- Clinical Overview
- Establishing Zones/Patient Flow
- Scene Layout
- Rescuer Protection
- Triage, Decontamination Triage
- Prioritization for Decontamination
- Victim Self Decontamination
- Mass Casualty Triage Standards
- Sarin Specific Triage
- ABC Reminders
- Treatment
- Victim Removal
- Decontamination Zone
- Support Zone
Nerve Agents - Prehospital Management
Cinical Overview
Agent Identification
- Sarin (military designation GB) is a nerve agent that is one of the most toxic of the known chemical warfare agents. It is generally odorless and tasteless. Exposure to sarin can cause death in minutes. A fraction of an ounce (1 to 10 mL) of sarin on the skin can be fatal. Nerve agents are chemically similar to organophosphate pesticides (OP) and exert their effects by interfering with the normal function of the nervous system.
- Sarin is odorless and is the most volatile nerve agent.
- Sarin (GB) can be absorbed into the body by inhalation, ingestion, skin contact, or eye contact. Ingestion is an uncommon route of exposure. Responders should obtain assistance in identifying the chemical(s) from container shapes, placards, labels, shipping papers, and analytical tests. General information on these identification techniques is located in Emergency Response Guidebook.
- Identification Tools - CHEMM Intelligent Syndromes Tool (CHEMM-IST), WISER, Sarin Chemical Properties.
- Devices - A limited selection of portable devices for rapid field detection or measurement of sarin is available.
- Colorimetric detection systems include the Chemical Agent Detector C2 Kit (vapor, aerosol, liquid), M256A1 Chemical Agent Detector Kit (vapor, liquid), No. 1 Mark 1 Detector Kit (vapor, aerosol), Draeger CDS Kit (vapor, aerosol) or comparable colorimetric detector tube kit.
- Some electronic handheld devices are capable of sarin detection and measurement (e.g., the HAZMATCAD Chemical Agent Detector). Ion mobility spectrometry devices capable of detecting sarin include the SABRE 2000 (particles and vapor).
- For additional information see: Guide for the Selection of Chemical Detection Equipment for Emergency First Responders, Guide 100-06, January 2007, 3rd Edition published by the Department of Homeland Security.
Route of Exposure
Exposure to Sarin (GB) can occur by all routes, inhalation, ingestion, and dermal contact. Liquid Sarin (GB) may produce health effects within minutes. Health effects from mild to moderate exposure may be delayed up to 18 hours; larger exposures may cause death within minutes to hours.
- Inhalation - nerve agents are readily absorbed from the respiratory tract. Runny nose and tightness in the throat or chest begin within seconds to minutes after exposure; bronchorrhea – rapid accumulation of water in the lungs – is one of the most serious effects and can rapidly be fatal. Nerve agent vapors are heavier than air. Odor does not provide adequate warning of detection.
- Skin/Eye Contact - nerve agent liquids are readily absorbed into the skin and eyes. Vapors are not absorbed through the skin except at very high concentrations. Ocular effects may result from both direct contact and systemic absorption and include lacrimation and miosis. Ocular symptoms following remote site (ingestion; skin absorption) systemic exposure may be the last to develop because of the time required for absorbed agent to reach the eye. On the other hand, direct deposition of sarin on the conjunctiva may result in almost immediate ocular symptoms. The nature and timing of symptoms following dermal contact with liquid nerve agents depend on exposure dose; effects may be delayed for several hours.
- Ingestion - ingestion of nerve agents is expected to be relatively rare compared to inhalation exposure or skin contact; however, they are readily absorbed from the GI tract and are highly toxic.
Clinical Signs and Symptoms
Nerve agents are potent acetylcholinesterase inhibitors causing the same signs and symptoms regardless of the exposure route. However, the initial effects depend on the dose and route of exposure. Prolonged muscarinic receptor activation by Ach (acetylcholine) leads to symptoms characterized by over production of secretions (lacrimation, salivation, bronchial secretions, GI fluid loss, sweating) and smooth muscle activation (diarrhea, urination). This constellation of symptoms is characterized by the mnemonic SLUDGE.
- Manifestations of nerve agent exposure include:
- Ocular pinpoint pupils: (highly indicative of nerve agent exposure in a mass casualty situation), eye pain, conjunctivitis, and increased tearing are common (with systemic absorption pinpoint pupils may not occur immediately). Symptoms may occur from local effects secondary to vapor exposure or as a manifestation of systemic absorption.
- CNS: High dose - seizures, confusion, loss of consciousness, apnea. Other signs and symptoms include; irritability, memory loss, fatigue, memory loss, behavioral and psychological changes.
- In many instances children may present with only neurological signs and symptoms.
- Skeletal muscles: Nerve agents stimulate skeletal muscle contraction, producing twitching and fasciculations. This leads to fatigue and flaccid paralysis.
- Pulmonary: Inhalation of nerve agent vapor causes respiratory tract effects within seconds to minutes, increased, rhinorrhea, and bronchial secretions, chest tightness, wheezing, shortness of breath, and respiratory failure.
- Cardiovascular: Potentially up to three phases in variable length - transient tachycardia/with or without hypertension (minutes) followed by bradycardia and hypotension. The final phase starts hours to days after exposure with QT interval prolongation and a tendency toward malignant dysrhythmias.
- Gastrointestinal: Abdominal pain, nausea, vomiting, diarrhea, and involuntary defecation. If GI symptoms occur within one hour of dermal contamination, severe intoxication is present.
- Other: Runny nose, excessive salivation and sweating, and urination.
- Link to CHEMM-IST
- Link to Toxic Syndromes
- Link to Primary and Secondary Survey
Differential Diagnosis
- Recognition of potential nerve agent exposure clinical signs and symptoms may be most apparent when multiple patients present with the same syndrome.
- The diagnosis in a severely intoxicated individual is straightforward. The combination of miosis, copious secretions, bronchospasm, generalized muscle fasciculations, and seizures is characteristic.
- Look carefully for miosis (if present will be helpful). Miosis may not be present initially following a low volatility nerve agent exposure. Onset may be delayed following an exposure route that does not involve direct liquid or vapor deposition on the eye.
- A mild vapor exposure may mimic a child having allergic rhinitis/conjunctivitis.
- A mild vapor exposure may present with only visual complaints such as narrowing of the visual field or a sense that everything is going dark.
- GI symptoms by themselves may be the only presenting signs, and might be expected to present as the initial symptoms following ingestion.
- Opioid abuse or weaponized opioids can include miosis, apnea, and coma. However, patients should not have significant vomiting and diarrhea. Pulmonary edema, a rare side effect of rapid reversal of the opioid toxidrome with naloxone, should not be mistaken for the bronchorrhea associated with nerve agent exposure.
- Link to Chemical Hazards Emergency Medical Management Intelligent Syndromes Tool (CHEMM-IST)
Pediatric/Obstetric/Geriatric Vulnerabilities
Pediatric:
Children are more vulnerable to sarin because:
- Children are likely to have more severe nicotinic effects than adults (weakness, tachycardia, as well as CNS effect (seizures). On the other hand, children tend to have less muscarinic effects than adults (bradycardia, salivation, bronchial secretions, and spasm) potentially making the toxic syndrome more difficult to recognize in a child.
- Sarin may penetrate the blood brain barrier more easily in children than adults. Children may only exhibit CNS effects.
- Children exposed to the same levels of sarin as adults may receive a larger dose because they have greater lung surface area: body weight ratios and increased minute volumes: weight ratios.
- Infants and toddlers do not have the motor skills to escape from the scene of an incident.
- The high vapor density of gases places the highest concentration close to the ground which is in the breathing zone of children.
- Children have a higher respiratory rate and inhale a greater volume per minute.
- Children have smaller diameter airways, anatomic subglottic narrowing, omega shaped epiglottic structure, relatively large tongue size, and less rigid ribs and trachea which make them more vulnerable to pulmonary agent induced pathology such as bronchospasm, copious secretions, and pulmonary edema.
- Children’s skin is thinner and has more moisture content therefore being more vulnerable to inflammatory effects and to toxin absorption.
- Children have less fluid reserve, which increases the risk of rapid dehydration following vomiting and diarrhea.
Obstetric:
Pregnant women have several unique vulnerabilities when exposed to nuclear, biological, or chemical disaster agents. The physiologic changes that occur during pregnancy have the potential to alter a pregnant woman’s response to chemical exposures when compared to non-pregnant adults:
- Increased blood volumes and the addition of the placental-fetal circulation can result in a dilutional effect, not only of the toxic agents but also of the antidotes administered.
- Increased tidal volumes may result in increased likelihood of the pregnant victim being exposed to a greater amount of respiratory chemicals in a given unit of time.
- Increased renal blood, glomerular filtration, and renal excretion may result (depending on the elimination characteristics of the specific agent) in the victim rapidly excreting not only the noxious agent but also the antidotes that may have been administered.
- The enlarged pregnant uterus may cause compression of the vena cava (supine hypotension syndrome) when the victim is laid flat on her back either for immobilization or for resuscitation. This may have implications for fetal well-being if prolonged.
- The mother’s safety and well-being should always take priority over any concerns related to potential fetal harm by the toxic agents or by the antidotal treatments. Therefore, any treatments that would be given to a non-pregnant woman should not be withheld because of the pregnancy.
- Antidote dosages should be calculated with taking into consideration the increased blood volume in pregnancy. If this evaluation is inconclusive, use general adult dosing recommendations. Follow dosing recommendations, but be aware that these may not take into account the physiological changes in pregnancy.
Geriatric:
No systematic human studies that have evaluated the differences in the clinical effects of sarin between older and younger adults are available. Therefore information regarding age differences in responses must be developed from animal experiments or extrapolated from the known differences in the response to pharmaceuticals. Alterations in both pharmacokinetics and pharmacodynamics as people age contribute to altered responses in older adults. Therefore, older adults could be more vulnerable to sarin due to:
- Thinner skin which may alter the absorption of poisons following dermal exposure (particularly applicable to vesicants, which may cause more damage in older adults).
- Increased ratio of body fat to body water which may increase the distribution of fat-soluble poisons.
- Decreased renal function which may decrease the elimination of some poisons.
- Decreased serum acetylcholinesterase activity which may alter the effects of nerve agents.
- Loss of reserve in several key organ systems. Older patents have less cardiovascular, CNS, and pulmonary reserve. Therefore an exposure that may be tolerable in a younger adult could be life-threatening in an older patient.
These changes also increase the risk of adverse events from treatment for chemical exposures. Older patients are more likely to develop delirium from anticholinergic medications (e.g., atropine) and from benzodiazepines. Therefore dosing should be slower in cases where immediate reversal of nerve agent effects is not required for stabilization. Note that since age-related changes are directly related to an individual’s physiological condition, it is impossible to predict the extent of age-related changes in an individual.
Link to Primary and Secondary Survey
Establishing Zones/Patient Flow/Initial Treatment
In addition to the safety assessment, the First Responder should gain control of the incident site and establish Initial Isolation and Protective Action Distances (a.k.a. "Hot," "Warm," "Cold" Zones) IAW the latest printed edition Emergency Response Guidebook with respect to the incident particulars (such as wind direction, weather conditions, degree of hazard, identified physical properties of the release, location, number of victims affected, etc.).
Establishing Zones/Patient Flow/Initial Treatment
Scene Layout
- Victims are evacuated from the hazard area (Hot/Warm Zones, exclusion zone).
- A First Responder performs initial decontamination triage/”regular triage” on the victims in the Warm Zones. Victims with no apparent exposure are sent to a safe/refuge observation area to monitor for delayed symptoms and signs of contamination. When clinically indicated, antidotes and key supportive care medications are administered prior to decontamination.
- Victims with likely exposure are sent to the water shower deluge and undergo mass casualty decontamination.
- Following decontamination victims without additional visible symptoms are sent to a safe/refuge observation area for monitoring.
- Symptomatic and ambulatory victims undergo additional medical triage, treatment, and are transported to a medical facility if required for further medical treatment. Secondary decontamination should be set up as necessary with decontamination occurring prior to the victim entering the medical facility. (Note: Secondary decontamination can also be set up between the mass decontamination and the safe/refuge area, as necessary. A second pass through the primary water shower deluge may suffice (if resources are available).
- Victims are released from the safe/refuge observation area or medical facility as directed.
- Link to scene layout appendix 1
Rescuer Protection
PPE selection, training, and use should be based on applicable regulations (OSHA18), standards (NIOSH19), and/or guidance (NFPA20), SME recommendation, and manufacturers’ specifications, in conjunction with scene evaluation and risk assessment and Authority Having Jurisdiction standard operating procedures or standard operating guidelines. OSHA, NIOSH, and NFPA advise a risk-based approach when determining the type and use of PPE. The level of PPE and respiratory protection needed by HAZMAT personnel varies greatly and depends on their anticipated work activities. The factors to consider in the selection of PPE include the anticipated substance, toxicity of the substance, potential routes of exposure, degree of contact, and the specific task assigned to HAZMAT personnel. Personnel must be provided with appropriate respiratory and dermal protection specific to the contaminant. Currently, no PPE can protect the wearer from exposure to all possible hazards. Therefore, responders and receivers must determine the appropriate combination of PPE based on the specific substance and a risk assessment.
Victims exposed only to nerve agent vapor do not pose secondary contamination risks to rescuers, but do not attempt resuscitation without a barrier. Victims whose clothing or skin is contaminated with liquid nerve agent can secondarily contaminate response personnel by direct contact or through off-gassing vapor. Avoid dermal contact with nerve agent contaminated victims or with gastric contents of victims who may have ingested nerve agent-containing materials. Persons whose clothing or skin is contaminated with nerve agent-containing solutions can secondarily contaminate response personnel by direct contact or through off-gassing vapor. In addition, vomitus and bodily secretions (sweat-saturated clothing) which contain adsorbed nerve agent may pose a risk of secondary contamination through direct contact or off-gassing.
- Personal Protection Equipment (PPE) required if contaminated environment is not characterized, liquid nerve agent is present or likely to cause skin exposure and/or nerve agent airborne exposure levels are above the AEGL-2 or 1/2 the IDLH: Level A
- Level A - protective clothing is the highest level of protection. Level A includes a Chemical, Biological, Radiological, Nuclear (CBRN) Self Contained Breathing Apparatus (SCBA) with a fully encapsulating vapor tight suit with gloves and booties attached to the suit (tanks last from 1/2 hour to 1 hour).
- Respiratory Protection: Positive-pressure, Chemical, Biological, Radiological, Nuclear (CBRN) self-contained breathing apparatus (SCBA) is recommended in response situations that involve exposure to potentially unsafe levels of nerve agent.
- Skin Protection: Chemical-protective clothing is recommended because both nerve agent vapor (high exposure doses) and liquid can be absorbed through the skin to produce systemic toxicity.
- PPE required for decontamination team:
- Level A recommended for team members closest to the contaminated area and are assisting entry teams or patients just leaving the contaminated areas where outer garments/clothing removal is conducted;
- Level B is recommended for rest of decontamination team in the Contamination Reduction Zone (CRZ).
- If you are a First Responder and you think you have been exposed, see either Nerve Agent Specific Triage or Clinical Signs and Symptoms
- Link to reference section for acute event PPE related safety information
Triage, Decontamination Triage
Chemical casualty triage is based on walking feasibility, respiratory status, age, and additional conventional injuries. The triage officer must know the natural course of a given injury, the medical resources immediately available, the current and likely casualty flow, and the medical evacuation capabilities.
For the victim exposed to the nerve agent who presents with severe multisystem symptoms, antidotes and supportive care can be lifesaving if initiated immediately. Decontamination, including self-care steps like clothing removal, may be performed in conjunction with triage and the initiation of lifesaving actions such as antidote administration and supportive care.
Prioritization for Decontamination
Prioritize patients for decontamination by estimating relative risk and grouping patients into immediate and delayed decontamination groups. Risk assessment should take into consideration the following criteria (and others as appropriate) in preferential order.
- Need for decontamination in order to receive immediate lifesaving care including antidotal therapy
- Children should be prioritized before adults within the same decontamination group
- Age, pregnancy and chronic medical conditions should also be considered when estimating relative risk and prioritizing patients for decontamination
- The appearance of visible contamination (liquid) on skin or clothing;
- Signs and symptoms consistent with exposure;
- A reasonable probability of exposure based on distance from the likely source, time spent in proximity to the release source, and environmental conditions.
- Contamination detected on patient using appropriate detection technology
Victim Self Decontamination
ENCOURAGE PATIENTSTO REMOVE AS MUCH CLOTHING AS POSSIBLE, BUT AT LEAST REMOVE OUTER GARMENTS DOWN TO UNDERWEAR. Cutting and/or unbuttoning is preferred to pulling clothing over the head.
If clothes must be lifted over the head, instruct victims to do so carefully by placing hands and arms inside the garment and using the hands to pull the head opening away from the face and head as much as possible
Victims suspected or known to have liquid and/or vapor exposures to sarin need to immediately move away (upwind) from the contaminated area. Avoidance measures to: (a) prevent inhaling the toxic vapors is critical; (b) avoid touching contaminated clothing or personal effects of others; (c) avoid physical contact of uncontaminated individuals (e.g., family members, friends, responders, bystanders) in order to prevent further exposures. Then, while in a relatively safe area, self-care is recommended, until first responders arrive on-scene to remove contaminated material from skin, eyes, and clothing. Contaminated clothing should immediately be removed and placed away from clean material (e.g., in a bag). Skin and eyes, if water is available, should be thoroughly washed. Water should deluge the eyes for approximately 15 minutes. If liquid soap is available, use soap with the water to enhance skin decontamination (not in the eyes). Hair should also be washed with water and preferably soap, to remove chemical agent. Thoroughly wash hands.
Mass Casualty Triage Standards
Sarin Specific Triage
- Severe symptoms - these include unconsciousness, convulsions, apnea, and flaccid paralysis.
- Mild/ Moderate symptoms - these include localized swelling, muscle fasciculations, nausea and vomiting, weakness, shortness of breath.
- Patients who are conscious and have full muscular control will need minimal care.
- Patients with a history of possible exposure to vapor only (with no possibility of liquid exposure) who have no signs of exposure by the time they reach the medical facility have not been exposed (because these effects occur within seconds to minutes after exposure). They can be discharged.
- Delayed Effects from skin exposure to liquid nerve agent may not develop for up to 18 hours following exposure. Therefore, patients exposed primarily through skin, had conducted skin decontamination, and were initially asymptomatic need awareness that nerve agent symptoms may occur up to 18 hours later.
- Patients who have inhalation exposure and who complain of chest pain, chest tightness, or cough should be observed and examined periodically for 6 to 12 hours to detect delayed-onset bronchitis, pneumonia, pulmonary edema, or muscle weakness/respiratory failure.
- Patients exposed to nerve agent vapor that have only miosis and/or mild rhinorrhea when they reach the medical facility do not need to be admitted. All other symptomatic patients who have had exposure to nerve agent should be hospitalized and observed closely.
- Miosis can be the last symptom to resolve and take days, even weeks (Kato, 1996 on post-Tokyo attack ophthalmologic care).
| Triage for Nerve Agent Casualties | |
|---|---|
| Immediate (1) | Effects - Unconscious, talking but not walking, moderate to severe effects in two or systems more systems (e.g., respiratory, GI, cardiac arrest. muscular, CNS) |
| Clinical Signs - seizing or postictal, severe respiratory distress, recent cardiac arrest | |
| Delayed (2) | Effects - recovering from agent exposure or antidote |
| Clinical Signs - diminished secretions, improving respiration. | |
| Minimal (3) | Effects - walking and talking |
| Clinical Signs - pinpoint pupils, runny nose, and mild to moderate difficulty breathing | |
| Expectant (4) (limited resources) |
Effects - Unconscious for prolonged period of time |
| Clinical Signs - Cardiac/respiratory arrest of long duration. | |
ABC Reminders
Quickly ensure that the victim has a patent airway. Maintain adequate circulation. If trauma is suspected, maintain cervical immobilization manually and apply a decontaminable cervical collar and a backboard when feasible. In pregnant women, avoid supine hypotension syndrome by maintaining a left lateral tilt. Apply direct pressure to stop arterial bleeding, if present.
Treatment
- Treatment consists of supportive measures and repeated administration of atropine and pralidoxime. These drugs appear to be safe during pregnancy and should be used in pregnancy without delay.
- If clinically warranted and situationally feasible for IV therapy - link to treatment in the Hot/Warm Zones for Hospital Providers
- Atropine and supportive treatment may have to be initiated prior to decontamination.
- Link to Nerve Agent Specific Antidotes in Hospital section
- Link to Nerve Agent Treatment - Autoinjector Instructions
- Link to Basic and Advanced Life Support
- Link to Pediatric Basic and Advanced Life Support
- Link to Key Acute Care Adult Medications section
- Link to Key Acute Care Pediatric Medications section
Antidote/Seizure Medication Dosing: (Autoinjector Instructions)
Consult with a medical toxicologist or a poison center at the national toll-free number 1-800-222-1222 for further guidance on appropriate antidote dosing.
Currently two atropine/pralidoxime autoinjector formulations exist:
- Mark 1 Kit - each kit contains one 600 mg pralidoxime autoinjector, one 2 mg atropine autoinjector
- Duodote - a single autoinjector contains approximately 600 mg of pralidoxime and 2 mg of atropine
- In general treatment of severe nerve agent poisoning requires lower total doses of atropine than required for treatment of organophosphorous compounds
- In severe cases of nerve agent toxicity following vapor exposure (i.e. apneic and unconscious) it may take up to 15 mg of atropine to restore consciousness and breathing. Typically atropine has not been required for more than 3 hours to treat the life threatening effects. Non - life threatening effects such as nausea and vomiting have required atropine for 6-36 hours.
- Organophosphate ingestions have required hundreds of mgs a day of atropine
Mild effects:
- Miosis alone (no respiratory symptoms)- No antidotes. However, if eye/head pain or N&V (in the absence of other systemic signs suggesting a liquid exposure) are severe use atropine ophthalmic drops
- Miosis and severe rhinorrhea - Atropine
Atropine Autoinjector/IM† * Infant (0-2 yrs) 0.05 mg/kg given IM
or via autoinjector (0.25 and 0.50 mg sizes are available)Child (3-7 yrs) 1 mg autoinjector/IM Child (8-14 yrs) 2 mg autoinjector/IM Adolescent/Adult 2 mg autoinjector/IM Pregnant women 2 mg autoinjector/IM Senior 1 mg autoinjector/IM
Mild/Moderate effects:
- These include localized swelling, muscle fasciculations, nausea and vomiting, weakness, shortness of breath. Utilize auto-injectors if available. May use a 600 mg 2PAM Cl auto-injector in an infant as small as 12 kg.
Atropine Autoinjector/IM† * 2-PAM Cl - 600 mg Autoinjector/IM† * Infant (0-2 yrs) 0.05 mg/kg given IM
or via autoinjector (0.25 and 0.50 mg sizes are available)15 mg/kg‡ Child (3-7 yrs)
13-25 kg1 mg autoinjector/IM 15 mg/kg
May use 1 autoinjector (600 mg)‡Child (8-14 yrs)
26-50 kg2 mg autoinjector/IM 15 mg/kg
May use 1 autoinjector (600 mg)‡Adolescent/Adult 2-4 mg autoinjector/IM 1 autoinjector (600 mg) Pregnant Women 2-4 mg autoinjector/IM 1 autoinjector (600 mg) Seniors, frail 2 mg autoinjector/IM 10 mg/kg IM
1 autoinjector (600 mg) - Repeat initial dose (2 mg max) of atropine every 5 - 10 minutes until dyspnea, resistance to ventilation, and secretions are minimized.
- If resistance to ventilation is significant , requiring repeat dosing in less than 5 minutes utilize the higher doses and increase frequency depicted in the severe effects section below
- Treat vomiting and diarrhea from a liquid exposure in a similar way.
- Regular IM atropine dosing may take 20-25 minutes to have a therapeutic effect (vs. 8 minutes with an autoinjector).
- May repeat pralidoxime - up to a total of 45 mg/kg during the first hour
- May repeat pralidoxime - up to 45 mg/kg 1 hour after initial treatment
Severe Effects - Initial Dosing:
- These include unconsciousness, convulsions, apnea, flaccid paralysis and requiring assisted ventilation (severe respiratory distress). I.V. atropine has produced ventricular fibrillation in hypoxic animals with nerve agent poisoning. Therefore it is recommended that hypoxia be corrected prior to atropine administration. However atropine should not be withheld due to fears of this complication. It would be preferable to utilize an atropine autoinjector for the first dose in the hypoxic nerve agent exposed patient.
- Repeat atropine 2 mg (child 8-14, adolescents, adults, pregnant women, and seniors), 0.05 mg/kg (0.25 mg - 0.50 mg) (infant 0-3) and 1 mg (child 3-7) at 2 -5 minute intervals until secretions have diminished, breathing is comfortable, and airway resistance has returned to normal.
- Repeat 2PAM Cl dose hourly X 2, if clinically possible start 2 PAM Cl via continuous infusion
Link to Advanced Treatment Emergency Department/Hospital Management
| Atropine Autoinjector/IM† * | 2-PAM Cl Autoinjector/IM† * | |
|---|---|---|
| Infant (0-3 yrs) | 0.1 mg/kg given IM or via autoinjector (0.25 and 0.50 mg sizes are available) | 45 mg/kg IM‡ |
| Child (3-7 yrs) 13-25 kg | 0.1 mg/kg IM 1 (2 mg) autoinjector | 45 mg/kg Use 1 autoinjector (600 mg)‡ |
| Child (8-14 yrs) 26-50 kg | 4 mg 2 (2 mg) autoinjector | 45 mg/kg IM Use 2 autoinjector (1200 mg)‡ |
| Adolescent (>14 yrs) | 6 mg 3 (2 mg) autoinjectors | Use 3 autoinjectors (1800 mg) |
| Adult | 6 mg 3 (2mg) autoinjectors | Use 3 autoinjectors (1800 mg) |
| Pregnant Women | 6 mg 3 (2 mg) autoinjectors | Use 3 autoinjectors (1800 mg) |
| Seniors, frail | 2-4 mg 1-2 (2 mg) autoinjectors | 25 mg/kg IM Use 2-3 autoinjectors (1200-1800 mg) |
Treatment of seizures:
- Diazepam or midazolam should be given to all patients having seizure activity, unconsciousness, diffuse muscle twitching, and if >1 organ is involved. The military gives diazepam as part of initial therapy for any seriously ill NA exposed patients. Utilized early, atropine may function as an anticonvulsant. The benzodiazepines are the most effective seizure medication for nerve agent toxicity.
Initial Dosing:
Note: 1 CANA autoinjector contains 10 mg diazepamDiazepam† * Midazolam ‡ * Initial Dosing Infant 0.2-0.5 mg/kg IM repeat Q2-5 min 0.15 mg/kg IM, repeat PRN 10 minutes X 2, 0.2-0.5 mg/kg IV/IO Q15-30 minutes X 1 Total max dose 5 mg Total max dose 0.3 mg/kg Child 0.2-0.5 mg/kg IM repeat Q2-5 min 0.15 mg/kg IM, not to exceed 10 mg, repeat PRN 10 minutes X 2, 0.2-0.5 mg/kg IV/IO Q15-30 minutes x 1 Total max dose 5 mg (<5 yrs) Total max dose 0.3 mg/kg, not to exceed 20 mg Total max dose 10 mg (≥5 yrs)
1 CANA autoinjectorAdolescent 2-3 CANA autoinjectors 0.15 mg/kg IM, max 10 mg, repeat PRN 10 minutes 5-10 mg IV/IO Q15 min X 1 Total max dose 30 mg Total max dose 20 mg Adult 2-3 CANA autoinjectors 10 mg IM, repeat PRN 10 minutes 5-10 mg IV/IO Q15 min X 1 Total max dose 30 mg Total max dose 20 mg Pregnant Women 2-3 CANA autoinjectors 10 mg IM, repeat PRN 10 minutes 5-10 mg IV/IO Q15 min X 1 Total max dose 30 mg Total max dose 20 mg, Category D * Senior 2-3 CANA autoinjectors 10 mg IM, repeat PRN 10 minutes 5-10 mg IV/IO Q15 min X 1 Total max dose 30 mg Total max dose 20 mg
† FDA approved for this indication
2-PAM auto-injector usage in pediatrics is off-label
‡ Not FDA approved for this indication/Off-label use
* Pregnancy Categories: Refer to DailyMed regarding Pregnancy Categories and additional pregnancy-related information.
Victim Removal
If victims can walk, lead them out of the Hot/Warm Zones to the decontamination location. Victims who are unable to walk may be removed on backboards or gurneys; if these are not available, carefully carry or drag victims to safety. Avoid supine hypotension syndrome in pregnant women by maintaining a left lateral tilt. Should there be a large number of casualties, and if decontamination resources permit, separate decontamination corridors should be established for ambulatory and non-ambulatory victims.
Consider appropriate management of chemically contaminated children, such as measures to reduce separation anxiety if a child is separated from a parent or other adult, clothing to prevent hypothermia in children, and tagging or tracking children to prevent loss.
- Link to Management of the Deceased
Decontamination Zone
Decontamination Zone
Rescuer Protection
- PPE required for decontamination team
- Level A recommended for team members closest to the contaminated area and who are assisting entry teams or patients just leaving the contaminated areas where outer garments/clothing removal is conducted;
- Level B is recommended for rest of decontamination team in the Contamination Reduction Zone (CRZ).
- Link to Rescuer Protection
ABC Reminders
Speed is critical. If the victim is symptomatic, immediately institute emergency life support measures including the use nerve agent specific antidotes. Treatment should be given simultaneously with decontamination procedures. Quickly ensure that the victim has a patent airway and is ventilating well. Assist ventilation with a bag-valve-mask device equipped with a canister or air filter, if necessary. Maintain adequate circulation. Atropine administration may be required to enable ventilation. Stabilize the cervical spine with a decontaminable collar and a backboard if trauma is suspected. In pregnant women, avoid supine hypotension syndrome by maintaining a left lateral tilt. Direct pressure should be applied to control heavy bleeding, if present.
Antidotes
If clinically indicated, administer initial or repeat dosing of atropine, pralidoxime, and seizure medications. If possible, a system should be employed to track antidotes administered.
Link to Treatment
Principles of Decontamination
The key to successful mass casualty decontamination is to use the fastest approach that will cause the least harm and do the most good for the majority of the people.
- Decontamination is potentially a three tiered process; immediate self, followed by a timely high volume low pressure water shower, and if warranted a repeat decontamination (technical patient decontamination) can be performed with soap and water or just water.
- Conduct decontamination triage prior to administering a high-volume, low-pressure water shower.
- Rapid decontamination is critical to prevent further absorption by the patient and to prevent exposure to others.
- The immediate removal of clothing outside the contaminated area for patients who have been visibly contaminated or who have been suspected of having been contaminated, and
- Processing the victims through a high-volume low-pressure water shower (~50 to 60 psi) is priority. This may aid in the removal of 80-90% of physical contamination in almost all cases.
- Provide effective mass casualty decontamination. Other activities, such as setting up commercial decontamination tents, tarps, additional decontamination equipment, and/ or creating a soap-water solution should be accomplished when time and resources permits, but should not delay the initiation of decontamination.
- Decontamination should be performed as necessary, where the set-up of secondary decontamination should not delay primary decontamination.
- Non-liquid If responders suspect the contamination is biological, radiological, or a gas/vapor, a water-only shower is typically adequate. Persons whose skin is exposed only to nerve agent vapor pose no risk of secondary contamination; however, clothing and hair can trap vapor and this can lead to secondary contamination.
- Liquid A secondary decontamination shower that includes a soap-water solution will likely be required for liquid contamination to ensure effective physical removal of agent. Victims whose skin or clothing is contaminated with liquid nerve agent can contaminate rescuers by direct contact or through off-gassing vapor.
Basic Decontamination
Decontamination Setup
Once the Initial Isolation and Protective Action Distances (a.k.a. “Hot,” “Warm,” “Cold” Zones) have been established IAW the latest printed edition Emergency Response Guide-book, decontamination setup should occur. This includes the primary and secondary decontamination lines.
The Ladder Decontamination System (LDS) is one example of an expedient equipment set up for establishing high-volume, low-pressure decontamination. The dimensions of the corridor should be approximately 20 feet in width (between fire trucks) and approximately 40 feet in length. The LDS provides a large capacity, high-volume, low-pressure water shower (approximately 60 psi).
Two engines can create a corridor of water spray from both sides using hose lines and deck guns, while the ladder pipe provides high-volume, low-pressure water flow from above.
Multiple LDSs use more than one ladder pipe to increase the length of the decontamination corridor to accommodate larger groups of victims and can be established to provide decontamination for different groups, such as ambulatory and non-ambulatory victims or even to provide decontamination at hospitals.
Note: Containment of runoff must be considered at every mass casualty decontamination incident. However, the speed of decontamination, especially in the case of chemically contaminated victims, is of paramount importance.
Use pictorial and written posted instructions for victims to self decon when able, use locale-appropriate multilingual signage.
Double bag contaminated clothing etc. (place hearing aids, valuables in small bag). Place bag in container by showers.
Children and the elderly are at increased risk for hypothermia - provide warm showers, blankets.
The decontamination system should be designed for use in children of all ages, by parentless children, the non-ambulatory child, the child with special needs, and also allow families to stay together.
Use step-by-step child friendly instructions that explain to the children and parents what they need to do, why they are doing it, and what to expect.
Take into consideration that infants when wet are slippery and will need a way to get them through the decontamination process - i.e. plastic buckets, car seats, and stretchers.
Designate a holding area and provide staff to support and supervise the children. Recommended age appropriate staffing ratios for untended children:
- 1 adult to 4 infants
- 1 adult to 10 preschool children
- 1 adult to 20 school-age children
Mass Casualty Decontamination Conduct
This step addresses procedures for performing decontamination on a large number of victims, including providing victim instructions for properly removing clothing and proceeding through a decontamination shower corridor.
Clothing Removal:
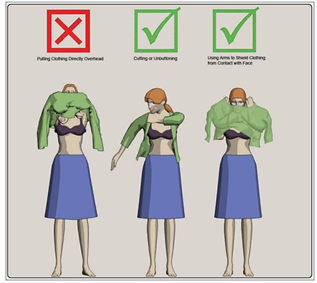
Figure 1. Proper removal of clothing
While it is not critical that victims remove their clothing for this process, it should be recommended that victims do so to the point of their own comfort level. Removal of all clothing would be most effective. Making this action a requirement may cause many citizens to become uncooperative and potentially delay the mass decontamination process. No delay should be caused by arguing the point. Removal of clothing down to the underwear is an effective compromise for all situations, with the exception of liquid contamination that has saturated outer clothing and contacted undergarments.
Note: The effectiveness of disrobing prior to decontamination rapidly decreases with time following exposure.
If clothes must be lifted over the head, instruct victims to do so carefully by closing their mouth to avoid ingestion or inhalation, and by placing hands and arms on the inside of the garment and using the hands to pull the head opening away from the face and head as much as possible. These precautions will reduce the chance of exposing the head, face and eyes to inhalation or ingestion contamination. Whenever possible, victims should unbutton or cut clothes away rather than lift them over their head (Figure 7-4). This will reduce the chance of exposing the head, face and eyes to contamination.
Cover all open wounds with plastic wrap prior to performing decontamination (particular attention should be made to open wounds because nerve agent is readily absorbed through abraded skin).
Scraping with a wooden stick, i.e. a tongue depressor or Popsicle stick, can remove bulk agent.
Water Shower Deluge:

Figure 2. Proper body positioning for mass decontamination
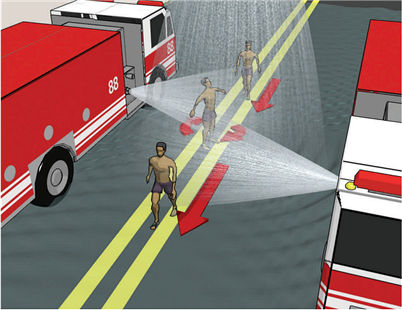
Figure 3. Proper decontamination corridor walk-through techniques
The most expedient approach following the removal of clothing is to immediately apply an emergency deluge of high-volume, low-pressure (approximately 60 psi) water shower. Thorough washing increases the effectiveness of decontamination, depending on the type of contamination, ambient environment, number of victims, and resources available.
Use available water source. Warm water temperatures may aid in the rate of chemical evaporation. Use of cool water (i.e., less than 77 degrees Fahrenheit) should be avoided unless no other means of decontamination is available.
First Responders should direct victims to proceed through the water deluge shower to enable victims to receive an initial decontamination water shower as soon as possible. First Responders should adjust the shower time to enable as many victims as possible to receive an initial decontamination water shower deluge as rapidly as possible. Prolonged skin contact with water during decontamination should be avoided. The effectiveness of wet decontamination varies according to the volatility of the contaminating chemical.
Studies have demonstrated that the effectiveness of wet decontamination varies according to the volatility of the contaminating chemical. In most cases, the use of excessive amounts of water can cause an increase in the rate of chemical absorption into the skin. Shorter durations of showering should reduce this effect. Wash time should be at least 30 seconds, but not longer than 3 minutes to ensure thorough soaking.
Time is critical. Self-care and immediate decontamination is priority! DO NOT DELAY in order to set up decontamination tents, shelter tents, or to add soap, etc. Initial research supports 30 seconds to 3 minutes timing based on minimal levels of effectiveness at the 30 second level and possible tissue damage with increased chemical absorption at 3 minutes. Actual times must be determined at the scene and take into consideration multiple factors including number of victims, environmental temperatures, index of suspicion, and clinical symptomatology.
When liquid contamination is involved, soap should be included as soon as possible in the process, HOWEVER, not to the extent that application delays initial decontamination with water. Soap may be delayed until secondary decontamination if adding it would delay initial decontamination.
When removing liquid chemical contamination (e.g., sulfur mustard), use of a wash cloth may significantly aid in decontamination by gently rubbing the contaminated area. Caution should still be exercised to prevent the spread of contamination. Rubbing without the aid of soap is not recommended, as it may increase spread of the liquid agent over a larger surface area of the body, resulting in increased medical risk.
Segregation for Observation & Monitoring
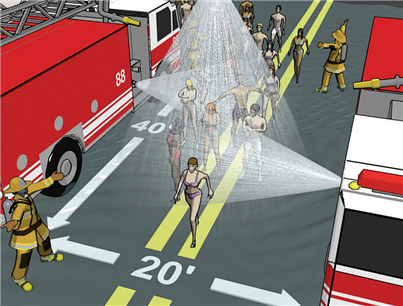
Figure 4. Decontamination using decontamination corridor setup
Actions to be taken following completion of initial mass decontamination includes re-robing, observing victims for delayed symptoms and determining visual evidence of residual contamination (such as off-gassing); performing repeat decontamination as necessary; arranging for clothing/cover for decontaminated victims; recovering personal items (if possible); and transporting victims to medical facilities for follow-on care.
Providing Victims a Means of Clothing/Cover
Whenever practical, victims should be provided a means of clothing or cover; both to restore modesty and provide warmth. Common items employed by response agencies during response exercises include a wide variety commercial off the shelf items (e.g., disposable paper suits / gowns, socks, or slippers, foil rescue blankets, sheets, and/or large plastic garbage bags).
Tag Victims to Identify Decontamination Status
Decontaminated victims should be identified to aid medical personnel and others in determining potential risk to themselves when treating or assisting victims. Identification should include a method that can account for both initial mass decontamination and repeat decontamination. Some examples include the use of colored rubber bands and specially developed triage tags.
Technical Patient Decontamination
Secondary decontamination, with an emulsifier (such as soap) may be necessary if an oily liquid hazard (e.g., sulfur mustard) is involved, whereas initial decontamination is performed. While the use of a soap-water solution is best for physical removal of gross contamination of all hazards, it will likely be required for oily liquid agents in order to provide the most effective physical removal of the agent from the victims’ skin. Rubbing without the aid of soap is not recommended, as it may increase spread of the liquid agent over a larger surface area of the body, resulting in increased medical risk.
Use this method only if responders are capable of immediately applying a soap and water solution as this method represents the best solution for all HAZMAT/WMD mass casualty decontamination situations. In the absence of soap, application of water via the LDS is the preferred primary method of decontamination.
Secondary decontamination can also be set up between the mass decontamination and the safe/refuge observation area, as necessary. A second pass through the primary water shower deluge may suffice if resources are available. Follow the prescribed procedure for secondary decontamination illustrated in Step 4.
For comprehensive overview of soap and water decontamination, link to Hospital Decontamination.
Cold Weather Guidelines
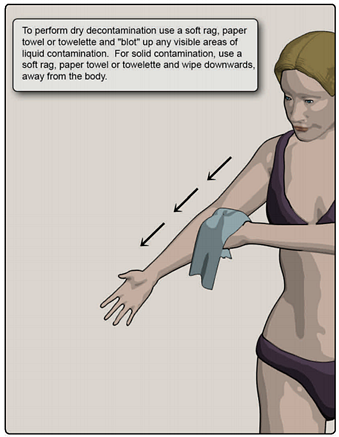
Figure 5. Dry decontamination method
Even in cold weather conditions, it is still most practical to conduct decontamination outdoors. In general, the human body can withstand very low temperatures for a brief amount of time. Children and the elderly are at increased risk for hypothermia.
The recommended methods for mass casualty decontamination, immediate clothing removal and a high-volume, low-pressure shower, remain the same for outdoor air temperatures as low as 36º F. Once victims are decontaminated, they should be provided with clothing/cover and moved to a heated facility.
For outdoor air temperatures 35º F and below, removal of clothing and an alternate decontamination method for removal of liquid contamination is recommended, such as blotting with paper towel, followed by high-volume, low-pressure water shower at a heated facility.
Symptoms of hypothermia include sensation of cold, exhaustion, and numbness. Signs of hypothermia include shivering, pallor (i.e., deficiency of color, especially of the face; aka paleness) in adults, flushed skin in children, decrease hand coordination, confusion, and slurred speech.
Mild hypothermia can be treated with passive re-warming by using blankets. Moderate hypothermia requires active re-warming with warm intravenous fluids, oxygen, lavage, or immersion baths. Sever hypothermia might require both active re-warming and cardiopulmonary bypass. The core body temperature should be re-warmed by 2° to 4° F [1° to 2° C] per hour. As needed, cardiopulmonary resuscitation and supportive care should be provided, cardiac rhythm monitored, and electrolytes replenished.
Note: In a mass casualty decontamination situation in extreme cold, decontamination with water could create a greater hazard and result in more cold weather casualties due to hypothermia than the contamination hazard.
Link to - Cold weather decontaminations pictorial guidelines
Link to - Decontamination of first responders
Decontamination of Infants and Children
- Video: Decontamination of Infants and Children (HHS/AHRQ, Children's Hospital Boston) (Watch video)
- Decontamination of Children (HHS/AHRQ) provides a step-by-step decontamination demonstration in real time, and trains clinicians about the nuances of treating infants and children, who require special attention during decontamination.
Wound Management
- Link to Wound Management
DOD reference for decontamination
- Link to Updated Guidelines for Mass Casualty Decontamination During a HAZMAT/Weapon of Mass Destruction Incident, Volumes I and II
Support Zone
Support Zone
Re-triage
Following decontamination the patient should be reassessed; noting changes in triage category (if any), the need for or the modification of supportive therapy as well as the initiation or continuation of nerve agent specific antidotes (See ABC reminders/Advanced Treatment) .
ABC Reminders
Quickly access airway patency. If trauma is suspected, maintain cervical immobilization manually and apply a cervical collar and a backboard when feasible. In pregnant women, avoid supine hypotension syndrome by maintaining a left lateral tilt. Ensure adequate respiration and pulse. Document oxygen saturation. Place on a cardiac monitor.
Link to Primary and Secondary Survey
If the patient is symptomatic, immediately institute emergency life support measures, including the use of nerve agent specific antidotes.
Advanced Treatment
In cases of respiratory compromise, secure airway and respiration via endotracheal intubation or laryngeal mask airway (LMA) (use Bag Valve Mask (BVM) if unable to secure airway). If clinically indicated, perform cricothyroidotomy or place 14 gauge intracath in the cricothryroid membrane (if equipped and trained to do so).
Link to 14 gauge intracath placement instructions.
Patients who are in cardiorespiratory failure or have seizures should be treated according to advanced life support (ALS) protocols.
In cases of ingestion, do not induce emesis. If the victim is alert, asymptomatic, and has a gag reflex, administer slurry of activated charcoal (administer at 1 gm/kg, usual adult dose 60-90 g, child dose 25-50 g). A soda can and a straw may be of assistance when offering charcoal to a child (consider naso-gastric tube - if possible contact ED prior to use of NG tube in infants and children [risk vs. benefit of inducing emesis with NG tube placement]).
Link to Basic and Advanced Life Support
Antidotes
If clinically indicated, administer initial or repeat dosing of atropine, pralidoxime, and seizure medications. If possible, a system should be employed to track antidotes administered.
Transfer to Medical Facility
Only decontaminated patients or patients not requiring decontamination should be transported to a medical facility.
If a nerve agent containing solution has been ingested, prepare the ambulance in case the victim vomits toxic material. Have ready several towels and open plastic bags to quickly clean up and isolate.
Link to Sarin Specific Triage for aid in patient admission/release decisions.
If the triage decision is to release the patient, the patient’s names, addresses, and telephone numbers should be recorded. They should be advised to seek medical care promptly if symptoms develop or recur. (see Patient Information Sheet  ).
).
PDF documents can be viewed with the free Adobe® Reader™


 Show all sections
Show all sections