arterial blood.
Renard C, Borron SW, Renaudeau C, Baud FJ. Sodium thiosulfate for acute cyanide poisoning: study in a rat model. Ann Pharm Fr. 2005 Mar;63(2):154-61. [PubMed Citation]
-
Alpha-ketoglutaric acid and sodium thiosulfate antagonize the toxic effects of cyanide. The present study was performed to test whether a synergistic effect may occur. The alpha-ketoglutaric acid/sodium thiosulfate solutions were injected intraperitoneally into mice prior to exposure to hydrogen cyanide (HCN) in a dynamic inhalation chamber or preceding an intraperitoneal injection of sodium cyanide (NaCN). All lethal concentration (LCT) and lethal dose (LD) values were determined after a period of 24 h. Alpha-ketoglutaric acid alone provided no protection at 250 mg/kg when challenged with HCN. Sodium thiosulfate 500 mg/kg provided a 5% protection. However, when these doses of alpha-ketoglutaric acid and sodium thiosulfate were combined, protection was increased by 18%. Alpha-ketoglutaric acid (250 mg/kg) and sodium thiosulfate (1000 mg/kg) provided an additional 48% protection against a LCT88 of HCN. A single dose of alpha-ketoglutaric acid (500 mg/kg) and sodium thiosulfate (1000 mg/kg) solutions afforded a 70% increase in survivability of the exposed animals. When mice were injected ip with 100 mg/kg of alpha-ketoglutaric acid 15 min prior to the injection of 5.5 mg/kg (LD50) of NaCN, the lethality was reduced to an LD30. Two hundred mg/kg alpha-ketoglutaric acid, challenged with the same dose of NaCN, reduced the lethality to 23%. When mice were challenged with 6.0 mg/kg of NaCN (LD70) pretreated with 100 mg/kg of alpha-ketoglutaric acid or 200 mg/kg of sodium thiosulfate, the LD was not altered in the former but reduced to an LD15 in the latter. At higher doses of sodium thiosulfate (500 mg/kg), an LD60 occurred at 13.6 mg/kg NaCN (2.5 x LD50).
Hume AS, Mozingo JR, McIntyre B, Ho IK. Antidotal efficacy of alpha-ketoglutaric acid and sodium thiosulfate in cyanide poisoning. J Toxicol Clin Toxicol. 1995;33(6):721-4. [PubMed Citation]
-
The object of this study was to evaluate the effectiveness of five regimens in treating cyanide poisoning. A series of anesthetized adult beagle dogs were instrumented to record hemodynamic and respiratory function and given 2.5 mg/kg sodium cyanide intravenously. The 10 control animals given only cyanide died at from 5 to 7 minutes. Therapy, as described below, was given to other groups at from 2 to 3 minutes following the cyanide administration. Artificial respiration did not alter the lethal effects of cyanide nor prolong survival time in any of the 10 animals. Amyl nitrite given by inhalation or by the intravenous route allowed survival of all 15 animals. Sodium nitrite (20 mg/kg), dimethylaminophenol (DMAP) (5 mg/kg), and hydroxylamine hydrochloride (5 mg/kg) given intravenously with no artificial ventilation also allowed for 100% survival (15 animals). Amyl nitrite, sodium nitrite, and sodium thiosulfate were ineffective when given intramuscularly (I.M.) (0 of 12 dogs); however, I.M. DMAP (5 mg/kg) and I.M. hydroxylamine hydrochloride (50 mg/kg) increased heart rate and blood pressure and restored spontaneous breathing. All 15 animals treated with I.M. doses of either of these drugs survived the lethal dose of cyanide. Results of these studies indicate that intravenous sodium nitrite, DMAP, and hydroxylamine hydrochloride, and amyl nitrite by inhalation, are all effective in reversing the lethal effects of cyanide poisoning. Only DMAP and hydroxylamine hydrochloride are effective when given by the intramuscular route. These results provide data to support an approach to therapy that is more practical and applicable where expert medical care may not be available following cyanide exposure.
Vick JA, Froehlich H. Treatment of cyanide poisoning. Mil Med. 1991 Jul;156(7):330-9. [PubMed Citation]
-
To test the efficacies of various antidotes to cyanide (CN) poisoning, the lethal dose of cyanide in dogs was estimated during constant infusion of potassium cyanide at a rate of 0.1 mg/kg/min. Additionally, arterial blood pressure, right ventricular pressure, heart rate, electrocardiogram, blood-gas and pH values, and whole blood and tissue CN concentrations were measured. The lethal dose in animals whose lungs were ventilated with room air was 2.4 +/- .2 mg/kg (mean +/- SE), while the whole-blood CN concentration was 438 +/- 40 micrograms/dl and the gracilis muscle concentration was 2.0 +/- .3 micrograms/100 g. A low dose of vitamin B12a (100 mg/kg), an infusion of thiosulfate (12 mg/kg/h), or ventilation with 100 per cent O2 increased the amount of CN needed to cause death. A bolus injection of nitrite (5 mg/kg), thiosulfate (150 mg/kg), or cysteine (450 mg/kg) increased the protection from lethality even further. Protection against CN administration for the total 150-min period of observation was provided by a bolus injection plus a constant infusion of nitrite (5 mg/kg bolus plus 5 mg/kg/h). thiosulfate (30 mg/kg bolus plus 60 mg/kg/h), or vitamin B12a (50 mg/kg bolus plus 100 mg/kg/h). However, nitrite infusion produced high levels of methemoglobin 7.2 +/- 1.1 g/dl, while vitamin B12a infusion and cysteine injection, at the stated doses, did not prevent cyanide-induced circulatory failure. Therefore, thiosulfate appears to be the most effective and safest prophylactic agent against cyanide toxicity in dogs.
Ivankovich AD, Braverman B, Kanuru RP, Heyman HJ, Paulissian R. Cyanide antidotes and methods of their administration in dogs: a comparative study. Anesthesiology. 1980 Mar;52(3):210-6. [PubMed Citation]
-
Murine carrier erythrocytes containing bovine rhodanese and sodium thiosulfate are being explored as a new approach to antagonize the lethal effects of potassium cyanide in mice. Prior studies indicated that these carrier erythrocytes persist in the vascular system for the same length of time as normal erythrocytes and can enhance metabolism of cyanide to thiocyanate. The present studies demonstrate the ability of these carrier red blood cells containing rhodanese and thiosulfate to antagonize the lethal effects of cyanide either alone or in various combinations with sodium nitrite and/or sodium thiosulfate. Potency ratios are compared in groups of mice treated with sodium nitrite, sodium thiosulfate, and carrier erythrocytes containing rhodanese and sodium thiosulfate either alone or in various combinations prior to the administration of potassium cyanide. These results indicate that the administration of carrier erythrocytes containing rhodanese and thiosulfate alone can provide significant protection against the lethal effects of cyanide. These carrier erythrocytes potentiate the antidotal effect of sodium thiosulfate alone or the combination of sodium nitrite and sodium thiosulfate. The mechanisms of cyanide antagonism by these carrier erythrocytes and their broader conceptual significance to the antagonism of other chemical toxicants are discussed.
Cannon EP, Leung P, Hawkins A, Petrikovics I, DeLoach J, Way JL. Antagonism of cyanide intoxication with murine carrier erythrocytes containing bovine rhodanese and sodium thiosulfate. J Toxicol Environ Health. 1994 Mar;41(3):267-74. [PubMed Citation]
Pregnant animal studies
-
In animal studies, there are no teratogenic effects in offspring of hamsters treated during pregnancy with sodium thiosulfate in doses similar to those given intravenously to treat cyanide poisoning in humans. Other studies suggest that treatment with sodium thiosulfate ameliorates the teratogenic effects of maternal cyanide poisoning in hamsters. In other studies, sodium thiosulfate was not embryotoxic or teratogenic in mice, rats, hamsters, or rabbits at maternal doses of up to 550, 400, 400 and 580 mg/kg/day, respectively.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
-
A previous study reported that the co-infusion of IV sodium thiosulfate (STS) with sodium nitroprusside (SNP) to near-term gravid ewes prevented both maternal and fetal cyanide toxicity. We questioned whether maternally administered STS crossed the ovine placenta to enhance fetal transulfuration of cyanide, or whether the fetus was dependent on maternal detoxification of cyanide after diffusion of cyanide into the maternal circulation. Ten anesthetized, near-term gravid ewes underwent hysterotomies with delivery of fetal heads for venous catheterization. Five control ewes received IV isotonic sodium chloride solution, whereas five experimental ewes received IV STS (50 mg/kg over 15 min). Serial plasma thiosulfate concentrations in ewes and fetuses were measured over 135 min. Areas under the time-plasma thiosulfate concentration curves were calculated for experimental and control ewes at 2758+/-197 and 508+/-74 min x mg(-1) x L(-1), respectively (P < 0.008). Mean areas under the curve for experimental and control fetuses were 236+/-34 and 265+/-23 min x mg(-1) x L(-1), respectively (P > 0.5). Maternally administered STS may prevent fetal cyanide poisoning from SNP administration without relying on STS crossing the placenta into the fetal circulation. Fetal cyanide may cross down a concentration gradient from fetal to maternal circulation, to be transulfurated to thiocyanate in maternal tissues. IMPLICATIONS: We evaluated the mechanism of action of sodium thiosulfide (STS) in sodium nitroprusside-induced cyanide toxicity in the ewe. Fetal cyanide poisoning is alleviated by maternal administration of STS, although this cyanide antidote apparently does not cross the placenta.
Graeme KA, Curry SC, Bikin DS, Lo Vecchio FA, Brandon TA. The The lack of transplacental movement of the cyanide antidote thiosulfate in gravid ewes. Anesth Analg. 1999 Dec;89(6):1448-52. [PubMed Citation]
-
Coadministration of sodium thiosulfate with sodium nitroprusside (SNP) to children and adults prevents increases in cyanide concentrations during anesthesia or long-term SNP infusions. We wondered whether maternally administered sodium thiosulfate would prevent increases in fetal red cell cyanide concentrations in gravid ewes receiving SNP infusions. Under anesthesia, the fetal head was delivered through a lateral hysterotomy for catheterization of the jugular vein; the fetus was left in utero. Six control ewes near term received SNP at 25 micrograms.kg-1.min-1 for 4 h. Norepinephrine was used to maintain maternal mean arterial pressure at 80% baseline values. Six experimental ewes received the same treatment except that sodium thiosulfate was infused with SNP (1 g sodium thiosulfate per 100 mg SNP). Serial red cell cyanide concentrations in ewes and fetuses were followed. One control fetal death resulted from abruptio placenta, and this ewe and fetus were excluded from analysis. An additional control ewe and fetus died from apparent cyanide poisoning late during the course of the experiment. While control ewes and fetuses suffered progressive increases in red cell cyanide concentrations into the toxic range, experimental ewes and fetuses never developed toxic red cell cyanide levels (ewes P <0.003, fetuses P <0.004). These data, if applicable to humans, suggest that coadministration of sodium thiosulfate with SNP to pregnant women at doses currently in use for nonpregnant patients will prevent fetal, as well as maternal, cyanide toxicity.
Curry SC, Carlton MW, Raschke RA. Prevention of fetal and maternal cyanide toxicity from nitroprusside with coinfusion of sodium thiosulfate in gravid ewes. Anesth Analg. 1997 May;84(5):1121-6. [PubMed Citation]
-
It is well-known that the majority of malformations found in the human population is based on complex gene-environment interactions. As an industrial chemical sodium thiosulfate (STS) is used heavily in many industries. Nevertheless, there is little known about the effects of STS on embryo development. In the present study, we have investigated the effects of STS on cardiac development in rat cardiomyocyte H9C2 cell line and chick embryos. As determined by MTT assays, the proliferation of H9C2 cells was inhibited by STS in a dose-dependent manner. Fertilized eggs injected via the yolk sac with STS at Hamburger-Hamilton (HH) stages 6, 9 and 12 showed significantly increased cardiotoxicity at HH stage 18, including cardiomyocyte apoptosis and animal mortality. Western blot analysis showed that STS significantly affected the expression of the apoptosis-related genes bcl-2, bax, and caspase-3 in a dose-dependent manner in the H9C2 cell line and in chick embryos. Dysregulation of apoptosis was correlated with embryonic heart malformations. Thus, STS may be a potent cardiac teratogen during embryo development.
Cui Y, Liu L, Xia H, Han Z, Hu Y, Ma X. Sodium thiosulfate exposure disrupts in vitro and in vivo heart development. Front Biosci (Elite Ed). 2011 Jan 1;3:469-75. [PubMed Citation]
Other non-clinical studies
-
The mutagenic potential of sodium thiosulfate has been examined in the in vitro Bacterial Reverse Mutation Assay (Ames Assay). Sodium thiosulfate was not mutagenic in the absence of metabolic activation in S. typhimurium strains TA98, TA100, TA1535, TA537, or TA1538. Sodium thiosulfate was not mutagenic in the presence of metabolic activation in strains TA 98, TA1535, TA1537, TA1538 or E. coli strain WP2.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Thiosulfate taken orally is not systemically absorbed. Most of the thiosulfate is oxidized to sulfate or is incorporated into endogenous sulphur compounds; a small proportion is excreted through the kidneys. Approximately 20-50% of exogenously administered thiosulfate is eliminated unchanged via the kidneys. After an intravenous injection of 1 g sodium thiosulfate in patients, the reported serum thiosulfate half-life was approximately 20 minutes. However, after an intravenous injection of a substantially higher dose of sodium thiosulfate (150 mg/kg, that is, 9 g for 60 kg body weight) in normal healthy men, the reported elimination half-life was 182 minutes.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
-
A volunteer study examined the pharmacokinetics of sodium thiosulfate and the fate of thiosulfate. After injection of 150 mg/kg, volume of distribution (Vd) was 0.15 L/kg, distribution half-life was 23 minutes, and elimination half-life was 3 hours. The peak serum thiosulfate concentration rose 100-fold. Approximately 50% of the drug was eliminated in 18 hours, most of it within 3 hours. Baseline thiosulfate concentrations were higher in starved patients and children, presumably because of their higher protein utilization and metabolism to thiosulfate. Normally, the kidney actively reabsorbs thiosulfate, but this study found that with exogenous administration, thiosulfate clearance equaled creatinine clearance.
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1692-94
Animal
-
The duration of action of the cyanide antidotes sodium thiosulphate and hydroxocobalamin was investigated in guinea-pigs after prophylactic administration before a long-term infusion of NaCN. The parameter for the diminution of the antidote action was the point of time at which the concentration of HCN in the exhaled air of the animals exceeded 100 nmol/kg per min. The time taken to reach this threshold level in the control animals was 12 min. While the threshold level could be extended only to 35 min with hydroxocobalamin (300 mg/kg i.v.) the protective action of sodium thiosulphate (100, 500 and 1000 mg/kg i.v.) persisted dose dependently for about 1, 2 and 4 h, respectively. Additionally we found a plasma half-life of sodium thiosulphate in guinea-pigs of 26 min. This value corresponds approximately to the plasma half-life of sodium thiosulphate in humans given in the literature. Because of the large injection volume necessary, sodium thiosulphate is not suitable for prophylactic use in man.
Mengel K, Krär W, Isert B, Friedberg KD. Thiosulphate and hydroxocobalamin prophylaxis in progressive cyanide poisoning in guinea-pigs. Toxicology. 1989 Mar;54(3):335-42. [PubMed Citation]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
-
Cyanide poisoning: sodium nitrite injection and sodium thiosulfate injection are administered by slow intravenous injection. They should be given as early as possible after a diagnosis of acute life-threatening cyanide poisoning has been established.
- Sodium Nitrite -10 mL of a 3% solution (300 mg) of sodium nitrite at the rate of 2.5 to 5 mL/minute
- Sodium Thiosulfate - 50 mL of a 25% solution (12.5 g) of a sodium thiosulfate solution immediately following administration of sodium nitrite.
NOTE: If signs of poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate. Chemical incompatibility has been reported between NITHIODOTE and hydroxocobalamin and these drugs should not be administered simultaneously through the same IV line. No chemical incompatibility has been reported between sodium thiosulfate and sodium nitrite, when administered sequentially through the same IV line.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
Children (FDA)
-
Cyanide poisoning: sodium nitrite injection and sodium thiosulfate injection are administered by slow intravenous injection. They should be given as early as possible after a diagnosis of acute life-threatening cyanide poisoning has been established.
Sodium Nitrite -0.2 mL/kg of a 3% solution (6 mg/kg or 6-8 mL/m2 BSA) of sodium nitrite at the rate of 2.5 to 5 mL/minute not to exceed 10 mL (300 mg)
Sodium Thiosulfate - 1 mL/kg of body weight using a 25% solution (250 mg/kg or approximately 30-40 mL/m2 of BSA) not to exceed 50 mL (12.5 g) total dose immediately following administration of sodium nitrite.
NOTE: If signs of poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate. Chemical incompatibility has been reported between NITHIODOTE and hydroxocobalamin and these drugs should not be administered simultaneously through the same IV line. No chemical incompatibility has been reported between sodium thiosulfate and sodium nitrite, when administered sequentially through the same IV line.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
Pregnancy (FDA)
-
Both sodium nitrite and sodium thiosulfate are Pregnancy Category C. There are no adequate and well-controlled studies in pregnant women. NITHIODOTE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
Nursing Mothers (FDA)
-
It is not known whether sodium nitrite or sodium thiosulfate is excreted in human milk. Because NITHIODOTE may be administered in life-threatening situations, breast-feeding is not a contraindication to its use. Because many drugs are excreted in human milk, caution should be exercised following NITHIODOTE administration to a nursing woman. There are no data to determine when breastfeeding may be safely restarted following administration of sodium nitrite and sodium thiosulfate.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
Geriatric (FDA)
-
Cyanide poisoning: sodium nitrite and sodium thiosulfate are known to be substantially excreted by the kidney, and the risk of adverse reactions to these drugs may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
Renal Impairment (FDA)
-
Sodium nitrite and sodium thiosulfate are known to be substantially excreted by the kidney, and the risk of toxic reactions to these drugs may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Sodium Thiosulfate has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (Public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Formulation:
-
NITHIODOTE Injection consists of:
-
One vial of sodium nitrite injection, USP 300 mg/10mL (30 mg/mL) and
-
One vial of sodium thiosulfate injection USP 12.5 grams/50 mL (250 mg/mL)
Administration of one vial of each medication constitutes a single dose.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
-
Sodium Thiosulfate Injection, 12.5 grams/50 mL (250 mg/mL).
Product label: Sodium Thiosulfate Injection [Hope Pharmaceuticals]. Approval 2/2012.
-
The New Drug Application (NDA) for Nithiodote (NDA 201444) only permits the marketing of the two drug products, sodium nitrite and sodium thiosulfate, as components of one package. Since the drug products have different expiry dates, this has necessitated that product users, such as the military and hospitals, replace the entire package when only one of the components has reached its expiry date. By submitting separate applications for the two products, the Applicant intends to market the components separately, thus increasing the flexibility for product users as they manage their supply stocks.
Center for Drug Evaluation and Research: Summary Review for Regulatory Action. February 14, 2012 (FDA)
Shelf life:
Shelf life
-
18 months, when stored at controlled room temperature between 20°C and 25°C (68°F to 77°F), with excursions permitted to 15°C to 30°C (59°F to 86°F).
Center for Drug Evaluation and Research: Summary Review for Regulatory Action. February 14, 2012 (FDA)
Storage:
-
Store at controlled room temperature between 20°C and 25°C (68°F - 77°F); excursions permitted to 15-30°C (59 to 86°F). Protect from direct light. Do not freeze.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Cyanide Antidote Package (Amyl Nitrite Inhalants: 0.3 mL [12 ampoules], Sodium Nitrite: 200 mg/10 mL [2 ampoules], Sodium Thiosulfate: 12.5 mg/50 mL [2 vials]). Cyanide poisoning (adult dosage): apply 1 ampoule of Amyl Nitrite to a handkerchief and hold in front of patient's mouth for 15 seconds followed by a rest for 15 seconds. Then reapply until Sodium Nitrite can be administered. Discontinue Amyl Nitrite and give Sodium Nitrite IV 300 mg at a rate of 2.5 to 5 mL/minute. Immediately after inject 12.5 g of Sodium Thiosulfate.
Bartlett JG, Greenberg MI, eds. PDR Guide to Terrorism Response. Montvale, NJ: Thomson PDR, 2005 p.321-41
-
Cisplatin-induced nephrotoxicity: 4 g/sq m as an IV bolus followed by 12 g/sq m as an IV infusion over 6 hours.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.682
Children
-
Cyanide Antidote Package (Amyl Nitrite Inhalants: 0.3 mL [12 ampoules], Sodium Nitrite: 200 mg/10 mL [2 ampoules], Sodium Thiosulfate: 12.5 mg/50 mL [2 vials]). Cyanide poisoning (children dosage): apply 1 ampoule of Amyl Nitrite to a handkerchief and hold in front of patient's mouth for 15 seconds followed by a rest for 15 seconds. Then reapply until Sodium Nitrite can be administered. Discontinue Amyl Nitrite and give 6-8 mL/sq m of Sodium Nitrite IV, max of 12.5 g. Immediately after inject 7 g/sq m of Sodium Thiosulfate, max of 12.5 g.
Bartlett JG, Greenberg MI, eds. PDR Guide to Terrorism Response. Montvale, NJ: Thomson PDR, 2005 p.321-41
-
Cysplatin-induced nephrotoxicity: in the case of accidental cysplatin overdose in a child 14 years of age, sodium thiosulfate was gi.ven IV as a 4 g/ sq m loading dose followed by 2.7 g/ sq m/day in 3 divided doses and continued until urinary platinum levels fell below 1 mcg/mL.
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.682
8. Route of Administration/Monitoring
-
Sodium nitrite injection and sodium thiosulfate injection are administered by slow intravenous injection. They should be given as early as possible after a diagnosis of acute life-threatening cyanide poisoning has been established.
Patients should be monitored for at least 24-48 hours after NITHIODOTE administration for adequacy of oxygenation and perfusion and for recurrent signs and symptoms of cyanide toxicity. When possible, hemoglobin/hematocrit should be obtained when treatment is initiated. Measurements of oxygen saturation using standard pulse oximetry and calculated oxygen saturation values based on measured PO2 are unreliable in the presence of methemoglobinemia. Methemoglobin level: Administrations of sodium nitrite solely to achieve an arbitrary level of methemoglobinemia may be unnecessary and potentially hazardous. The therapeutic effects of sodium nitrite do not appear to be mediated by methemoglobin formation alone and clinical responses to sodium nitrite administration have been reported in association with methemoglobin levels of less than 10%. Administration of sodium nitrite beyond the initial dose should be guided primarily by clinical response to treatment (i.e., a second dose should be considered only if there is inadequate clinical response to the first dose). It is generally recommended that methemoglobin concentrations be closely monitored and kept below 30%. Serum methemoglobin levels should be monitored during treatment using co-oximetry, and administration of sodium nitrite should generally be discontinued when methemoglobin levels exceed 30%. Intravenous methylene blue and exchange transfusion have been reported in the literature as treatments for life-threatening methemoglobinemia.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
-
All patients should be monitored in an emergency or intensive care setting. Monitoring should include regular assessments of metabolic acidosis using serial arterial blood gases, metabolic panels, or serum lactate determination, If hypotension develops, the infusion or inhalation of nitrites should be slowed or stopped accordingly, and the patient should be given supplemental fluids or pressors. If clinical or laboratory signs of cyanide toxicity do not improve within 15 or 30 minutes of Cyanide Antidote Package (CAP) administration, the sodium nitrite and sodium thiosulfate portions should be repeated at half the recommended initial dose. They should also be repeated in patients who develop recurrent toxicity after an initial improvement. Recurrent toxicity may also represent ongoing cyanide absorption, so further decontamination would be considered. Patients who do not respond to repeat doses of the CAP should have the diagnosis of cyanide toxicity reconsidered. A MetHgb level should be checked approximately 30 and 60 minutes after nitrite infusion - earlier if signs and methemoglobinemia develop. The MetHgb level needed for optimal benefit has not been established. Some authors describe a target MetHgb level of 25% to 30%; however, recent evidence suggests that high levels are not necessary for therapeutic benefit. Clinical improvement rather than MetHgb level should be the therapeutic goal.
Dart RC, ed. Medical Toxicology 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2004 p.172-5
-
Chemical incompatibility has been reported between NITHIODOTE and hydroxocobalamin and these drugs should not be administered simultaneously through the same IV line. No chemical incompatibility has been reported between sodium thiosulfate and sodium nitrite, when administered sequentially through the same IV line.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
9. Adverse effects
-
Traditional treatment of cyanide poisoning involves a two-step process: administration of amyl or sodium nitrite followed by administration of sodium thiosulfate. In a mass casualty scenario, there are issues with administering the traditional antidotes amyl nitrite and sodium nitrite: (1) the degree of methemoglobinemia can be exceeded, which is critical in pediatric treatment, and (2) because the nitrites are vasodilators, hypotension may be induced.
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
-
Adverse effects associated with sodium thiosulfate:
-
Cardiovascular system: hypotension
-
Central nervous system: headache, disorientation
-
Gastrointestinal system: nausea, vomiting
-
Hematological: prolonged bleeding time
-
Body as a Whole: salty taste in mouth, warm sensation over body
-
In humans, rapid administration of concentrated solutions or solutions not freshly prepared, and administration of large doses of sodium thiosulfate have been associated with a higher incidence of nausea and vomiting. However, administration of 0.1 g sodium thiosulfate per pound up to a maximum of 15 g in a 10-15% solution over 10-15 minutes was associated with nausea and vomiting in 7 of 26 patients without concomitant cyanide intoxication.
In a series of 11 human subjects, a single intravenous infusion of 50 mL of 50% sodium thiosulfate was associated with increases in clotting time 1-3 days after administration. However, no significant changes were observed in other hematological parameters.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
-
Sodium thiosulfate drug product may contain trace impurities of sodium sulfite. The presence of a trace amount of sulfites in this product should not deter administration of the drug for treatment of emergency situations, even if the patient is sulfite-sensitive.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
-
The toxicity of sodium thiosulfate is low. The LD50 (median lethal dose of 50% of the test subjects) for animals is approximately 3 to 4 g/kg, with death attributed to metabolic acidosis, elevated sodium concentration, and decreased blood pressure and PO2. Sodium thiosulfate delivers a significant sodium load and is hyperosmolar. Administering the infusion over 10 to 30 minutes attenuates some of these adverse effects. Adverse effects associated with therapeutic dosing include hypotension, nausea, and vomiting. The osmotic and diuretic effects presumably result from both the formation of thiocyanate and the intrinsic osmotic properties of the drug.
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1692-94
10. Contraindication(s)
None
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues-
Title: Development of a field-deployable device to rapidly measure blood cyanide levels
The objective of this proposal is to assess the feasibility of a fully integrated device for the rapid and early diagnosis of cyanide poisoning in whole blood using the spectral shift of the vitamin B12 precursor cobinamide upon binding with cyanide as an indicator. Cyanide is an extremely potent and rapid acting poison with as little as 50 mg fatal to humans. Currently there are no portable rapid tests for the detection of cyanide in whole blood available. The primary goal of this proposal is to demonstrate feasibility of the cobinamide-cyanide chemistry in a rapid test using a whole blood sample from a finger-stick. The total assay time from the sample collection to a valid result will be less than 5 minutes. The innovation in this proposal is to incorporate the cobinamide chemistry with blood separation technology, fluid path designs, and a detection area, all integrated in a compact, disposable device which can be interpreted with a handheld visualization system. Feasibility work of this novel rapid assay for detecting blood cyanide through cobinamide will initially focus on several technical approaches for the device design. Parallel paths to pursue a wet chemistry design, a design where the reagents are dried in the device, and a combination thereof will be considered, compared and evaluated. In all of the proposed methods the whole blood sample will be added to the device, followed by an integrated whole blood processing step to yield a plasma sample. Either prior to or immediately after the whole blood separation and lysis, the sample will be mixed with the on-board reagent, dried or wet, and possibly buffers. The processed sample-reagent mixture then moves through the device through various fluid paths to a detection area. To allow for sufficient mixing time between the sample and the reagents, different fluid paths, mixing chambers, polymeric time-gates, and dissolvable films will be evaluated with the goal of maximizing the interaction time. The spectral shift of cobinamide upon binding of cyanide to cobinamide will be measured in the detection area using a handheld or small, portable reader instrument. Upon successful completion of this Phase 1 project we intend to further develop our concept/prototype assay, complete the device design and integrate the finished test with a reader instrument through a Phase 2 proposal. Specific aims for a Phase 2 of this project include the validation of the cobinamide-based device for measuring cyanide in blood. Results will be compared to two established methods for measuring cyanide in biological samples. The sensitivity, specificity, accuracy, precision, and reproducibility of the devices will be determined. Additional studies will be designed to obtain regulatory approval and subsequently we will commercialize the test. If successful, this test will provide the medical community and first responders with a fast, reliable and economic alternative to determine cyanide poisoning. PUBLIC HEALTH RELEVANCE: Cyanide poisoning has been recognized as a threat from smoke inhalation and potentially through weapons of mass destruction, but there are currently no methods available to rapidly detect cyanide in blood from at-risk patients. The objective of the proposed work is to assess the feasibility of a fully integrated method for the rapid and early detection and diagnosis of cyanide poisoning in whole blood using the spectral shift of the vitamin B12 precursor cobinamide upon binding with cyanide as an indicator. Successful accomplishment of the goals of this project will be the basis for the development of a test to detect and measure cyanide blood poisoning which will allow healthcare providers to treat patients quickly and effectively.
RePORTER. NIH. Development of a field-deployable device to rapidly measure blood cyanide levels.
-
Title: Countermeasures against chemical threats: countermeasures against cyanide
Sources for potential exposure to cyanide of both civilians and military personnel include combustion of nitrogenous materials, commercial accidents and the deliberate release of a cyanogenic chemical. The relative ease in obtaining and releasing cyanide means that the risk of cyanide use in a terrorist attack resulting in a mass casualty situation should not be ignored. Rapid intervention is critical for effective medical intervention in cases of cyanide exposure: treatments require a "three minute solution." The availability of a rapid, IM injectable antidote should meet this requirement. However, there are no such available treatments - the current cyanide antidotes are administered intravenously. Because intravenous administration is time consuming and requires well trained medical personnel mass exposure to cyanide would likely leave many victims untreated. Because IM administration can be performed via an autoinjector and can therefore be done rapidly by minimally trained personnel there is a critical need for a rapid-acting, IM-injectable antidote for the treatment of mass cyanide casualties. In order to address this need /the authors/ are advancing /a/preclinical lead, sulfanegen to clinical development. /They/ have previously demonstrated efficacy of sulfanegen in murine, swine and rabbit models of cyanide toxicity. In these models sulfanegen is effective in reversal of cyanide toxicity by IM injection and therefore should meet the three minute solution. /The authors/ have recently held a pre-IND meeting with the FDA and /their/ goal is to advance this cyanide antagonist to the clinic by validation of animal models, demonstrate efficacy of sulfanegen in these animal models and perform the required GLP pharmacokinetic and safety evaluation required for clinical trials. /The authors/ will then commence a Phase 1 human safety study while simultaneously performing the required GLP studies for NDA submission under the animal rule. Successful completion of the aims of this project should lead to a clinical countermeasure for cyanide toxicity that will be applicable in all cases of cyanide exposure from individuals to mass casualty settings. PUBLIC HEALTH RELEVANCE: /The authors/ have discovered a novel cyanide antidote that /they/ have named sulfanegen and demonstrated that it is more effective than existing antidotes in models of cyanide toxicity. /They/will perform the necessary studies to translate this antidote from the bench to the bedside. Successful completion of the goals of this project will therefore result in a clinical antidote that could be useful in the case of a terrorist attack involving cyanide.
RePORTER. NIH. Countermeasures against chemical threats: countermeasures against cyanide.
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations- No data available at this time.
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations-
A better understanding of the mechanism of action of cyanide at the molecular level is needed.
-
The use of antidotes to cyanide poisoning in patients exposed to other toxic hazards such as smoke or industrial chemicals needs further evaluation.
-
Models should be developed to characterize the long-term effects of acute exposure to cyanide and identify how cyanide-containing compounds are metabolized.
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
-
Studies of pediatric fire victims
-
Hydroxycobalamin versus cyanide antidote kit
-
Need more sensitive measure of blood cyanide
-
Consider three-arm trial (one group receives all)
-
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
15. Study-related ethical concerns
— including review panel recommendations- No data available at this time.
16. Global regulatory status
U.S.
-
NITHIODOTE (sodium nitrite and sodium thiosulfate) kit is indicated for the treatment of acute cyanide poisoning that is judged to be life-threatening. When the diagnosis of cyanide poisoning is uncertain, the potentially life-threatening risks associated with NITHIODOTE should be carefully weighed against the potential benefits, especially if the patient is not in extremis.
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
-
Sodium thiosulfate is used topically in the treatment of tinea versicolor. Sodium thiosulfate is applied topically in the form of a 25% lotion or solution (no longer commercially available in the US). A commercially available lotion also containing salicylic acid may be used. Alternatively, aqueous solutions of the drug are prepared extemporaneously. For the treatment of tinea versicolor, a thin film of sodium thiosulfate 25% lotion or solution is applied to the involved areas twice daily; these areas should be thoroughly cleansed and dried before applying the lotion or solution. Although evidence of active infection may disappear within a few days, treatment is usually continued for several weeks to months.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012
-
Sodium thiosulfate is used to reduce the risk of nephrotoxicity associated with cisplatin therapy.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012
E.U.
-
Thiosulphate should be considered together with the other cyanide antidotes in sequential treatment but should not be administered as a mixture at the same time as hydroxocobalamin.
Sodium thiosulfate 25% or 250 mg/ml in 50 ml vials used as sequential treatment following methaemoglobin inducers (amyl nitrite, sodium nitrite, 4-dimethylaminophenol hydrochloride).
Adults: 50 ml=12.5 g dose IV or 180 mg/kg over at an infusion rate of 2.5 ml/min
Children: 1.6 ml/kg or 400 mg/kg, up to 50 ml at an infusion rate of 2.5 ml/min
NOTE: Additional half-sized dose, if not response in 30 to 60 min
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
U.K.
-
Sodium thiosulphate is used in conjunction with sodium nitrite for cyanide poisoning.
By intravenous injection over 10 minutes (as sodium thiosulphate injection 500 mg/mL), 12.5 g; dose may be repeated in severe cyanide poisonings if dicobalt edentate not available.
Children: 400 mg/kg (maximum 12.5g); dose may be repeated in severe cyanide poisonings if dicobalt edentate not available.
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 34
17. Other potentially useful information
-
PURPOSE: Thiosulfate has been shown to reduce the risk of cyanide toxicity during nitroprusside administration. Admixtures containing both agents may provide a safe and effective alternative to more expensive agents used to reduce blood pressure in the critically ill patient. This study determined the physical and chemical stability of a 1:10 nitroprusside:thiosulfate admixture, stored up to 48 hours. The economic consequences of a shift toward using thiosulfate and nitroprusside, and away from higher cost alternatives, are considered. METHODS: Seven samples of 50 mg nitroprusside and 500 mg thiosulfate were prepared and stored away from light, at room temperature, and in a refrigerator prepared in D5W and NS. Each sample was analyzed via a novel high-performance liquid chromatographic (HPLC) method at time 0, 8, 24, and 48 hours. The method was tested and passed specifications for linearity, reproducibility, and accuracy. A visual inspection by 9 licensed pharmacists was used to demonstrate physical stability. A cost evaluation comparing nitroprusside and thiosulfate to alternative agents was completed. RESULTS: The concentration of both nitroprusside and thiosulfate remain greater than 95% of the initial concentration through 48 hours. Physical compatibility was confirmed in all samples tested through 72 hours. CONCLUSION: The combination of nitroprusside and thiosulfate is chemically and physically stable as a single compounded dose for up to 48 hours when stored at room temperature and protected from light. The admixture represents an inexpensive option to other higher cost alternatives such as nicardipine or clevidipine.
Schulz LT, Elder EJ Jr, Jones KJ, Vijayan A, Johnson BD, Medow JE, Vermeulen L. Stability of sodium nitroprusside and sodium thiosulfate 1:10 intravenous admixture. Hosp Pharm. 2010;Oct;45(10):779-84. [PubMed Citation]
-
Sources of cyanide exposure are many, including combustion of plastic and vinyl, such as in a house fire, laboratory or industrial exposures including exposure in the electroplating industry both of printed circuit boards and in jewelry work. Rapid and definitive diagnosis of cyanide poisoning is unavailable in the emergency department setting. It is desirable to make a definitive diagnosis in order to prevent potential complications of empiric treatment of presumptive cyanide poisoning from the cyanide antidote kit currently approved by the US Food and Drug Administration (FDA). We investigated a technique to detect cyanide currently utilized by water treatment facilities to determine if it can be applied to rapidly detect concentrations of cyanide in the clinically important range. Varying standardized dilutions of KCN ranging from 0.25 microg/mL to 30 microg/mL were acidified with a drop of sulphuric acid in a closed system under a ventilation hood. Cyantesmo test strips were placed into the test tubes above the fluid level where liberated HCN gas interacted with the test strip to effect a color change. Color changes were compared to negative controls and to each other. The test strips demonstrated an incrementally increasing deep blue color change over a progressively longer portion of the test strip in less than 5 minutes for each concentration of KCN including 1, 3, 10, and 30 microg/mL. The concentrations of 0.25, 0.5, and 0.75 required more than 2 hours to begin demonstration of any color change. The Cyantesmo test strips accurately and rapidly detected, in a semi-quantifiable manner, concentrations of CN greater than 1 microg/mL contained in each test sample. Future work to validate this test in blood and in clinical specimens is planned.
Rella J, Marcus S, Wagner BJ. Rapid cyanide detection using the Cyantesmo kit. J Toxicol Clin Toxicol. 2004; 42(6):897-900. [PubMed Citation]
-
Exposure to cyanide can occur in a variety of ways, including exposure to smoke from cigarettes or fires, accidental exposure during industrial processes, and exposure from the use of cyanide as a poison or chemical warfare agent. Confirmation of cyanide exposure is difficult because, in vivo, cyanide quickly breaks down by a number of pathways, including the formation of both free and protein-bound thiocyanate. A simple method was developed to confirm cyanide exposure by extraction of protein-bound thiocyanate moieties from cyanide-exposed plasma proteins. Thiocyanate was successfully extracted and subsequently derivatized with pentafluorobenzyl bromide for GC-MS analysis. Thiocyanate levels as low as 2.5 ng mL(-1) and cyanide exposure levels as low as 175 μg kg(-1) were detected. Samples analyzed from smokers and non-smokers using this method showed significantly different levels of protein-bound thiocyanate (p<0.01). These results demonstrate the potential of this method to positively confirm chronic cyanide exposure through the analysis of protein-bound cyanide in human plasma.
Youso SL, Rockwood GA, Lee JP, Logue BA. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2010 Sep 10;677(1):24-8. [PubMed Citation]
-
Diaquacobinamide (H(2)O)(2)Cbi(2+) or its conjugate base hydroxyaquacobinamide (OH(H(2)O)Cbi(+))) can bind up to two cyanide ions, making dicyanocobinamide. This transition is accompanied by a significant change in color, previously exploited for cyanide determination. The reagent OH(H(2)O)Cbi(+) is used in excess; when trace amounts of cyanide are added, CN(H(2)O)Cbi(+) should be formed. But the spectral absorption of CN(H(2)O)Cbi(+) is virtually the same as that of OH(H(2)O)Cbi(+). It has been inexplicable how trace amounts of cyanide are sensitively measured by this reaction. It is shown here that even with excess OH(H(2)O)Cbi(+), (CN)(2)Cbi is formed first due to kinetic reasons; this only slowly forms CN(H(2)O)Cbi(+). This understanding implies that CN(H(2)O)Cbi(+) will itself be a better reagent. We describe a single valve merging zone flow analyzer that allows both sample and reagent economy. With a 50cm liquid core waveguide (LCW) flow cell and an inexpensive fiber optic - charge coupled device array spectrometer, a S/N=3 limit of detection of 8nM, a linear dynamic range to 6μM, and excellent precision (RSD 0.49% and 1.07% at 50 and 100nM, respectively, n=5 each) are formed. At 1% carryover, sample throughput is 40h(-1). The setup is readily used to measure thiocyanate with different reagents. We demonstrate applicability to real samples by analyzing human saliva samples and hydrolyzed extracts of apple seeds, peach pits, and almonds.
Ma J, Dasgupta PK, Zelder FH, Boss GR. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2012 Jul 29;736:78-84. [PubMed Citation]
-
When cyanide is introduced into the body, it quickly transforms through a variety of chemical reactions, normally involving sulfur donors, to form more stable chemical species. Depending on the nature of the sulfur donor, cyanide may be transformed into free thiocyanate, the major metabolite of cyanide transformation, 2-amino-2-thiazoline-4-carboxylic acid or protein-bound thiocyanate (PB-SCN) adducts. Because protein adducts are generally stable in biological systems, it has been suggested that PB-SCN may have distinct advantages as a marker of cyanide exposure. In this study, plasma was analyzed from 25 smokers (chronic low-level cyanide exposure group) and 25 non-smokers for PB-SCN. The amount of PB-SCN found in the plasma of smokers, 1.35 μM, was significantly elevated (p < 0.0001) when compared to non-smokers, 0.66 μM. Differences in sub-groups of smokers and non-smokers were also evaluated. The results of this study indicate the effectiveness of analyzing PB-SCN in determining instances of chronic cyanide exposure with possible extension to confirmation of acute cyanide exposure.
Youso SL, Rockwood GA, Logue BA. The analysis of protein-bound thiocyanate in plasma of smokers and non-smokers as a marker of cyanide exposure. J Anal Toxicol 2012 May;36(4):265-9. [PubMed Citation]
-
50 g Soluble in 100 mL water; 231 g at 100 deg C.
HSDB. Sodium thiosulfate
18. Publications
Bartlett JG, Greenberg MI, eds. PDR Guide to Terrorism Response. Montvale, NJ: Thomson PDR, 2005 p.321-41
Baskin SI, Horowitz AM, Nealley EW. The antidotal action of sodium nitrite and sodium thiosulfate against cyanide poisoning. J Clin Pharmacol. 1992 Apr;32(4):368-75. [PubMed Citation]
Bebarta VS, Pitotti RL, Dixon P, Lairet JR, Bush A, Tanen DA. Hydroxocobalamin versus sodium thiosulfate for the treatment of acute cyanide toxicity in a swine (Sus scrofa) model. 2012; Jun;59(6):532-9. [PubMed Citation]
Bebarta VS, Tanen DA, Lairet J, Dixon PS, Valtier S, Bush A. Hydroxocobalamin and Sodium Thiosulfate Versus Sodium Nitrite and Sodium Thiosulfate in the Treatment of Acute Cyanide Toxicity in a Swine (Sus scrofa) Model. Ann Emerg Med. 2010 Apr;55:345-351. [PubMed Citation]
Berlin CM The treatment of cyanide poisoning in children. The treatment of cyanide poisoning in children. Pediatrics. 1970; 46(5):793-796. [PubMed Citation]
Biodefense Meeting. Best Pharmaceuticals for Children Act. Eunice Kennedy Shriver National Institute of Child Health and Human Development, September 8-9, 2008, Rockville, MD (NICHD)
Cambal LK, Swanson MR, Yuan Q, Weitz AC, Li H, Pitt BR, Pearce LL, and Jim Peterson J. Acute, sublethal cyanide poisoning in mice is ameliorated by nitrite alone: complications arising from concomitant administration of nitrite and thiosulfate as an antidotal combination. Chem. Res. Toxicol. 2011;Jul 24(7):1104-12. [PubMed Citation]
Cannon EP, Leung P, Hawkins A, Petrikovics I, DeLoach J, Way JL. Antagonism of cyanide intoxication with murine carrier erythrocytes containing bovine rhodanese and sodium thiosulfate. J Toxicol Environ Health. 1994 Mar;41(3):267-74. [PubMed Citation]
Chen KK, Rose CL. Treatment of acute cyanide poisoning. J Am Med Assoc. 1956;162(12):1154-5.
Center for Drug Evaluation and Research: Summary Review for Regulatory Action. February 14, 2012 (FDA)
Cui Y, Liu L, Xia H, Han Z, Hu Y, Ma X. Sodium thiosulfate exposure disrupts in vitro and in vivo heart development. Front Biosci (Elite Ed). 2011 Jan 1;3:469-75. [PubMed Citation]
Curry SC, Carlton MW, Raschke RA. Prevention of fetal and maternal cyanide toxicity from nitroprusside with coinfusion of sodium thiosulfate in gravid ewes. Anesth Analg. 1997 May;84(5):1121-6. [PubMed Citation]
Dart RC, ed. Medical Toxicology 3rd Edition. Philadelphia, PA: Lippincott Williams & Wilkins, 2004 p.172-5
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
EMEA/CPMP Guidance Document on the use of Medicinal Products for the Treatment of Patients Exposed to Terrorist Attacks with Chemical Agents (April 2003) (EMA)
Garcia R, Sherpherd G. Cyanide poisoning and its treatment. Pharmacotherapy. 2004 Oct;24(10):1358-65. [PubMed Citation]
Geller RJ, Ekins BR, Iknoian RC. Cyanide toxicity from acetonitrile-containing false nail remover. Am J Emerg Med. 1991 May;9(3):268-70. [PubMed Citation]
Geller RJ, Barthold C, Saiers JA, Hall AH. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006 Nov;118(5):2146-58. [PubMed Citation]
Graeme KA, Curry SC, Bikin DS, Lo Vecchio FA, Brandon TA. The The lack of transplacental movement of the cyanide antidote thiosulfate in gravid ewes. Anesth Analg. 1999 Dec;89(6):1448-52. [PubMed Citation]
Hall AH , Linden CH, Kulig KW, Rumack BH; Cyanide poisoning from laetrile ingestion: role of nitrite therapy. Pediatrics. 1986 Aug;78:269-272
Hall AH, Doutre WH, Ludden T, Kulig KW, Rumack BH. Nitrite/thiosulfate treated acute cyanide poisoning: Estimated kinetics after antidote. Clin Toxicol. 1987; 25(1-2):121-133. [PubMed Citation]
Hall AH, Dart R, Bogdan G. Sodium thiosulfate or hydroxocobalamin for the empiric treatment of cyanide poisoning? Ann Emerg Med. 2007 Jun;49(6):806-13. [PubMed Citation]
Hall AH, Saiers J, Baud F. Which cyanide antidote? Crit Rev Toxicol 2009;39(7):541-52. [PubMed Citation]
Hamel J; A Review of acute cyanide poisoning with a treatment update. Critical Care Nurse. 2011 Feb;31(1):72-82. [PubMed Citation]
Heintz B, Bock TA, Kierdorf H, Sieberth HG. Cyanide poisoning: treatment with hyperoxygenation and sodium thiosulphate. Dtsch Med Wochenschr. 1990 Jul 13;115(28-29):1100-3. [PubMed Citation]
HSDB. Sodium thiosulfate
Hume AS, Mozingo JR, McIntyre B, Ho IK. Antidotal efficacy of alpha-ketoglutaric acid and sodium thiosulfate in cyanide poisoning. J Toxicol Clin Toxicol. 1995;33(6):721-4. [PubMed Citation]
Ivankovich AD, Braverman B, Kanuru RP, Heyman HJ, Paulissian R. Cyanide antidotes and methods of their administration in dogs: a comparative study. Anesthesiology. 1980 Mar;52(3):210-6. [PubMed Citation]
Johnson WS, Hall AH, Rumack BH. Cyanide poisoning successfully treated without 'therapeutic methemoglobin levels'. Am J Emerg Med. 1989 Jul;7(4):437-40. [PubMed Citation]
Kastrup EK et al., eds. Drug Facts and Comparisons St Louis, MO: Wolters Kluwer Health, 2012 p.682
Keim ME. Terrorism involving cyanide: the prospect of improving preparedness in the prehospital setting. Prehosp Disaster Med. 2006 Mar-Apr;21(2):s56-60. [PubMed Citation]
Lasch EE and Shawa RE. Multiple cases of cyanide poisoning by apricot kernels in children from Gaza. Pediatrics. 1981; 68(1):5-7.
Ma J, Dasgupta PK, Zelder FH, Boss GR. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2012 Jul 29;736:78-84. [PubMed Citation]
Mannaioni G, Vannacci A, Marzocca C, Zorn AM, Peruzzi S, Moroni F. Acute cyanide intoxication treated with a combination of hydroxycobalamin, sodium nitrite, and sodium thiosulfate. J Toxicol Clin Toxicol. 2002;40(2):181-3. [PubMed Citation]
Martin J, et al., eds. British National Formulary, No. 58. London, UK: BMJ Group, RPS Publishing, 2009 p. 34
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012
Mégarbane B, Delahaye A, Goldgran-Tolédano D, Baud FJ. Antidotal treatment of cyanide poisoning. J Chin Med Assoc. 2003 Apr;66(4):193-203. [PubMed Citation]
Mehta C. Antidotal effect of sodium thiosulfate in mice exposed to acrylonitrile. Res Commun Mol Pathol Pharmacol. 1995 Feb;87(2):155-65. [PubMed Citation]
Mengel K, Krär W, Isert B, Friedberg KD. Thiosulphate and hydroxocobalamin prophylaxis in progressive cyanide poisoning in guinea-pigs. Toxicology. 1989 Mar;54(3):335-42. [PubMed Citation]
Nakatani T, Kosugi Y, Mori A, Tajimi K, Kobayashi K; Changes in the parameters of oxygen metabolism in a clinical course recovering from potassium cyanide. Am J Emerg Med. 1993 May; 213-7. [PubMed Citation]
Nelson LS, Lewin NA, Howland M, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfranks's Toxicologic Emergencies, 9th Edition. New York, NY: McGraw-Hill Medical, 2011 p. 1692-94
Product label: NITHIODOTE (sodium nitrite and sodium thiosulfate) kit [Hope Pharmaceuticals]. Revised 1/2011. [DailyMed]
Product label: Sodium Thiosulfate Injection [Hope Pharmaceuticals]. Approval 2/2012.
Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC. Review article: management of cyanide poisoning. Emerg Med Australas. 2012 Jun;24(3):225-38. [PubMed Citation]
Rella J, Marcus S, Wagner BJ. Rapid cyanide detection using the Cyantesmo kit. J Toxicol Clin Toxicol. 2004; 42(6):897-900. [PubMed Citation]
Renard C, Borron SW, Renaudeau C, Baud FJ. Sodium thiosulfate for acute cyanide poisoning: study in a rat model. Ann Pharm Fr. 2005 Mar;63(2):154-61. [PubMed Citation]
RePORTER. NIH. Countermeasures against chemical threats: countermeasures against cyanide.
RePORTER. NIH. Development of a field-deployable device to rapidly measure blood cyanide levels.
Ruangkanchanasetr S, Wananukul V, Suwanjutha S. Cyanide poisoning, 2 cases report and treatment review. J Med Assoc Thai. 1999 Nov;82 Suppl 1:S162-7. [PubMed Citation]
Schulz LT, Elder EJ Jr, Jones KJ, Vijayan A, Johnson BD, Medow JE, Vermeulen L. Stability of sodium nitroprusside and sodium thiosulfate 1:10 intravenous admixture. Hosp Pharm. 2010;Oct;45(10):779-84. [PubMed Citation]
Summary of the NIAID Expert Panel Review on Medical Chemical Defense Research, March 19, 2003, Bethesda, MD (NIH/NIAID)
Sylvester DM, Hayton WL, Morgan RL, Way JL. Effects of thiosulfate on cyanide pharmacokinetics in dogs. Toxicol Appl Pharmacol. 1983 Jun 30;69(2):265-71. [PubMed Citation]
Tadić V. The in vivo effects of cyanide and its antidotes on rat brain cytochrome oxidase activity. Toxicology. 1992 Nov 22;76(1):59-67. [PubMed Citation]
Turchen SG, Manoguerra AS, Whitney C. Severe cyanide poisoning from the ingestion of an acetonitrile-containing cosmetic. Am J Emerg Med. 1991 May;9(3):264-7. [PubMed Citation]
Vick JA, Froehlich H. Treatment of cyanide poisoning. Mil Med. 1991 Jul;156(7):330-9. [PubMed Citation]
Yen D, Tsai J, Wang LM, Kao WF, Hu SC, Lee CH, Deng JF. The clinical experience of acute cyanide poisoning. Am J Emerg Med. 1995 Sep;13(5):524-8. [PubMed Citation]
Youso SL, Rockwood GA, Logue BA. The analysis of protein-bound thiocyanate in plasma of smokers and non-smokers as a marker of cyanide exposure. J Anal Toxicol 2012 May;36(4):265-9. [PubMed Citation]
Youso SL, Rockwood GA, Lee JP, Logue BA. Determination of cyanide exposure by gas chromatography-mass spectrometry analysis of cyanide-exposed plasma proteins. Anal Chim Acta. 2010 Sep 10;677(1):24-8. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
