You are here: Home > Medical Countermeasures Database > Statins
Statins - Medical Countermeasures Database
- Name of Chemical Defense therapeutic agent/device
- Chemical Defense therapeutic area
- Evidence-based medicine for Chemical Defense
- Pharmacokinetic and toxicokinetics data
- Indication/dosing
- Formulation/shelf life
- Off-label use & dosing
- Route of administrating/monitoring
- Adverse effects
- Contraindications
- Clinical studies in progress
- Non-clinical studies in progress
- Needed studies for Chemical Defense
- Needed studies for non-Chemical Defense
- Study-related ethical concerns
- Global regulatory status
- Other potentially useful information
- Publications
- Web sites
1. Name of Chemical Defense therapeutic agent/device
Statins
2. Chemical Defense therapeutic area(s)
— including key possible usesStatins can be used as anti-inflammatory treatment for acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) induced by pulmonary agent such as phosgene.
3. Evidence-based medicine for Chemical Defense
— including efficacy and safetyA. Summary
Structure
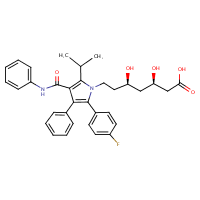
US NLM. ChemIDplus Lite. Atorvastatin
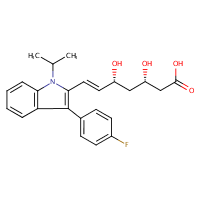
US NLM. ChemIDplus Lite. Fluvastatin
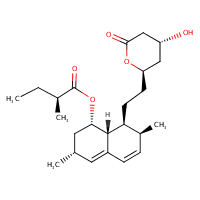
US NLM. ChemIDplus Lite. Lovastatin
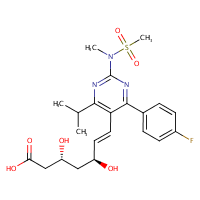
US NLM. ChemIDplus Lite. Rosuvastatin
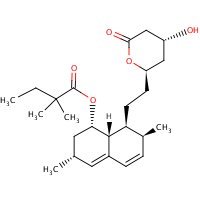
US NLM. ChemIDplus Lite. Simvastatin
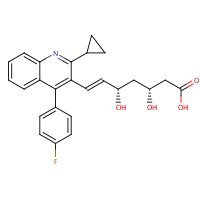
US NLM.ChemIDplus Lite. Pitavastatin
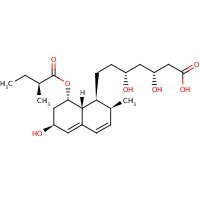
US NLM. ChemIDplus Lite. Pravastatin
Mechanism of action
-
Statins are 3-hydroxy-3-methylglutaryl-co-enzyme A reductase inhibitors of cholesterol biosynthesis, and have been reported to exert pleiotropic effects on cellular signalling and cellular functions involved in inflammation. Recent reports have demonstrated that previous statin therapy reduced the risk of pneumonia or increased survival in patients with community-acquired pneumonia. However, the precise mechanisms responsible for these effects are unclear. In the present study, we examined the effects of statins on cytokine production from lipopolysaccharide (LPS)-stimulated human bronchial epithelial cells (BEAS-2B). Interleukin (IL)-6 and IL-8 mRNA expression and protein secretion in LPS-stimulated cells were inhibited significantly by the lipophilic statin pitavastatin and the hydrophilic statin pravastatin. As these inhibitory effects of statin were negated by adding mevalonate, the anti-inflammatory effects of statins appear to be exerted via the mevalonic cascade. In addition, the activation levels of Ras homologue gene family A (RhoA) in BEAS-2B cells cultured with pitavastatin were significantly lower than those without the statin. These results suggest that statins have anti-inflammatory effects by reducing cytokine production through inhibition of the mevalonic cascade followed by RhoA activation in the lung.
Iwata A, Shirai R, Ishii H, Kushima H, Otani S, Hashinaga K, Umeki K, Kishi K, Tokimatsu I, Hiramatsu K, Kadota J. Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells. Clin Exp Immunol. 2012 May;168(2):234-40. [PubMed Citation]
-
The 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) have potent anti-inflammatory, vasodilatory and anti-platelet effects that are independent of the lipid-lowering effects. These non-lipid-lowering or pleiotropic effects are dependent on HMG-CoA reductase inhibition in tissues other than the liver. In animal models, high-dose statins upregulate cytosolic phospholipase A(2) and cyclooxygenase-2, leading to increased production of prostacyclin and 15-deoxy-PGJ(2). In addition, statins activate protein kinase A, which phosphorylates 5-lipoxygenase, resulting in decreased production of the pro-inflammatory leukotrienes and increased production of 15-epi-lipoxin A4, an eicosanoid with potent anti-inflammatory and inflammation-resolution properties. It is unclear, however, whether these effects occur in the clinical setting and whether these effects (partially) explain the anti-inflammatory effects of statins in patients.
Birnbaum Y, Ye Y. Pleiotropic effects of statins: the role of eicosanoid production. Curr Atheroscler Rep. 2012 Apr;14(2):135-9. [PubMed Citation]
-
Results from large-scale clinical trials of lipid lowering with 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) have led to a revolution in the management of atherosclerosis. In addition to their potent effect on serum lipid levels, statins influence several other cellular pathways, including those involving inflammatory, oxidative, and thrombotic processes. These effects clearly have the potential to beneficially modify the atherogenic process, and it has been suggested that they contribute to the impressive results seen in the clinical trials. We review the clinical evidence for benefits of statin therapy that are distinct from their effect on lipid biology. In particular, we address three key issues: the role of statins in diseases not traditionally associated with elevated cholesterol levels; whether clinical benefits are seen with statin therapy before an effect on lipid levels; and whether the magnitude of clinical benefit observed with statin therapy is unrelated to the degree of cholesterol reduction. At present, low-density-lipoprotein lowering seems to be the primary mechanism underlying the clinical benefits of statin therapy and should remain the focus of risk-reduction strategies in clinical practice.
Halcox JP, Deanfield JE. Beyond the laboratory: clinical implications for statin pleiotropy. Circulation. 2004 Jun 1;109(21 Suppl 1):II42-8. [PubMed Citation]
Summary of clinical and non-clinical studies
Acute lung injury is a life-threatening condition defined by intense pulmonary inflammation and alveolar collapse triggering respiratory failure and multi-organ dysfunction; the more severe form of this is acute respiratory distress syndrome (Ware and Matthay; 2000). Statins have anti-inflammatory properties, for example the ability to directly mediate endothelial cell signaling and induce characteristic actin cytoskeletal rearrangement leading to endothelial cell barrier protection (Jacobson; 2009), making them a good candidate as a supportive therapy in the acute treatment of ALI and ARDS stemming from toxic exposure. Pravastatin and Pitavastatin suppress the production of cytokines thereby reducing inflammation (Iwata et al; 2012). In mice pravastatin inhibited TGF-β1, CTGF, RhoA and cyclin D1 pathways attenuating the bleomycin induced ALI and pulmonary fibrosis (Kim et al; 2010). Simvastatin inhibited the production of IL-6 by human monocytes in a dose-dependent manner (Li and Chen; 2003). A double blind placebo study was done to test the effects of statins on patients with ALI using 80mg simvastatin. Patients with ALI were given 80mg simvastatin or a placebo while on mechanical ventilation for no longer than 14 days. Treatment with simvastatin reduced pulmonary and systemic inflammation and also improved pulmonary function and systemic organ dysfunction. Although 80mg is no longer the prescribed dose for prolonged use in the treatment of hypercholesterolemia, it was well tolerated in the patients participating in the study (Craig et al; 2011).
B. Link to clinical studies
Adult
-
Preclinical studies suggest that HMG-CoA reductase inhibitors (statins) may attenuate organ dysfunction. Investrigators evaluated whether statins are associated with attenuation of lung injury and prevention of associated organ failure in patients with ALI/ARDS. From a database of patients with ALI/ARDS, we determined the presence and timing of statin administration. Main outcome measures were the development and progression of pulmonary and nonpulmonary organ failures as assessed by changes in PaO2/FiO2 ratio and Sequential Organ Failure Assessment score (SOFA) between days 1 and 7 after the onset of ALI/ARDS. Secondary outcomes included ventilator free days, ICU and hospital mortality, and lengths of ICU and hospital stay. From 178 patients with ALI/ARDS, 45 (25%) received statin therapy. From day 1 to day 7, the statin group showed less improvement in their PaO2/FiO2 ratio (27 vs. 55, P = 0.042). Ventilator free days (median 21 vs. 16 days, P = 0.158), development or progression of organ failures (median ΔSOFA 1 vs. 2, P = 0.275), ICU mortality (20% vs. 23%, P = 0.643), and hospital mortality (27 vs. 37%, P = 0.207) were not significantly different in the statin and non-statin groups. After adjustment for baseline characteristics and propensity for statin administration, there were no differences in ICU or hospital lengths of stay. In this retrospective cohort study, statin use was not associated with improved outcome in patients with ALI/ARDS. Investigators were unable to find evidence for protection against pulmonary or nonpulmonary organ dysfunction (Class IV).
Kor DJ, Iscimen R, Yilmaz M, Brown MJ, Brown DR, Gajic O. Statin administration did not influence the progression of lung injury or associated organ failures in a cohort of patients with acute lung injury. Intensive Care Med. 2009 Jun;35(6):1039-46. [PubMed Citation]
-
There is no effective pharmacological treatment for acute lung injury (ALI). Statins are a potential new therapy because they modify many of the underlying processes important in ALI. The objective of this study was to test whether simvastatin improves physiological and biological outcomes in ALI. Researchers conducted a randomized, double-blinded, placebo-controlled trial in patients with ALI. Patients received 80 mg simvastatin or placebo until cessation of mechanical ventilation or up to 14 days. Extravascular lung water was measured using thermodilution. Measures of pulmonary and nonpulmonary organ function were assessed daily. Pulmonary and systemic inflammation was assessed by bronchoalveolar lavage fluid and plasma cytokines. Systemic inflammation was also measured by plasma C-reactive protein. Sixty patients were recruited. Baseline characteristics, including demographics and severity of illness scores, were similar in both groups. At Day 7, there was no difference in extravascular lung water. By Day 14, the simvastatin-treated group had improvements in nonpulmonary organ dysfunction. Oxygenation and respiratory mechanics improved, although these parameters failed to reach statistical significance. Intensive care unit mortality was 30% in both groups. Simvastatin was well tolerated, with no increase in adverse events. Simvastatin decreased bronchoalveolar lavage IL-8 by 2.5-fold (P = 0.04). Plasma C-reactive protein decreased in both groups but failed to achieve significance in the placebo-treated group. Treatment with simvastatin appears to be safe and may be associated with an improvement in organ dysfunction in ALI. These clinical effects may be mediated by a reduction in pulmonary and systemic inflammation (Class III).
Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O'Kane CM, Elborn JS, McAuley DF. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med. 2011 Mar 1;183(5):620-6. [PubMed Citation]
Pregnancy, breastfeeding studies
-
Cholesterol and other products of the cholesterol biosynthetic pathway are essential for fetal development, including synthesis of steroids and cell membranes. Because of the ability of statins to decrease the synthesis of cholesterol and possibly other products of the cholesterol biosynthetic pathway, these agents may cause fetal harm when administered to pregnant women. ... Severe congenital skeletal malformation, tracheoesophageal fistula, and atresia were reported in a neonate whose mother received lovastatin concomitantly with dextroamphetamine sulfate during the first trimester of pregnancy (Class IV).
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
-
Pravastatin is distributed into human milk and pitavastatin is distributed into milk in animals (Class IV).
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
C. Link to non-clinical (e.g. animal) studies
Adult animal models
-
Therapies to limit the life-threatening vascular leak observed in patients with acute lung injury (ALI) are currently lacking. We explored the effect of simvastatin, a 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitor that mediates endothelial cell barrier protection in vitro, in a murine inflammatory model of ALI. C57BL/6J mice were treated with simvastatin (5 or 20 mg/kg body wt via intraperitoneal injection) 24 h before and again concomitantly with intratracheally administered LPS (2 μg/g body wt). Inflammatory indexes [bronchoalveolar lavage (BAL) myeloperoxidase activity and total neutrophil counts assessed at 24 hr with histological confirmation] were markedly increased after LPS alone but significantly reduced in mice that also received simvastatin (20 mg/kg; ~35-60% reduction). Simvastatin also decreased BAL albumin (~50% reduction) and Evans blue albumin dye extravasation into lung tissue (100%) consistent with barrier protection. Finally, the sustained nature of simvastatin-mediated lung protection was assessed by analysis of simvastatin-induced gene expression (Affymetrix platform). LPS-mediated lung gene expression was significantly modulated by simvastatin within a number of gene ontologies (e.g., inflammation and immune response, NF-κB regulation) and with respect to individual genes implicated in the development or severity of ALI (e.g., IL-6, Toll-like receptor 4). Together, these findings confirm significant protection by simvastatin on LPS-induced lung vascular leak and inflammation and implicate a potential role for statins in the management of ALI.
Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005 Jun;288(6):L1026-32. [PubMed Citation]
-
Lung infections are associated with acute lung injury (ALI), systemic inflammation, and vascular events. Clinical studies suggest that statins improve health outcomes of patients with pneumonia and ALI. The mechanisms by which this occurs are unknown. The aim of this study was to determine whether statins attenuate systemic inflammation and cardiovascular dysfunction related to ALI in mice. Simvastatin (SS; 20 mg/kg) or vehicle solution was instilled intraperitoneally into mice 24 h before and again just prior to intratracheal LPS instillation (1 μg/g). These mice were then anesthetized with 2.0% isoflurane and underwent a short surgical procedure to instill LPS. Four hours later, IL-6 levels in bronchoalveolar lavage fluid and in arterial and venous serum were measured. Cardiac function was evaluated using 2-D echocardiography, and endothelial function was determined using wire myography on aortic sections. LPS induced a significant increase in serum IL-6 levels. SS reduced venous (P = 0.040) but not arterial concentrations of IL-6 (P = 0.112). SS improved the maximal vasodilatory response of the aorta to ACh (P = 0.004) but not to sodium nitroprusside (P = 1.000). SS also improved cardiac output (P = 0.023). Vasodilatory response to ACh was impaired when aorta from untreated mice was incubated with ex vivo IL-6 (P = 0.004), whereas in the aorta from mice pretreated with SS, the vasodilatory response did not change with IL-6 incubation (P = 0.387). SS significantly improved LPS-induced cardiovascular dysfunction possibly by reducing systemic expression of IL-6 and its downstream signaling pathways. These findings may explain how statins improve health outcomes in patients with ALI.
Suda K, Eom J, Jaw JE, Mui T, Bai N, Or C, Ngan D, Li Y, Wang X, Tsuruta M, Tam S, Man SP, Van Eeden S, Sin DD. Endotoxin-induced cardiovascular dysfunction in mice: effect of simvastatin. J Appl Physiol. 2011 Oct;111(4):1118-24. [PubMed Citation]
-
Objectives to elicit whether pre-treatment with pravastatin will prevent or ameliorate the acute lung injury that occurs following lower torso ischemia-reperfusion (IR) in an experimental animal model. Male Sprague-Dawley rats were randomized into three groups (n=7/group). The control group underwent a sham laparotomy and aortic dissection. The second group underwent infrarenal aortic cross clamping for 30 min followed by reperfusion for 120 min. The third group pre-treated with pravastatin sodium (0.4 mg/kg/day over 5 days) were again subjected to an ischemia-reperfusion (IR) injury. The parameters used to assess lung injury included: Wet to dry lung weight ratio (W:D), myeloperoxidase activity (MPO), protein concentration (BALprot) and neutrophil count (BAL PMN) of bronchoaveolar lavage fluid. Western blotting was used to determine the expression of constitutive endothelial nitric oxide synthase (ecNOS) within lung tissue. IR causes an acute lung injury as indicated by statistically significant differences in W:D lung weight ratios, MPO activity, neutrophil count and BAL protein concentration in the IR group over that of controls. Pre-treatment with pravastatin attenuated this neutrophil infiltration and microvascular leakage. The pravastatin group showed a marked increased expression of ecNOS over that of the IR group and controls. This data indicates that pre-treatment with pravastatin protects against ischemia-reperfusion induced lung injury in an experimental animal model. We believe that its mechanism of action involves an upregulation of ecNOS, which increases basal expression of nitric oxide providing protective effects on the pulmonary circulation against microvascular injury.
Joyce M, Kelly CJ, Chen G, Bouchier-Hayes DJ. Pravastatin attenuates lower torso ischaemia-reperfusion-induced lung injury by upregulating constitutive endothelial nitric oxide synthase. Eur J Vasc Endovasc Surg. 2001 Apr;21(4):295-300. [PubMed Citation]
-
The present study was designed to determine whether pravastatin, a 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor, could attenuate acute lung injury (ALI) induced by lipopolysaccharide (LPS) in BALB/c mice. Acute lung injury was induced successfully by intratracheal administraiton of LPS (3 mg/g) in BALB/c mice. Pravastatin (3, 10 and 30 mg/kg, i.p.) was administered to mice 24 hr prior to and then again concomitant with LPS exposure. Challenge with LPS alone produced a significant increase in lung index and the wet/dry weight ratio compared with control animals. Pulmonary microvascular leakage, as indicated by albumin content in the bronchoalveolar lavage fluid (BALF) and extravasation of Evans blue dye albumin into lung tissue, was apparently increased in LPS-exposed mice. Lipopolysaccharide exposure also produced a significant lung inflammatory response, reflected by myeloperoxidase activity and inflammatory cell counts in BALF. Furthermore, histological examination showed that mice exposed to LPS also exhibited prominent inflammatory cell infiltration and occasional alveolar hemorrhage. Pravastatin (3, 10 or 30 mg/kg, i.p.) produced a significant reduction in multiple indices of LPS-induced pulmonary vascular leak and inflammatory cell infiltration into lung tissue. Elevated tumor necrosis factor (TNF)-a levels in lung tissue homogenates of ALI mice were significantly decreased after administration of 10 or 30 mg/kg pravastatin. These findings confirm significant protection by pravastatin against LPS-induced lung vascular leak and inflammation and implicate a potential role for statins in the management of ALI. The inhibitory effect of pravastatin was associated with its effect in decreasing TNF-a.
Yao HW, Mao LG, Zhu JP. Protective effects of pravastatin in murine lipopolysaccharide-induced acute lung injury. Clin Exp Pharmacol Physiol. 2006 Sep;33(9):793-7.[PubMed Citation]
-
Pravastatin is best known for its antilipidemic action. Recent studies have shown that statins have immunomodulatory and anti-inflammatory effects. The present study aimed to determine whether or not pravastatin can attenuate acute lung injury and fibrosis in a mouse model. Bleomycin was given to C57BL6 mice through intratracheal instillation. Pravastatin was given through intraperitoneal injection. To study the effect of pravastatin on the early inflammatory phase and the late fibrotic phase, mice were killed on days 3, 7, 14 and 21. Pravastatin attenuated the histopathological change of bleomycin- induced lung injury and fibrosis. The accumulation of neutrophils and increased production of tumor necrosis factor-a in bronchoalveolar lavage fluid were inhibited in the early inflammatory phase. Pravastatin effectively inhibited the increase of lung hydroxyproline content induced by bleomycin. Furthermore, pravastatin reduced the increased expression of transforming growth factor (TGF)-b1, connective tissue growth factor (CTGF), RhoA and cyclin D1. The increased levels of TGF-b1 and CTGF mRNA expression were also significantly inhibited by pravastatin. Pravastatin effectively attenuated bleomycin-induced lung injury and pulmonary fibrosis in mice. Our results provide evidence for the therapeutic potential of pravastatin in the treatment of acute lung injury and pulmonary fibrosis.
Kim JW, Rhee CK, Kim TJ, Kim YH, Lee SH, Yoon HK, Kim SC, Lee SY, Kwon SS, Kim KH, Kim YK. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin Exp Pharmacol Physiol. 2010 Nov;37(11):1055-63. [PubMed Citation]
Other non-clinical studies
Human non-clinical studies-
The accumulating evidence suggests that C-reactive protein (CRP) may have direct inflammatory effects on the vascular wall and that statin therapy may have important non-lipid anti-inflammatory effects confirmed by decreasing serum inflammatory markers, such as CRP. However, the effect of simvastatin on interleukin-6 (IL-6) release in cultured human monocytes was not investigated. Design A prospective, human monocyte culture, simvastatin intervention study. Methods Monocytes were isolated from blood of healthy volunteers by the Ficoll density gradient and stimulated by broad concentrations of CRP (1-20 lg/ml) and lipopolysaccharide (LPS, 1-10 ng/ml) at indicated time points (0, 2, 4, 8, 16 and 24 hr). Also 10-8-10-6 mol/l simvastatin was coincubated with cells in the presence of CRP and LPS. Measurements of IL-6 were performed from supernatants of cultured medium in duplicate, using a commercial assay kit. Results CRP and LPS induced the rapid release of IL-6, with significantly elevated levels in cultured supernatants at 4 hr in the CRP group and at 2 h in the LPS group. The effects of CRP and LPS on IL-6 release of monocytes were dose and time dependent. A greater than 11-fold increase of IL-6 in the CRP group (20 lg/ml) and a greater than 26- fold increase in the LPS group (10 ng/ml) were observed at 24 h compared with the control group (945.7± 98.3 pg/ml compared with 94.3± 12.4 pg/ml and 1720.4 ± 690.1 pg/ml compared with 70.1± 16.7pg/ml, P < 0.001, respectively). However, 10-8-10-6 mol/l simvastatin inhibited significantly the production of IL-6 in monocytes stimulated by CRP and LPS in a dose-dependent manner, with the maximal inhibiting effect at a concentration of 10-6 mol/L (945.7 ± 98.3 pg/ml compared with 180.9 ± 31.2 pg/ml and 1720.4 ±690.1 pg/ml compared with 824.0 ± 206.2 pg/ml, P < 0.001 respectively). Conclusions CRP and LPS could induce IL-6 release in human monocytes and simvastatin could inhibit this response in a dose-dependent manner, which may provide an insight into the mechanisms of anti-inflammatory or anti-atherosclerotic actions of simvastatin.
Li JJ, Chen XJ. Simvastatin inhibits interleukin-6 release in human monocytes stimulated by C-reactive protein and lipopolysaccharide. Coron Artery Dis. 2003 Jun;14(4):329-34.[PubMed Citation]
-
The statins, hydroxy-3-methylglutaryl-CoA reductase inhibitors that lower serum cholesterol, exhibit myriad clinical benefits, including enhanced vascular integrity. One potential mechanism underlying increased endothelial cell (EC) barrier function is inhibition of geranylgeranylation, a covalent modification enabling translocation of the small GTPases Rho and Rac to the cell membrane. While RhoA inhibition attenuates actin stress fiber formation and promotes EC barrier function, Rac1 inhibition at the cell membrane potentially prevents activation of NADPH oxidase and subsequent generation of superoxides known to induce barrier disruption. We examined the relative regulatory effects of simvastatin on RhoA, Rac1, and NADPH oxidase activities in the context of human pulmonary artery EC barrier protection. Confluent EC treated with simvastatin demonstrated significantly decreased thrombin-induced FITC-dextran permeability, a reflection of vascular integrity, which was linked temporally to simvastatin-mediated actin cytoskeletal rearrangement. Compared with Rho inhibition alone (Y-27632), simvastatin afforded additional protection against thrombin-mediated barrier dysfunction and attenuated LPS-induced EC permeability and superoxide generation. Statin-mediated inhibition of both Rac translocation to the cell membrane and superoxide production were attenuated by geranylgeranyl pyrophosphate (GGPP), indicating that these effects are due to geranylgeranylation inhibition. Finally, thrombin-induced EC permeability was modestly attenuated by reduced Rac1 expression (small interfering RNA), whereas these effects were made more pronounced by simvastatin pretreatment. Together, these data suggest EC barrier protection by simvastatin is due to dual inhibitory effects on RhoA and Rac1 as well as the attenuation of superoxide generation by EC NADPH oxidase and contribute to the molecular mechanistic understanding of the modulation of EC barrier properties by simvastatin.
Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol. 2008 Oct;295(4):L575-83. [PubMed Citation]
-
The statins, a class of HMG-CoA reductase inhibitors, directly affect multiple vascular processes via inhibition of geranylgeranylation, a covalent modification essential for Rho GTPase interaction with cell membrane-bound activators. We explored simvastatin effects on endothelial cell actomyosin contraction, gap formation, and barrier dysfunction produced by the edemagenic agent, thrombin. Human pulmonary artery endothelial cells exposed to prolonged simvastatin treatment (5 microM, 16 h) demonstrated significant reductions in thrombin-induced (1 U/ml) barrier dysfunction ( approximately 70% inhibition) with accelerated barrier recovery, as measured by transendothelial resistance. Furthermore, simvastatin attenuated basal and thrombin-stimulated (1 U/ml, 5 min) myosin light chain diphosphorylation and stress fiber formation while dramatically increasing peripheral immunostaining of actin and cortactin, an actin-binding protein, in conjunction with increased Rac GTPase activity. As both simvastatin-induced Rac activation and barrier protection were delayed (maximal after 16 h), we assessed the role of gene expression and protein translation in the simvastatin response. Simultaneous treatment with cycloheximide (10 microg/ml, 16 h) abolished simvastatin-mediated barrier protection. Robust alterations were noted in the expression of cytoskeletal proteins (caldesmon, integrin beta4), thrombin regulatory elements (PAR-1, thrombomodulin), and signaling genes (guanine nucleotide exchange factors) in response to simvastatin by microarray analysis. These novel observations have broad clinical implications in numerous vascular pathobiologies characterized by alterations in vascular integrity including inflammation, angiogenesis, and acute lung injury.
Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol. 2004 May;30(5):662-70. [PubMed Citation]
Non-clinical reviews
-
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) represent a spectrum of diseases that are commonly encountered in the intensive care unit and are associated with high mortality. Although significant advances have been made with respect to the ventilatory management of patients with ALI/ARDS with proven beneficial effects on outcomes, pharmacologic therapies remain nonexistent. Because the cardinal feature of ALI/ARDS is an increase in lung vascular permeability, often precipitated by an exuberant inflammatory response with subsequent endothelial barrier disruption, strategies aimed at promoting endothelial barrier function could serve as novel therapies in this setting. We have identified several promising agonists in this regard including sphingosine 1-phosphate, activated protein C, and statins, a class of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. These agonists all have in common the ability to directly mediate endothelial cell signaling and induce characteristic actin cytoskeletal rearrangement leading to endothelial cell barrier protection. Our in vitro findings have been extended to animal models of ALI/ARDS and suggest that effective pharmacologic therapies for patients with ALI/ARDS may soon be available.
Jacobson JR. Pharmacologic therapies on the horizon for acute lung injury/acute respiratory distress syndrome. J Investig Med. 2009 Dec;57(8):870-3. [PubMed Citation]
4. Pharmacokinetic and toxicokinetics data
— including children-, pregnancy-, geriatric-, and obesity-related dataAdult
-
Pitavastatin calcium is a new addition to the class of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors ("statins") approved for use in the United States for the treatment of primary hyperlipidemia and mixed dyslipidemia. ... Based on findings from pharmacokinetic studies, pitavastatin may be given at any time of the day, with or without food. The drug had a mean plasma elimination t(1/2) of 12 hours, is expected to be associated with minimal drug-drug interactions because it is not metabolized by the cytochrome P450 3A4 isozyme, and is primarily excreted unchanged in the bile with little renal elimination. ...
Yee LL, Wright EA. Pitavastatin calcium: clinical review of a new antihyperlipidemic medication. Clin Ther. 2011 Aug; 33 (8): 1023-42. [PubMed Citation]
-
Elucidation of the rate-determining process in the overall hepatic elimination of drugs is critical for predicting their intrinsic hepatic clearance and the impact of variation of sequestration clearance on their systemic concentration. The present study investigated the rate-determining process in the overall hepatic elimination of the HMG-CoA reductase inhibitors pravastatin, pitavastatin, atorvastatin, and fluvastatin both in rats and humans. The uptake of these statins was saturable in both rat and human hepatocytes. Intrinsic hepatic clearance obtained by in vivo pharmacokinetic analysis in rats was close to the uptake clearance determined by the multiple indicator dilution method but much greater than the intrinsic metabolic clearance extrapolated from an in vitro model using liver microsomes. In vivo uptake clearance of the statins in humans (pravastatin, 1.44; pitavastatin, 30.6; atorvastatin, 12.7; and fluvastatin, 62.9 ml/min/g liver), which was obtained by multiplying in vitro uptake clearance determined in cryopreserved human hepatocytes by rat scaling factors, was within the range of overall in vivo intrinsic hepatic clearance (pravastatin, 0.84-1.2; pitavastatin, 14-35; atorvastatin, 11-19; and fluvastatin, 123-185 ml/min/g liver), whereas the intrinsic metabolic clearance of atorvastatin and fluvastatin was considerably low compared with their intrinsic hepatic clearance. Their uptake is the rate-determining process in the overall hepatic elimination of the statins in rats, and this activity likely holds true for humans. In vitro-in vivo extrapolation of the uptake clearance using a cryopreserved human hepatocytes model and rat scaling factors will be effective for predicting in vivo intrinsic hepatic clearance involving active uptake.
Watanabe T, Kusuhara H, Maeda K, Kanamaru H, Saito Y, Hu Z, Sugiyama Y. Investigation of the rate-determining process in the hepatic elimination of HMG-CoA reductase inhibitors in rats and humans. Drug Metab Dispos. 2010 Feb; 38 (2): 215-22. [PubMed Citation]
5. Current FDA/EUA approved indications and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data, and Emergency Use Authorization (EUA)Adults (FDA)
-
Hyperlipidemia (statins): Dosage of statins must be carefully adjusted according to individual requirements and response. Because higher dosages are associated with an increased risk of myopathy, the American College of Cardiology (ACC), the American Heart Association (AHA), and the National Heart, Lung and Blood Institute (NHLBI) clinical advisory panel on statins state that statin dosages generally should not exceed those required to attain the target LDL-cholesterol goal of therapy. The manufacturer of simvastatin states that higher dosages of the drug (i.e., 80 mg daily) should be restricted to patients who have been receiving long-term therapy (e.g., 12 months or longer) at this dosage without evidence of muscle toxicity. Serum lipoprotein concentrations should be determined periodically during statin therapy. Dosage should be increased at intervals of no less than 4 weeks until the desired effect on lipoprotein concentrations is observed; reduction of statin dosage can be considered in patients whose serum cholesterol concentrations fall below the desired target range.
Therapy with statins, in conjunction with dietary measures, should continue throughout the patient's lifetime pending further data defining a more appropriate treatment period.
Use of certain statins with potent inhibitors of cytochrome P-450 (CYP) isoenzyme 3A4, immunosuppressants (e.g., cyclosporine), fibric acid derivatives, antilipemic dosages (exceeding 1 g daily) of niacin, danazol, amiodarone, diltiazem, or verapamil may be associated with an increased risk of myopathy or rhabdomyolysis. If such combinations are used, lower initial dosages of the drugs should be administered, and titration to higher dosages should be done with caution.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
-
Hyperlipidemia (pitavastatin): Dosage of pitavastatin calcium is expressed in terms of pitavastatin and should be carefully adjusted according to individual requirements and response.The recommended initial adult dosage of pitavastatin for the management of primary hypercholesterolemia or mixed dyslipidemia is 2 mg once daily. The usual maintenance dosage is 1-4 mg once daily. The maximum dosage of pitavastatin is 4 mg once daily.
In adults receiving pitavastatin concomitantly with erythromycin, dosage of pitavastatin should not exceed 1 mg once daily.
In adults receiving pitavastatin concomitantly with rifampin, dosage of pitavastatin should not exceed 2 mg once daily.
In adults receiving pitavastatin concomitantly with antilipemic dosages of niacin, reduction in pitavastatin dosage should be considered.
The usual initial dosage of pravastatin sodium in adults is 40 mg daily. If antilipemic response is inadequate with the initial dosage, the manufacturer states that dosage may be increased to 80 mg daily.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
-
Concomitant cyclosporine therapy: Because of an increased risk of myopathy during concomitant therapy, patients receiving immunosuppressive drugs such as cyclosporine concomitantly with pravastatin should receive an initial pravastatin sodium dosage of 10 mg daily at bedtime; titration to higher dosages should be done with caution. Most patients treated with this drug combination received a maximum pravastatin sodium dosage of 20 mg daily.
-
Concomitant bile acid sequestrants therapy: The cholesterol-lowering effects of pravastatin sodium and bile acid sequestrants (e.g., cholestyramine) are additive or synergistic. However, since bile acid sequestrants may bind and delay absorption of pravastatin sodium, the manufacturer recommends that pravastatin sodium be administered 1 hour or more before and at least 4 hours after the bile acid sequestrant when these drugs are used concomitantly.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
-
Hyperlipidemia (simvastatin): The usual initial dosage of simvastatin in adults is 10 or 20 mg once daily in the evening.1 In patients with CHD or CHD risk equivalents (e.g., diabetes mellitus, peripheral arterial disease, history of stroke or other cerebrovascular disease), the recommended initial dosage of simvastatin is 40 mg daily. The usual maintenance dosage of simvastatin is 5-40 mg once daily in the evening;1 geriatric patients may respond to maintenance dosages of 20 mg or less daily. Because higher simvastatin dosages (e.g., 80 mg daily) have been associated with a greater risk of myopathy, including rhabdomyolysis, particularly during the first year of treatment, the manufacturer states that patients who are unable to achieve their LDL-cholesterol target goal with the 40-mg daily dosage of simvastatin should not be titrated to the 80-mg daily dosage but should be switched to alternative antilipemic agents that provide greater LDL-cholesterol reduction. The manufacturer also states that use of the 80-mg daily dosage of simvastatin should be restricted to patients who have been receiving long-term therapy (e.g., 12 months or longer) at this dosage without evidence of adverse muscular effects. Patients currently tolerating the 80-mg daily dosage of simvastatin who require therapy with an interacting drug (i.e., a drug with which concomitant use is contraindicated or is associated with a dose limit for simvastatin) should be switched to an alternative statin with less drug interaction potential.
-
Homozygous familial hypercholesterolemia: the recommended dosage of simvastatin is 40 mg daily in the evening. Simvastatin should be used as an adjunct to other lipid-lowering treatment (e.g., LDL apheresis) in these patients or as an alternative if such therapy is unavailable.
-
Concomitant therapy (simvastatin): Because of an increased risk of myopathy, including rhabdomyolysis, during concomitant therapy, particularly at higher dosages of simvastatin, the manufacturer of simvastatin states that concomitant use of simvastatin with potent inhibitors of cytochrome P-450 isoenzyme 3A4 (CYP3A4) (e.g., itraconazole, ketoconazole, posaconazole, HIV protease inhibitors, clarithromycin, erythromycin, telithromycin, nefazodone), cyclosporine, danazol, or gemfibrozil is contraindicated. Consumption of large quantities (more than one quart daily) of grapefruit juice should be avoided. In patients receiving amiodarone, diltiazem, or verapamil concomitantly with simvastatin, dosage of simvastatin should not exceed 10 mg daily. In patients receiving amlodipine or ranolazine concomitantly with simvastatin, dosage of simvastatin should not exceed 20 mg daily.
-
Chinese patients: The risk of myopathy appears to be increased among Chinese patients versus non-Chinese patients receiving simvastatin 40 mg daily concomitantly with preparations containing antilipemic dosages (1 g daily or higher) of niacin. The cause of the increased risk of myopathy is not known, and it is not known whether these findings apply to patients of other Asian ancestries. Because of such increased risk, caution is advised when Chinese patients receive simvastatin dosages exceeding 20 mg daily with preparations containing antilipemic dosages of niacin. Because the risk of myopathy is dose related, patients of Chinese descent should avoid concomitant use of simvastatin 80 mg daily with preparations containing antilipemic dosages of niacin.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
Children (FDA)
-
Hyperlipidemia: The recommended dosage of pravastatin sodium in children 8-13 or 14-18 years of age is 20 or 40 mg once daily, respectively. Safety and efficacy of dosages exceeding 20 or 40 mg daily in children 8-13 or 14-18 years of age, respectively, have not been evaluated. The manufacturer states that children and adolescents treated with pravastatin should be reevaluated in adulthood, and antilipemic therapy should then be modified appropriately to achieve adult target LDL-cholesterol goals..
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
-
Heterozygous familial hyper hypercholesterolemia: The recommended initial dosage of simvastatin for the treatment of heterozygous familial hypercholesterolemia in boys and postmenarchal girls 10-17 years of age is 10 mg once daily in the evening. Dosage should be increased at intervals of 4 weeks or longer until the desired effect on lipoprotein concentrations is observed or a maximum dosage of 40 mg daily is reached.1 Safety and efficacy of simvastatin dosages exceeding 40 mg daily have not been evaluated in this patient population.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
Pregnancy (FDA)
-
Cholesterol and other products of the cholesterol biosynthetic pathway are essential for fetal development, including synthesis of steroids and cell membranes. Because of the ability of statins to decrease the synthesis of cholesterol and possibly other products of the cholesterol biosynthetic pathway, these agents may cause fetal harm when administered to pregnant women. ... Severe congenital skeletal malformation, tracheoesophageal fistula, and atresia were reported in a neonate whose mother received lovastatin concomitantly with dextroamphetamine sulfate during the first trimester of pregnancy.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
Nursing Mothers (FDA)
-
Pravastatin is distributed into human milk and pitavastatin is distributed into milk in animals.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
Renal Impairment (FDA)
-
Since most statins (e.g., atorvastatin, fluvastatin, lovastatin, rosuvastatin, simvastatin) do not undergo substantial renal excretion, the manufacturers state that modification of dosage should not be necessary in patients with mild renal impairment (creatinine clearance of 61-90 mL/minute per 1.73 m2). However, pitavastatin and pravastatin undergo renal and hepatic elimination, with renal excretion reaching 15 and 20-47% of the administered dose, respectively. Because the possibility of accumulation of pravastatin and other statins cannot be ruled out, these drugs should be administered with caution in patients with moderate to severe renal impairment (creatinine clearance less than 60 mL/minute per 1.73 m2), initiating therapy with the drug under close monitoring and at reduced daily dosages.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
Hepatic Impairment (FDA)
-
Since statins are partially metabolized in the liver and potentially may accumulate in the plasma of patients with hepatic impairment, these drugs should be used with caution in patients who consume substantial amounts of alcohol and/or who have a history of liver disease; such patients should be monitored closely while receiving statin therapy. Statins should not be used in patients with active liver disease or unexplained, persistent increases in serum aminotransferase concentrations.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
Emergency Use Authorization (FDA/CDC)
No Emergency Use Authorization for Statins has been issued from the Food and Drug Administration under section 564 of the Federal Food, Drug and Cosmetic Act (FD&C Act) (21 U.S.C. 360bbb-3), amended by the Project Bioshield Act of 2004 (public Law 108-276).
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
6. Current available formulations/shelf life
Formulation
Atorvastatin calcium, 5mg, 10mg, 20mg, 40mg
Fluvastatin sodium, capsules, 20mg, 40mg
Lovastatin, 10mg, 20mg, 40mg
Rosuvastatin calcium, 5mg, 10mg, 20mg, 40mg
Pitavastatin calcium, tablets 1mg, 2mg, 4mg
Simvastatin, tablets 5mg, 10mg, 20mg, 40, 80mg
Pravastatin sodium, tablets 10mg, 20mg, 40mg, 80mg
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
Shelf life
Shelf life
When stored at 5-30°C statins are stable for 24 months after the date of manufacture.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
7. Current off label utilization and dosing
— including children-, pregnancy-, geriatric-, and obesity-related data- No data available at this time.
8. Route of Administration/Monitoring
-
All statins are administered orally.
-
Serum lipoprotein concentrations should be determined periodically during statin therapy.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
9. Adverse effects
-
Simvastatin dosages as high as 100 g/sq m in dogs were associated with emesis and mucus in the stool.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
-
The U.S. Food and Drug Administration (FDA) is recommending limiting the use of the highest approved dose of the cholesterol-lowering medication, simvastatin (80 mg) because of increased risk of muscle damage. Simvastatin 80 mg should be used only in patients who have been taking this dose for 12 months or more without evidence of muscle injury (myopathy). Simvastatin 80 mg should not be started in new patients, including patients already taking lower doses of the drug. In addition to these new limitations, FDA is requiring changes to the simvastatin label to add new contraindications (should not be used with certain medications) and dose limitations for using simvastatin with certain medicines.
FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. June 8, 2011 (FDA)
10. Contraindication(s)
-
Statins are contraindicated in patients with hypersensitivity to any component of the drug formulations. These drugs also are contraindicated in patients with active liver disease or unexplained persistent elevations of serum transaminases. Statins are contraindicated in pregnant or lactating women and in women of childbearing age, unless the latter are highly unlikely to conceive.
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
-
Drugs contraindicated with simvastatin: Itraconazole, Ketoconazole, Posaconazole (New), Erythromycin, Clarithromycin, Telithromycin, HIV protease inhibitors, Nefazodone, Gemfibrozil, Cyclosporine, Danazol
FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. June 8, 2011 (FDA)
11. Clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issuesTitle: Statins for Acutely Injured Lungs From Sepsis (SAILS) – Study Terminated, Results Available
Condition: Sepsis for Acutely Injured Lungs From Sepsis Induced ALI
Intervention: Drug: Rosuvastatin Drug: Placebo
ClinicalTrials.gov Statins
12. Non-clinical studies in progress
— including relevant ones and any others highlighting possible adverse effects and other effects/issues- No data available at this time.
13. Needed studies for Chemical Defense clinical indication
— including pharmacokinetics, safety, efficacy, pregnancy, breastfeeding, and review panel recommendations- No data available at this time.
14. Needed studies for non Chemical Defense clinical indications
— including review panel recommendations- No data available at this time.
15. Study-related ethical concerns
— including review panel recommendations- No data available at this time.
16. Global regulatory status
U.S.
-
Statins are a class of prescription drugs /that are approved to be/ used together with diet and exercise to reduce blood levels of low-density lipoprotein (LDL) cholesterol ("bad cholesterol")/ They are marketed as single-ingredient products, including ... Livalo (pitavastatin), Pravachol (pravastatin), Crestor (rosuvastatin), and Zocor (simvastatin). They are also marketed as combination products, including ... Simcor (simvastatin/niacin extended-release), and Vytorin (simvastatin/ezetimibe).
FDA Drugs: Statins. April 19, 2012 (FDA)
E.U.
-
Decision type W: decision granting a waiver in all age groups for the listed condition(s)
Type 2 diabetes mellitus and hypercholesterolaemia
The waiver applies to: All subsets of the pediatric population from birth to less than 18 years of age, for sitagliptin phosphate monohydrate / simvastatin, film-coated tablet, oral use, on the grounds that clinical studies cannot fulfill a therapeutic need of the pediatric population.
European Medicines Agency Decision: Granting of a product specific waiver. August 28, 2009. EMEA/531540/2009, P/170/2009 (EMEA)
17. Other potentially useful information
-
Statins represent a class of drugs that effectively lowers cholesterol, however they also possess pleiotropic effects, like promotion of angiogenesis, prevention of bone loss, immunomodulatory and anti-inflammatory effects. Thus, the aim of this study was to investigate the activity of simvastatin topically applied in mice in acute and chronic skin inflammation models. Skin inflammation was induced in mice ears by topical application of 12-O-tetradecanoyl-phorbol acetate (TPA). In the acute model, ear edema was measured by the increase of ear thickness 6 h after TPA (2.5 mg/ear). The chronic inflammatory process was induced by multiple applications of TPA (2.0 mg/ear) for nine alternate days, and the edema was measured daily as the increase in ear thickness. Topical treatment was applied immediately after TPA in acute model or started at 5th day of chronic experiment. For acute model treatment was simvastatin (0.24, 0.71 and 2.40 mM), dexamethasone (0.13 mM), both in acetone or vehicle alone (acetone). In chronic model simvastatin (1% and 3%) and dexamethasone (0.5%) were incorporated in ointment preparations, and a group received ointment alone (vehicle). Samples of ear tissue (6 mm) were taken from acute and chronic models, weighted and prepared for histological analysis and myeloperoxidase (MPO) enzymatic activity evaluation. Application of simvastatin in acetone reduced the ear edema after a single TPA application in a dose dependent manner [ID50 of 0.47 (0.22-1.13) mM], and the MPO enzymatic activity up to 61 +/- 10%. Also, both simvastatin ointment preparations 1% and 3% reduced acute TPA-induced ear edema in 55 +/- 7% and 65 +/- 8%, respectively. In the chronic model, simvastatin ointment 1% was able to reduce ear edema (25 +/- 3%) and ear weight (10 +/- 1%), though 3% formulation augmented both parameters. Histological analysis revealed a reduction of swelling and leukocyte migration in the acute model for both the formulations of simvastatin (1% and 3%), while in chronic model simvastatin 1% decreased ear swelling and epidermal thickness, but simvastatin 3% increased both parameters. The results confirm the anti-inflammatory activity of simvastatin when applied topically in both acute and chronic models of skin inflammation. Besides, the formulation of simvastatin ointment 1% shows to be a very effective formulation for a chronic usage.
Adami M, Prudente Ada S, Mendes DA, Horinouchi CD, Cabrini DA, Otuki MF. Simvastatin ointment, a new treatment for skin inflammatory conditions. J Dermatol Sci. 2012 May;66(2):127-35. [PubMed Citation]
-
Octanol/Water Partition Coefficient: log Kow = 4.68
HSDB. Simvastatin
18. Publications
Adami M, Prudente Ada S, Mendes DA, Horinouchi CD, Cabrini DA, Otuki MF. Simvastatin ointment, a new treatment for skin inflammatory conditions. J Dermatol Sci. 2012 May;66(2):127-35. [PubMed Citation]
Birnbaum Y, Ye Y. Pleiotropic effects of statins: the role of eicosanoid production. Curr Atheroscler Rep. 2012 Apr;14(2):135-9. [PubMed Citation]
Chen W, Pendyala S, Natarajan V, Garcia JG, Jacobson JR. Endothelial cell barrier protection by simvastatin: GTPase regulation and NADPH oxidase inhibition. Am J Physiol Lung Cell Mol Physiol. 2008 Oct;295(4):L575-83. [PubMed Citation]
ClinicalTrials.gov Statins
Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O'Kane CM, Elborn JS, McAuley DF. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study). Am J Respir Crit Care Med. 2011 Mar 1;183(5):620-6. [PubMed Citation]
Clinical trial registered with www.controlled-trials.com (ISRCTN70127774)
[DHHS/FDA; Emergency Preparedness and Response-Counterterrorism and Emerging Threats (12/01/2011)]
European Medicines Agency Decision: Granting of a product specific waiver. August 28, 2009. EMEA/531540/2009, P/170/2009 (EMEA)
FDA Drugs: Statins. April 19, 2012 (FDA)
FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of muscle injury. June 8, 2011 (FDA)
Halcox JP, Deanfield JE. Beyond the laboratory: clinical implications for statin pleiotropy. Circulation. 2004 Jun 1;109(21 Suppl 1):II42-8. [PubMed Citation]
HSDB. Simvastatin
Iwata A, Shirai R, Ishii H, Kushima H, Otani S, Hashinaga K, Umeki K, Kishi K, Tokimatsu I, Hiramatsu K, Kadota J. Inhibitory effect of statins on inflammatory cytokine production from human bronchial epithelial cells. Clin Exp Immunol. 2012 May;168(2):234-40. [PubMed Citation]
Jacobson JR. Pharmacologic therapies on the horizon for acute lung injury/acute respiratory distress syndrome. J Investig Med. 2009 Dec;57(8):870-3. [PubMed Citation]
Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005 Jun;288(6):L1026-32. [PubMed Citation]
Jacobson JR, Dudek SM, Birukov KG, Ye SQ, Grigoryev DN, Girgis RE, Garcia JG. Cytoskeletal activation and altered gene expression in endothelial barrier regulation by simvastatin. Am J Respir Cell Mol Biol. 2004 May;30(5):662-70. [PubMed Citation]
Joyce M, Kelly CJ, Chen G, Bouchier-Hayes DJ. Pravastatin attenuates lower torso ischaemia-reperfusion-induced lung injury by upregulating constitutive endothelial nitric oxide synthase. Eur J Vasc Endovasc Surg. 2001 Apr;21(4):295-300. [PubMed Citation]
Kim JW, Rhee CK, Kim TJ, Kim YH, Lee SH, Yoon HK, Kim SC, Lee SY, Kwon SS, Kim KH, Kim YK. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin Exp Pharmacol Physiol. 2010 Nov;37(11):1055-63. [PubMed Citation]
Kor DJ, Iscimen R, Yilmaz M, Brown MJ, Brown DR, Gajic O. Statin administration did not influence the progression of lung injury or associated organ failures in a cohort of patients with acute lung injury. Intensive Care Med. 2009 Jun;35(6):1039-46. [PubMed Citation]
Li JJ, Chen XJ. Simvastatin inhibits interleukin-6 release in human monocytes stimulated by C-reactive protein and lipopolysaccharide. Coron Artery Dis. 2003 Jun;14(4):329-34.[PubMed Citation]
McEvoy GK, ed. Drug Information 2012. Bethesda, MD: American Society of Health-System Pharmacists, 2012 p.1747-1784
Suda K, Eom J, Jaw JE, Mui T, Bai N, Or C, Ngan D, Li Y, Wang X, Tsuruta M, Tam S, Man SP, Van Eeden S, Sin DD. Endotoxin-induced cardiovascular dysfunction in mice: effect of simvastatin. J Appl Physiol. 2011 Oct;111(4):1118-24. [PubMed Citation]
US NLM. ChemIDplus Lite. Pravastatin
US NLM.ChemIDplus Lite. Pitavastatin
Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1334-49. [PubMed Citation]
Watanabe T, Kusuhara H, Maeda K, Kanamaru H, Saito Y, Hu Z, Sugiyama Y. Investigation of the rate-determining process in the hepatic elimination of HMG-CoA reductase inhibitors in rats and humans. Drug Metab Dispos. 2010 Feb; 38 (2): 215-22. [PubMed Citation]
Yao HW, Mao LG, Zhu JP. Protective effects of pravastatin in murine lipopolysaccharide-induced acute lung injury. Clin Exp Pharmacol Physiol. 2006 Sep;33(9):793-7. [PubMed Citation]
Yee LL, Wright EA. Pitavastatin calcium: clinical review of a new antihyperlipidemic medication. Clin Ther. 2011 Aug; 33 (8): 1023-42. [PubMed Citation]
19. Web sites
NIH CounterACT Program (HHS/NIH)
NIH RePORTER (HHS/NIH)
ClinicalTrials.gov (HHS/NIH)
PubMed (HHS/NIH)
DailyMed (HHS/NIH)
Record last updated 1/2/2013
